Abstract
Preeclampsia is a hypertensive complication of human pregnancy characterized by generalized maternal endothelial cell activation. Circulating pro-inflammatory cytokines derived from the placenta are thought to play a key role. We recently demonstrated that hypoxia-reoxygenation (H/R) of placental tissues in vitro causes equivalent oxidative stress to that seen in preeclampsia. Our aim was to determine whether H/R also increases production of tumor necrosis factor-α (TNF-α), and whether conditioned media from samples exposed to H/R causes activation of human umbilical vein endothelial cells (HUVECs). Concentrations of mRNA encoding TNF-α were significantly higher in placental tissues subjected to H/R compared to hypoxic or normoxic controls. Although there was no difference in the concentrations of TNF-α protein in tissue homogenates, levels of TNF-α protein in the medium were significantly higher after H/R compared to controls, indicating increased secretion. Furthermore, conditioned medium from samples subjected to H/R caused increased expression of E-selectin by HUVECs, and the addition of anti-TNF-α antibodies significantly reduced that activation. These results are consistent with our hypothesis that intermittent perfusion of the placenta, secondary to reduced trophoblast invasion, causes increased secretion of TNF-α, and that this contributes to the activation of maternal endothelial cells that characterizes preeclampsia.
Preeclampsia is a human pregnancy-specific disorder, diagnosed on the basis of newly acquired hypertension and proteinuria after 20 weeks gestation, that affects 3 to 5% of pregnancies. It is a major cause of maternal and perinatal mortality and morbidity, and is characterized by an enhanced maternal systemic inflammatory response associated with diffuse endothelial cell activation.1,2 Although the mechanisms underlying the pathogenesis are not fully understood, a key role for plasma cytokines, in particular tumor necrosis factor-α (TNF-α), has been hypothesized.3–5 Several lines of evidence support the “TNF-α hypothesis.” Firstly, plasma TNF-α has direct contact with the maternal endothelial cells in vivo. Secondly, elevated plasma concentrations of TNF-α have been observed in women with preeclampsia as compared to normal pregnant women.3 Thirdly, chronic infusion of TNF-α into rats during late pregnancy results in a significant increase in renal vascular resistance and arterial pressure.6 Finally, TNF-α has been shown to up-regulate the expression of numerous molecules such as platelet-derived growth factor,7 cell adhesion molecules,8 endothelin-1,9 and plasminogen activator inhibitor-110 in endothelial cells, indicating their activation. These molecules have detrimental effects on the vasculature, and also characterize preeclamptic pregnancy.2 Other effects of TNF-α on the endothelium include microvascular protein leakage3 and reduced acetylcholine-induced vascular relaxation.11
TNF-α can be produced by a variety of cell types on appropriate stimulation, including placental cells.12,13 The presence of a placenta is both necessary and sufficient to cause preeclampsia,1 and women with complete molar pregnancy have higher plasma levels of TNF-α14 and an increased risk of developing preeclampsia compared to normal pregnant women.15 In addition, elevated levels of TNF-α protein and mRNA have been demonstrated in the preeclamptic placenta.16,17 Therefore, it is likely that the placenta is one of the major sources of TNF-α production in preeclampsia.
The trigger for placental production of TNF-α in preeclampsia is unknown, although hypoxia has been suggested as a possible etiological factor.18 The most widely recognized predisposing factor for preeclampsia is deficient invasion of the endometrium by extravillous cytotrophoblast cells during the first trimester of pregnancy, leading to incomplete conversion of the spiral arteries.19 These arteries often additionally display acute atherotic changes.20 As a result, a consensus has gradually emerged that the placental lesions associated with preeclampsia arise from a state of chronic hypoxia. To support this hypothesis, it has been shown that cultured villous explants from term human placenta produced more TNF-α under hypoxia (2% O2) than those incubated under standard culture conditions (20% O2).18
However, a survey of the impact of oxygen on placental development and structure indicates that the concept of chronic placental hypoxia in preeclampsia may be overly simplistic.21 For example, examination of placental metabolism in preeclampsia reveals that there is no reduction in energy supplies as would be expected if there were indeed chronic hypoxia.22 Furthermore, despite the numerous claims of placental hypoxia in preeclampsia, no direct measurements on the dissolved oxygen tension within the intervillous space have been performed in vivo to confirm that this is the case. By contrast, pregnancy at high altitude is the one condition in which it is known that the oxygen tension of the maternal arterial supply to the placenta is significantly reduced.23 Examination of placentas from uncomplicated pregnancies at high altitude reveals that the organ is remarkably normal, and does not show an increased level of infarction.24 It would appear, therefore, that a reduced oxygen tension per se does not cause the lesion most characteristically associated with preeclampsia.
An alternative mechanism for the placental changes in preeclampsia is ischemia-reperfusion injury. The retention of vasoreactivity in the incompletely remodeled spiral arteries results in the maternal blood flow to the intervillous space being more variable or pulsatile than normal, as evidenced by Doppler studies.25 We suggest this leads to fluctuations in the oxygen tension within the placenta, so providing the basis for a hypoxia-reoxygenation (H/R) type insult. Our previous work has demonstrated that H/R leads to placental oxidative stress and apoptotic changes,26,27 two prominent features of the preeclamptic placenta. Here, we hypothesize that the stress induced may also regulate production of placental TNF-α.
The objectives of this study were therefore to determine whether H/R stimulates production of TNF-α in term human placental tissues compared to controls kept hypoxic or normoxic throughout, to investigate the role of TNF-α converting enzyme (TACE) in placental production of TNF-α after H/R, to determine the in vitro effects of conditioned medium from placental tissues subjected to H/R on cultured human umbilical vein endothelial cells (HUVECs), and to define the contribution of TNF-α to the activation of HUVECs.
Materials and Methods
Reagents were purchased from Sigma Chemical (Poole, UK), unless otherwise indicated.
Placental Tissue Collection, Culture, and Conditions
Human term placentas were obtained with ethical approval and informed consent from uncomplicated pregnancies, and villous samples cultured, as previously described.27 Conditions for hypoxia, normoxia, and standard H/R were established as previously detailed.26 For H/R experiments, villous samples were cultured under hypoxic conditions (dissolved PO2 12 to 16 mmHg) for 3 hours, and then transferred to medium equilibrated with air/5% CO2 (dissolved PO2 143 to 160 mmHg) in a separate humidified chamber continuously flushed with air/5% CO2 for an additional 4 hours. As controls, villous samples were kept under either hypoxic or normoxic (dissolved PO2 45 to 62 mmHg) conditions throughout the 7-hour period with a change of medium at 3 hours. Supernatant from villous tissue culture was designated as placental-conditioned medium, while freshly prepared placental-culture medium was designated as unconditioned medium.
In a separate set of control experiments villous samples were subjected to H/R using medium equilibrated with either air/5% CO2 or 5% O2/5% CO2 (dissolved PO2 45 to 62 mmHg) during the reoxygenation period.
Real-Time Quantitative RT-PCR
Total RNA was extracted from different villous samples using RNeasy Mini Kits (Qiagen, West Sussex, UK) and then subjected to reverse transcription using SUPERSCRIPT II RNase H Reverse Transcriptase (Invitrogen, Paisley, UK). Real-time quantitative PCR analysis was performed with an ABI PRISM 7700 sequence detector (Applied Biosystems, Foster City, CA, USA). The primers and probes for TNF-α and TACE were synthesized by MWG-Biotech Ltd. (Milton Keynes, UK) according to sequences reported previously.28 18S ribosomal RNA was used as endogenous control and each sample was run in triplicate. Thermal cycling was initiated with a 2-minute incubation at 50°C, followed by a first denaturation step of 10 minutes at 95°C and then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Relative quantification of TNF-α and TACE mRNA to 18S ribosomal RNA was calculated by the comparative cycle threshold method.28
In Situ Hybridization
To localize the transcripts of TNF-α mRNA, frozen unfixed villous samples were cut at 8 μm, fixed in ice-cold 4% paraformaldehyde in phosphate-buffered saline (PBS), washed, dehydrated, and then subjected to in situ hybridization as previously detailed.29 Single-stranded anti-sense and sense DNA oligonucleotide probes [Human TNF-α Probe Cocktail (R&D Systems, Oxon, UK)] were used. These were 3′ end-labeled by the addition of deoxyadenosine 5′-[α-35S] thiotriphosphate (Amersham Pharmacia Biotech, Buckinghamshire, UK), using terminal deoxynucleotidyl transferase (Roche Diagnostics, East Sussex, UK). After hybridization and washing, slides were subjected to emulsion autoradiography. Three placentas were used.
Immunohistochemistry for TNF-α and TACE
After quenching endogenous peroxidase activity and blocking non-specific binding, sections were reacted with the following primary antibodies: goat polyclonal anti-human TNF-α antibody (1:200; R&D Systems) and rabbit polyclonal anti-human TACE cytosolic domain antibody (1:4000; R&D Systems). Further processing for colorimetric detection was according to the instructions for the Vectastain Elite ABC Kit (Vector Laboratories, Peterborough, UK) using diaminobenzidine as the peroxidase substrate. Negative controls include substitution of the primary antibody with non-immune goat IgG or rabbit serum. Seven placentas were studied.
ELISA of TNF-α in Villous Homogenates and Placental-Conditioned Media
Frozen villous samples were homogenized, centrifuged, and protein concentrations determined in the supernatants. ELISA was performed using high sensitivity kits (R&D Systems). All samples (villous homogenates and corresponding conditioned media) and standards were assayed in duplicate. Results were normalized per mg villous protein. Fourteen placentas were analyzed.
Western Blots for TACE
Western blots were performed as previously detailed.26 Protein samples were separated by 8% SDS-PAGE, transferred to nitrocellulose membranes, and probed with a goat polyclonal antibody against human TACE (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA). Six placentas were studied.
Culture of HUVECs
HUVECs were obtained from BioWhittaker UK Ltd. (Wokingham, UK), and grown in complete endothelial cell growth medium (EGM; BioWhittaker) at 37°C with air/5% CO2. For individual experiments, the cells were seeded at a density of 20,000 cells/well in 24-well plates. Each well contained a 0.1% gelatin-coated coverslip (No. 1 thickness and 13-mm diameter). Cells were not used after the third passage. After the cells reached 90% confluence, the medium was aspirated, replaced with fresh EGM with incremental concentrations of placental-conditioned medium (1%, 10%, 50%) to a total volume of 0.5 ml in each well, and incubated for the periods indicated. Cells grown in EGM alone, or in EGM with the same concentrations of placental-unconditioned media, were used as controls. Each experimental condition was run in duplicate.
Viability Assay of HUVECs
Viability of HUVECs was measured by the trypan blue exclusion assay using a hemocytometer.
Immunofluorescence for E-Selectin on HUVECs
After incubating for the indicated time, cells were washed with ice-cold PBS, fixed with 2% paraformaldehyde in PBS at 4°C for 20 minutes, and permeabilized with 1% Tween-20 and 1% Triton-X 100 in PBS at room temperature for 5 minutes. After blocking non-specific binding, cells were reacted with mouse anti-human E-selectin monoclonal antibody (10 μg/ml; R&D Systems) at 4°C overnight. After washes, cells were incubated with FITC-conjugated goat anti-mouse IgG (1:50; CN Biosciences, Nottingham, UK) at room temperature for 1 hour. The coverslips were inverted, mounted with Vectashield-DAPI (Vector Laboratories) and observed with a Leica TCS-NT-UV confocal microscope (Leica Microsystems, Heidelberg, Germany). Negative controls were obtained by substitution of the primary antibody with mouse isotypic IgG1 (DAKO, Bucks, UK).
For quantification of surface E-selectin expression, all settings including laser power, the acousto-optic threshold filter, and detection photomultiplier tube gain were kept constant throughout. To avoid bleaching, digital images from 15 randomly selected fields for each coverslip were rapidly saved for later analysis. Quantitative assessment was achieved using the Leica true confocal scanner-Windows NT quantification software to measure total fluorescent intensity of each field blind to the culture conditions. The values for the negative controls served as an index of background fluorescence and were subtracted from the experimental results. Finally, the values were normalized to the number of cells within the field.
Effects of Anti-TNF-α Antibody on the Expression of E-Selectin
To determine the contribution of secreted TNF-α to the expression of E-selectin, placental-conditioned media from villous tissues subjected to H/R were pre-treated with a goat anti-human TNF-α antibody (R&D Systems) at a final concentration of 0.01 to 10 μg/ml at 37°C for 1 hour before mixing with the EGM. After 24 hours of incubation, cells were processed as above and the immunofluorescence of E-selectin was measured. Controls included omission of the anti-TNF-α antibody or substitution with non-immune goat IgG at a final concentration of 10 μg/ml.
Statistical Analysis
Data are presented as mean ± SEM or median and interquartile ranges, and statistical analysis was computed with analysis of variance or Kruskal-Wallis non-parametric tests, as appropriate. Sheffe’s post hoc tests were performed if significant effects were determined. Differences between two groups were evaluated with the Student’s t-test. Statistical significance was set at a P < 0.05.
Results
Expression of TNF-α mRNA after H/R
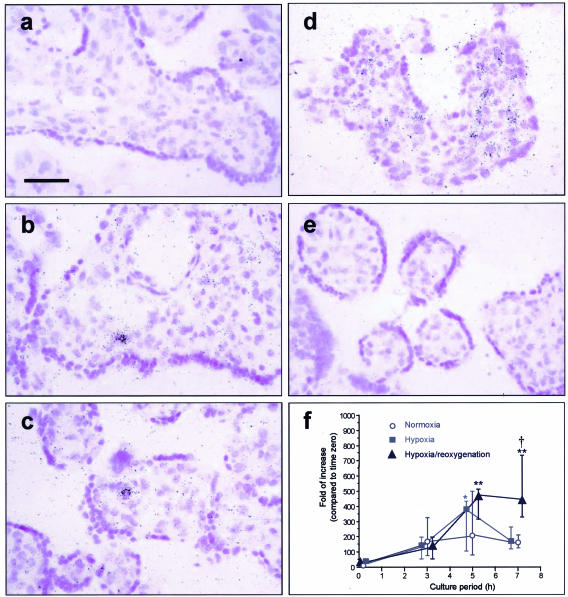
In situ hybridization showed that the signal for TNF-α mRNA was confined mainly within the stromal cells (Figure 1). In general, villous samples subjected to standard H/R had more clusters of signal in the stroma than hypoxic or normoxic controls. Using real-time quantitative PCR there was an increase in TNF-α mRNA at 3 hours under normoxic conditions, but concentrations then remained stable to 7 hours. Hypoxia caused a similar level at 3 hours, but this doubled at 5 hours and then went down at 7 hours. By contrast, concentrations in villous tissues subjected to H/R remained at a median level of 400-fold increase of TNF-α mRNA expression at 7 hours as compared to time 0 (Figure 1). There was no difference in concentrations between tissues reoxygenated with 21% or 5% O2 (P = 0.519, n = 3, data not shown)
Figure 1.
In situ hybridization showing signal for TNF-α mRNA were mainly localized within the stromal cells. In general, villous samples subjected to H/R (d) had more clusters of signal in the stroma than tissues sampled immediately after delivery (a), and kept under hypoxia (b) or normoxia throughout (c). Villous samples incubated with sense probes served as negative controls (e). Bar, 50 μm. f: Temporal expression of TNF-α mRNA under different culture conditions. Values presented as median and interquartile ranges for six placentas. *, P < 0.05; **, P < 0.01, compared to time 0; †, P < 0.05, compared to normoxic and hypoxic controls.
Immunohistochemistry for TNF-α
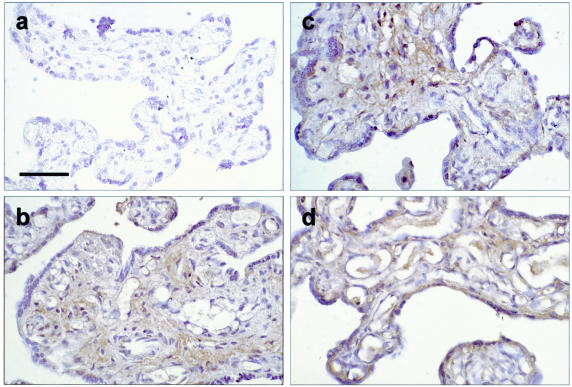
There was an apparent increase in the immunostaining after 7 hours of in vitro culture compared to time 0 in all three groups (Figure 2). The staining was mainly in the stromal area and occasionally in the endothelium. Furthermore, greater staining was noted in the syncytiotrophoblast after standard H/R. There was considerable variability among the placentas tested, however, so it was difficult to determine whether there was a genuine difference in the overall staining intensity between these three culture conditions.
Figure 2.
Immunohistochemistry of TNF-α in tissues sampled immediately after delivery (a), kept under hypoxia (b), normoxia (c), and subjected to H/R (d). There was an increase in the immunostaining, mainly in the stromal area and occasionally in the endothelium, after 7 hours of culture in all conditions as compared to time 0. More staining in the syncytiotrophoblast was also noted after H/R. Bar, 50 μm.
Concentrations of TNF-α in Villous Homogenates and Conditioned Media
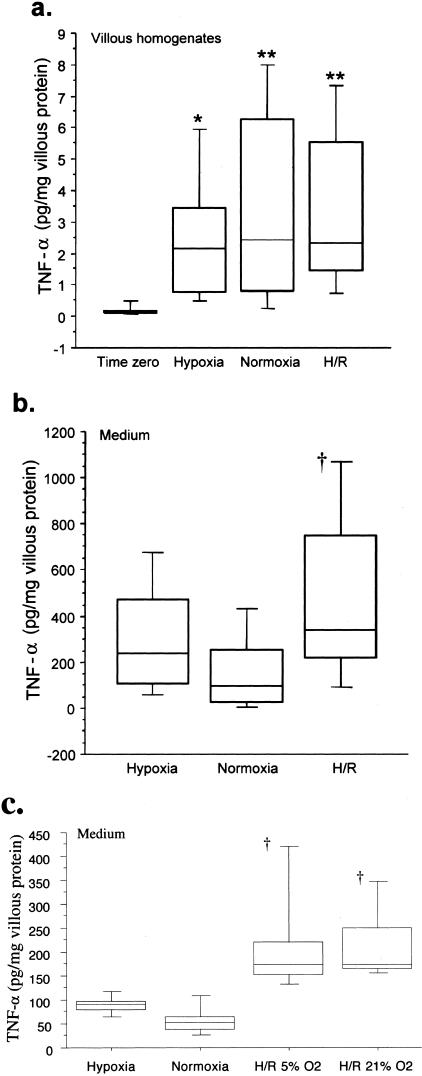
ELISA on villous tissue homogenates confirmed the impression gained from the immunohistochemistry. After 7 hours of culture there was a significant increase in tissue concentrations of TNF-α compared to time 0 (Figure 3a). There was no difference between hypoxia, normoxia, or standard H/R however, as a 6- to 8-fold range of variation between placentas was noted. Equally, there was no difference between tissues subjected to reoxygenation with either 21% or 5% O2 (P = 0.271, n = 6, data not shown). By contrast, TNF-α concentrations in the conditioned media were 1 to 200 times higher than those in the tissue homogenates (Figure 3b). In addition, concentrations were significantly higher in the medium from villous samples subjected to standard H/R compared to that from the normoxic controls. Again, no difference was observed between tissues rexoygenated with 21% or 5% O2 (P = 0.934, n = 6) (Figure 3c). Taken together, these data suggest that most of the TNF-α produced was released into the medium.
Figure 3.
TNF-α levels in villous homogenates (a), in the conditioned medium (b) after 7 hours of incubation under hypoxia, normoxia, or standard H/R, and in the conditioned medium (c) following reoxygenation under 5% O2 and 21% O2. Central bars represent median values; boxes represent interquartile ranges; and whiskers represent the 90th and 10th percentiles. *, P < 0.05; **, P < 0.01, compared with time 0; †, P < 0.05, compared to normoxia. Fourteen placentas were studied for (a) and (b), and six placentas for (c).
Temporal Expression of TACE mRNA after H/R
All three culture conditions showed a similar pattern of change in TACE mRNA expression; a mild increase at 3 hours that remained stable to 7 hours (Figure 4a). There was no significant difference between hypoxia, normoxia, or H/R.
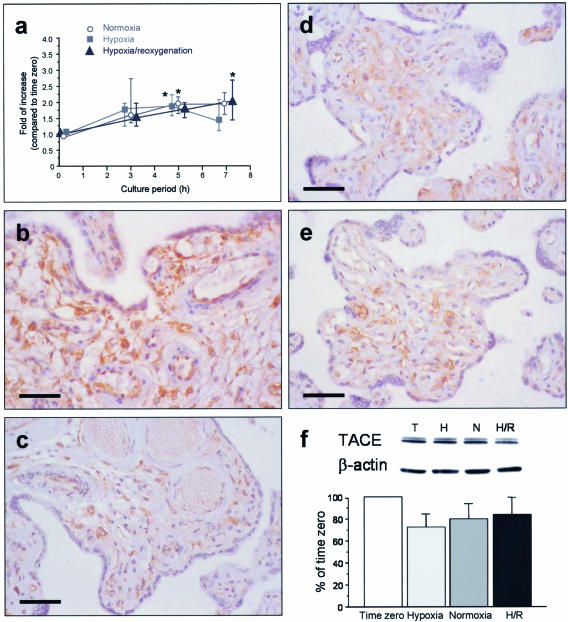
Figure 4.
a: Temporal expression of TACE mRNA under different culture conditions. *, P < 0.05, compared to time 0. However, no significant change was noted between hypoxia, normoxia, or H/R. Data presented as median and interquartile ranges for six placentas. Immunohistochemistry of TACE (b–e). In villous tissues sampled immediately after delivery (b), the immunostaining was mainly localized in the villous endothelium, some of the stromal cells and to a lesser extent the syncytiotrophoblast. After 7 hours of culture, there was generally a mild decrease in immunostaining, particularly in the syncytiotrophoblast, in villous samples kept under hypoxia (c), normoxia (d), and subjected to H/R (e). Bar, 50 μm. f: Western blot analysis revealed a decrease in tissue levels of TACE, though not statistically significant, after 7 hours of culture as compared to time 0. There was no significant difference in levels of TACE between hypoxia, normoxia, or H/R. Data presented as mean ± SEM for six separate experiments. Representative blots shown in the above. T, time 0; H, hypoxia; N, normoxia; H/R, hypoxia-reoxygenation.
Immunohistochemistry and Western Blot Analysis for TACE
In tissues sampled immediately after delivery, immunohistochemistry revealed that TACE was mainly localized in the villous endothelium, some of the stromal cells, and, to a lesser extent, in the syncytiotrophoblast (Figure 4b). After 7 hours of culture, there was a general mild decrease in the immunostaining, particularly in the syncytiotrophoblast (Figure 4, c to e). Western blot analysis also revealed that there was a decrease, though not statistically significant, in the tissue concentrations of TACE after 7 hours of culture (Figure 4f). There was no significant difference in the levels of TACE between hypoxia, normoxia, or H/R.
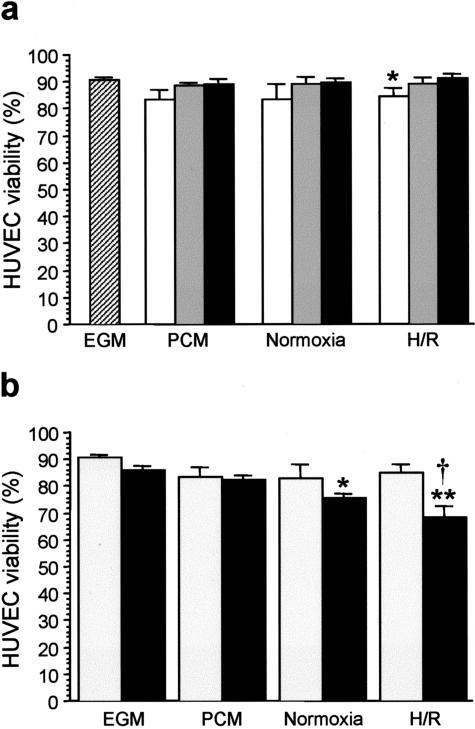
Placental-Conditioned Medium Reduces the Viability of HUVECs
Figure 5 shows the effects of incremental concentrations of placental-conditioned medium (1%, 10%, 50%) with EGM on the viability of HUVECs. After 24 hours of incubation, the overall viability of HUVECs grown in EGM alone was 90%. There was a concentration-dependent response, though not statistically significant, in cells grown in conditioned media from villous tissues kept at normoxia or in placental-unconditioned media (Figure 5a). The difference became statistically significant in cells incubated with conditioned media from villous tissues subjected to standard H/R. Incubation with 50% placental-conditioned medium caused significantly more endothelial cell death than 1% placental-conditioned medium (Figure 5a, P < 0.05). However, at a final concentration of 50% placental-conditioned medium, there was no difference in the overall viability between cells grown in conditioned media from villous tissues subjected to H/R and those grown in conditioned media from villous tissues kept at normoxia or in placental-unconditioned media. As a result, all subsequent experiments were carried out using a growth medium containing 50% placental-conditioned medium and 50% complete EGM.
Figure 5.
a: Effects of increasing concentrations of placental-unconditioned or -conditioned medium (black bars, 1%; gray bars, 10%; open bars, 50%) on the viability of HUVECs after 24 hours of incubation. *, P < 0.05; compared to 1% placental-unconditioned or -conditioned medium. Hatched bar, cells grown in endothelial cell growth medium (EGM) alone. b: Effects of incubation period on the viability of HUVECs grown in a final concentration of 50% placental-conditioned medium. Gray bars, 24 hours of incubation; black bars, 48 hours of incubation. *, P < 0.05; **, P < 0.01, compared to cells grown in EGM alone. †, P < 0.05, compared to 24 hours of incubation. PCM, cells grown in placental-unconditioned medium; Normoxia, cells grown in conditioned medium from villous tissues kept at normoxia throughout; H/R, cells grown in conditioned medium from tissues subjected to hypoxia-reoxygenation. Data presented as mean ± SEM from four and three different experiments in (a) and (b), respectively.
Next, the effects of the length of the incubation period on the viability of HUVECs grown in a final concentration of 50% placental-conditioned medium were examined (Figure 5b). When viability at 48 hours was compared between the four groups the overall viability was significantly lower in cells grown in conditioned media from villous tissues subjected to H/R (P < 0.01) or kept at normoxia (P < 0.05), compared to those grown in EGM alone. When comparisons were made between viability at 24 and 48 hours within each group, it was found that 48 hours of incubation generally resulted in fewer viable cells, particularly in cells grown in medium containing placental-conditioned medium from villous tissues subjected to H/R (P < 0.05).
Immunofluorescence for E-Selectin Expression on HUVECs
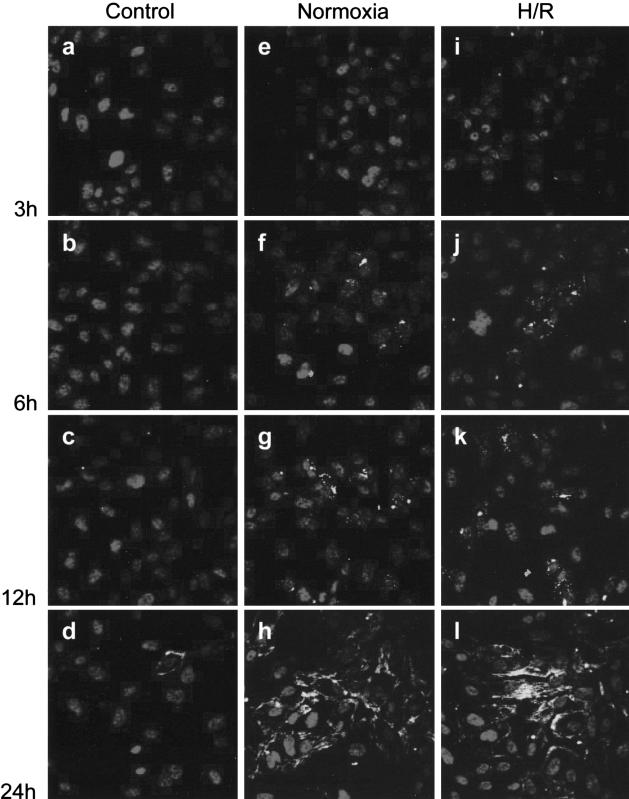
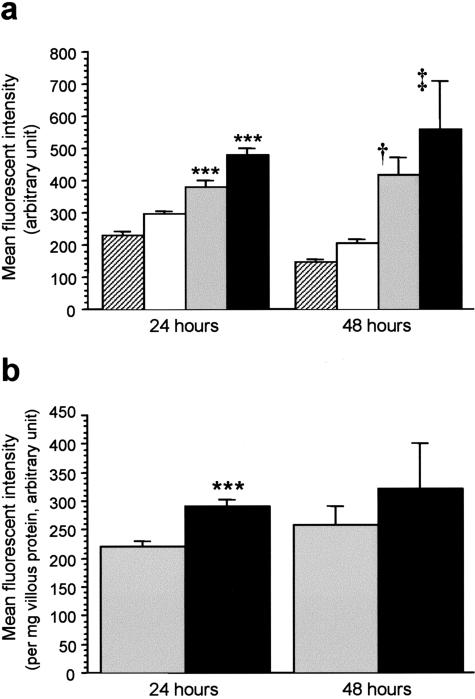
Immunofluorescence of E-selectin was first detectable after 6 hours of incubation and became more apparent at 24 hours on cells exposed to placental-conditioned media from villous tissues subjected to H/R or kept at normoxia throughout (Figure 6). In contrast, placental-unconditioned media caused only a few cells to express E-selectin at 24 hours of incubation. This was further confirmed by the quantitative analysis of the immunofluorescent intensity of E-selectin on endothelial monolayers (Figure 7). At 24 and 48 hours of incubation, E-selectin was significantly higher on cells grown in placental-conditioned media from villous tissues subjected to H/R or kept at normoxia throughout, as compared to controls with EGM alone or with placental-unconditioned media (Figure 7a). To compare the effects of placental-conditioned medium from villous tissues subjected to H/R and those from villous tissues kept at normoxia throughout, immunofluorescent intensity of E-selectin was further normalized per milligram of villous protein to correct for variation in the size of each villous sample when generating the conditioned medium. After 24 hours of incubation, conditioned medium from villous tissues subjected to H/R significantly caused more E-selectin expression than conditioned medium from villous tissues kept at normoxia (Figure 7b). However, the difference became non-significant at 48 hours of incubation due to increased variability within the groups.
Figure 6.
Temporal change of immunofluorescence for E-selectin on HUVECs after incubation with placental-unconditioned medium (a–d), conditioned medium from tissues kept under normoxia (e–h), or conditioned medium from tissues subjected to H/R (i–l). Immunofluorescence for E-selectin was first detectable after 6 hours of incubation and became more apparent at 24 hours in cells grown in media containing placental-conditioned media from tissues subjected to H/R or kept normoxic throughout. In contrast, placental-unconditioned media caused only a few cells to express E-selectin at 24 hours.
Figure 7.
a: Expression of E-selectin on HUVECs after 24 and 48 hours of incubation in EGM only (hatched bars), in placental-unconditioned medium (open bars) or in placental-conditioned media from tissues subjected to H/R (black bars), kept at normoxia (gray bars). ***, P < 0.001, compared to EGM controls at 24 hours. †, P < 0.01; ‡, P < 0.001, compared to EGM controls at 48 hours. Data presented as mean ± SEM for three separate experiments. b: HUVECs grown in conditioned medium from villous tissues subjected to H/R (black bars) expressed significantly more E-selectin than those grown in conditioned medium from villous tissues kept at normoxia (gray bars) at 24 hours of incubation. The difference became non-significant at 48 hours. ***, P < 0.001. Data presented as mean ± SEM for three separate experiments.
Effects of Anti-TNF-α Antibody on Expression of E-Selectin
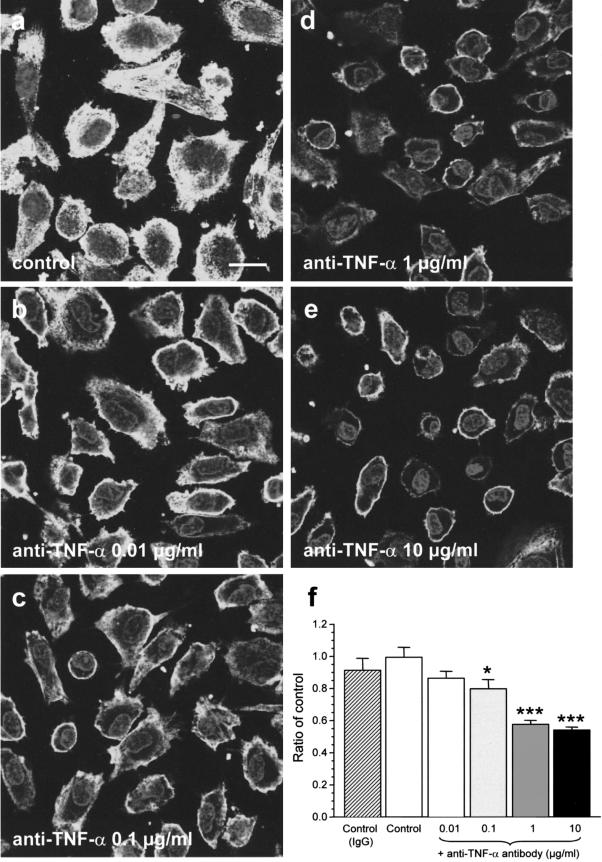
Figure 8 summarizes the effects of anti-TNF-α antibody on the expression of E-selectin. Pretreatment of the placental-conditioned medium from villous tissues subjected to H/R with the anti-human TNF-α neutralizing antibody at a final concentration of 1 to 10 μg/ml significantly reduced the E-selectin by 40%.
Figure 8.
Pretreatment of placental-conditioned medium from villous tissues subjected to H/R with incremental concentrations of anti-human TNF-α neutralizing antibody significantly attenuated expression of E-selectin (b–e), in a concentration-dependent response (f). *, P < 0.05; ***, P < 0.001, compared to cells grown in placental-conditioned medium only. Hatched bar, placental-conditioned medium with 10 μg/ml non-immune goat IgG. Data represented means ± SEM for three separate experiments. Optical sections were taken through the mid-position of the cells (a–e). Bar, 20 μm.
Discussion
In this study, hypoxia-reoxygenation caused an increase in placental TNF-α mRNA level, and increased production of TNF-α which was principally secreted into the culture medium. The fact that the rise in tissue TNF-α mRNA, and tissue and supernatant concentrations for TNF-α protein were the same following reoxygenation with 5% or 21% oxygen confirms that this is an H/R effect rather than a hyperoxic insult. Furthermore, conditioned medium from villous tissues subjected to H/R caused growth disturbance and expression of E-selectin on cultured HUVECs. It was found that TNF-α in the conditioned medium contributed, at least in part, to the activation of HUVECs.
The most likely source of TNF-α production in human placenta is the villous stromal cells, particularly the macrophages. Our in situ hybridization results localized TNF-α transcripts principally within the stromal cells, and are supported by studies using multi-label flow cytometry.30,31 However, other reports have shown TNF-α mRNA to be present in the syncytiotrophoblast of term placenta by in situ hybridization, and by RT-PCR on cytotrophoblast preparations.12,13 Differences in design and sensitivity of the probes (oliogonucleotide versus riboprobes) and the presence of contaminating cells in the cytotrophoblast preparations may explain these inconsistencies.
The cause for the increased placental expression and production of TNF-α is unclear, but generation of reactive oxygen species (ROS) as a result of H/R may play an important role. Our previous work has shown that re-oxygenation with either 5% or 21% oxygen causes extensive formation of ROS within placental tissues.26 Although little is known about the intracellular signaling cascade leading to placental production of TNF-α, considerable information exists concerning the roles of p38 mitogen-activated protein (MAP) kinase and nuclear factor-κB (NF-κB) in lipopolysaccharide (LPS)-induced TNF-α production in macrophages.32 Using placental explants incubated with LPS in the presence or absence of sulfasalazine, an inhibitor for NF-κB activation, Lappas and colleagues33 demonstrated that NF-κB activation regulates the production of TNF-α in human placentas. Furthermore, evidence from other organ systems such as heart and kidney shows that ROS can activate p38 MAP kinase and NF-κB.34 Therefore, the induction of TNF-α gene transcription following H/R is likely to be due to direct activation of p38 MAP kinase and NF-κB by locally formed ROS.
Another possible mechanism for the increased production of TNF-α involves increased post-translational processing by TACE.35 Up-regulation of TACE mRNA and protein with associated increased enzyme activity has been reported in in vitro models of ischemia-reperfusion on rat cortical cultures.36 However, we were unable to detect significant changes in the level of TACE mRNA and protein in our samples subjected to H/R. It seems unlikely, therefore, that TACE protein plays an important part in the increased secretion of TNF-α induced by H/R, although activity of the enzyme was not studied.
Because the activity of TNF-α is usually exerted over a short radius via autocrine or paracrine effects, detection of a substantial increase in the amount of this cytokine in the culture medium reflects not only excessive production but also saturation of the binding capacities of its receptors within the tissue. This could explain why there was no difference in the overall immunostaining and levels of immunoreactive TNF-α measured by ELISA in villous samples subjected to hypoxia, normoxia, or H/R.
The placenta is often thought to be the source of the increased circulating TNF-α in preeclampsia, but a recent study showed that while concentrations in peripheral and uterine venous blood were elevated in preeclamptic patients compared with normal pregnant women, the ratio between the two was not significantly different from 1.0 for either patient group.37 However, these measurements were performed at the time of caesarean delivery, by which time the syndrome was fully established and additional sources of TNF-α may have masked the placental contribution. The possibility that placental production plays an important role in initiating the disease, either directly or by activating maternal leukocytes during their passage through the organ, cannot be discounted. In an in vitro model treatment of cultured syncytiotrophoblast with TNF-α up-regulated apical expression of ICAM-1 in a concentration-dependent response, with enhanced adhesion of maternal monocytes.38 Furthemore, Mellembakken and co-workers39 compared the expression of adhesion molecules and complement-related markers on neutrophils and monocytes obtained simultaneously from the antecubital and uterine veins during caesarean sections and found significant activation of neutrophils and monocytes during utero-placental passage in preeclamptic, but not in normal pregnancies. Together, these findings suggest that a local inflammatory response involving enhanced leukocyte-syncytiotrophoblast interaction may contribute to an alternative pathogenesis of preeclampsia. When these activated leukocytes enter the maternal circulation they may cause peripheral endothelial cell activation.
There were several reasons why E-selectin was chosen as a marker for endothelial cell activation. First, E-selectin is endothelial cell-specific and is expressed only after de novo synthesis following activation.40 Second, E-selectin plays a key role in the neutrophil-endothelial adhesion.41 Third, soluble E-selectin has been suggested as an indicator of alteration in the functional state of the endothelial cells and elevated circulating levels of soluble E-selectin have been reported in patients with preeclampsia as compared to normal pregnant women.42 The kinetics of E-selectin expression in HUVECs following stimulation by TNF-α have been previously characterized.40 Expression peaks in 4 to 6 hours, declines to basal levels by 24 to 48 hours, and requires de novo mRNA and protein synthesis. Our efforts to define the temporal change of E-selectin expression after incubation with the conditioned medium revealed a persistent increase at 24 hours and even higher, though not statistically significant, at 48 hours. Other factors, such as interleukin-1β (IL-1β) and interferon-γ (IFN-γ), may be present in the conditioned medium and aggravate or regulate the expression of E-selectin. This is compatible with our finding that although pretreatment of the placental-conditioned medium from villous tissues subjected to H/R with the anti-human TNF-α antibody reduced the E-selectin expression in a concentration-dependent response, it did not abolish it entirely. IL-1β is another potent stimulus for the expression of E-selectin40 and IFN-γ, though not itself inducing E-selectin expression, appears to prolong its expression in endothelial cells in response to TNF-α stimulation.43
There is clearly an overlap in the effects caused by the hypoxia and H/R. Both may also arise from the same underlying problem of impaired conversion of the spiral arteries, and thus are difficult to separate on a clinical basis. We must therefore speculate as to which is the more likely scenario. It is well established that in many cases of late-onset preeclampsia fetal and placental weights are normal. This argues against chronic hypoxia being the causative agent, as does the constancy of energy levels within the placental tissues.22 Furthermore, our in vitro work has demonstrated that of the two insults, H/R is by far the most potent at inducing the placental changes seen in preeclampsia. Although hypoxia can induce some of the same changes, it should be noted that the level of hypoxia used to induce these changes was almost certainly incompatible with survival of a fetus in vivo. Finally, the demonstration of increased expression of xanthine oxidase in the preeclamptic placenta provides arguably the strongest evidence for the potential of an H/R injury.44 All these points reinforce the general concept that, at physiological levels, fluctuations in the oxygen concentration are more important than the absolute level in regulating cell behavior.
Acknowledgments
We thank the staff of the Delivery Unit of the Rosie Hospital, Cambridge for their help in obtaining placental material. The imaging was performed in the Multi-Imaging Centre of the School of Biological Sciences, University of Cambridge, which was established with grants from the Wellcome Trust.
Footnotes
Address reprint requests to Dr. Graham J. Burton, Department of Anatomy, University of Cambridge, Cambridge CB2 3DY, UK. E-mail: gjb2@cam.ac.uk.
Supported by Chang Gung Memorial Hospital (CMRP 1034 and BMRP688) and by the National Science Council of Taiwan (NSC92–2314-B182A-193).
References
- Redman CWG, Sargent IL. Placental debris, oxidative stress, and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-α induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15:170–175. doi: 10.1016/s0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Hajjar DP, Silverstein RL, Nachman RL. Tumor necrosis factor-mediated release of platelet-derived growth factor from cultured endothelial cells. J Exp Med. 1987;166:235–245. doi: 10.1084/jem.166.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read MA, Neish AS, Luscinskas FW, Palombella VJ, Maniatis T, Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995;2:493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-α. Am J Physiol. 1992;262:C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW, Kooistra T, van den Berg EA, Princen HM, Fiers W, Emeis JJ. Tumor necrosis factor increases the production of plasminogen activator inhibitor in human endothelial cells in vitro and in rats in vivo. Blood. 1988;72:1467–1473. [PubMed] [Google Scholar]
- Wang P, Ba ZF, Chaudry IH. Administration of tumor necrosis factor-α in vivo depresses endothelium-dependent relaxation. Am J Physiol. 1994;266:H2535–H2541. doi: 10.1152/ajpheart.1994.266.6.H2535. [DOI] [PubMed] [Google Scholar]
- Chen HL, Yang YP, Hu XL, Yelavarthi KK, Fishback JL, Hunt JS. Tumor necrosis factor α mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991;139:327–335. [PMC free article] [PubMed] [Google Scholar]
- Phillips TA, Ni J, Hunt JS. Death-inducing tumour necrosis factor (TNF) superfamily ligands and receptors are transcribed in human placentae, cytotrophoblasts, placental macrophages, and placental cell lines. Placenta. 2001;22:663–672. doi: 10.1053/plac.2001.0703. [DOI] [PubMed] [Google Scholar]
- Shaarawy M, Darwish NA. Serum cytokines in gestational trophoblastic diseases. Acta Oncol. 1995;34:177–182. doi: 10.3109/02841869509093953. [DOI] [PubMed] [Google Scholar]
- Page EW. The relation between hydatid moles, relative ischaemia of the gravid uterus, and the placental origin of eclampsia. Am J Obstet Gynecol. 1939;37:291–293. [Google Scholar]
- Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JNJ, Bennett WA. Expression of the placental cytokines tumor necrosis factor α, interleukin 1β, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181:915–920. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Walsh SW. TNF α concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol. 1996;32:157–169. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- Brosens I, Robertson WB, Dixon HG. The role of spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- De Wolf F, Robertson WB, Brosens I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am J Obstet Gynecol. 1975;123:164–174. doi: 10.1016/0002-9378(75)90522-0. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Hung T-H. Hypoxia-reoxygenation; a potential source of placental oxidative stress in normal pregnancy and preeclampsia. Fetal Maternal Med Rev. 2003;14:97–117. [Google Scholar]
- Bloxham DL, Bullen BE, Walters BNJ, Lao TT. Placental glycolysis and energy metabolism in preeclampsia. Am J Obstet Gynecol. 1987;157:97–101. doi: 10.1016/s0002-9378(87)80354-x. [DOI] [PubMed] [Google Scholar]
- Espinoza J, Sebire NJ, McAuliffe F, Krampl E, Nicolaides KH. Placental villus morphology in relation to maternal hypoxia at high altitude. Placenta. 2001;22:606–608. doi: 10.1053/plac.2001.0696. [DOI] [PubMed] [Google Scholar]
- Reshetnikova OS, Burton GJ, Milovanov AP. Effects of hypobaric hypoxia on the feto-placental unit: the morphometric diffusing capacity of the villous membrane at high altitude. Am J Obstet Gynecol. 1994;171:1560–1565. doi: 10.1016/0002-9378(94)90402-2. [DOI] [PubMed] [Google Scholar]
- Aardema MW, Oosterhof H, Timmer A, van Rooy I, Aarnoudse JG. Uterine artery Doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta. 2001;22:405–411. doi: 10.1053/plac.2001.0676. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol. 2001;159:1031–1043. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T-H, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia/reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- Satoh M, Nakamura M, Saitoh H, Satoh H, Maesawa C, Seawa I, Tashiro A, Hiramori K. Tumor necrosis factor-α converting enzyme and tumor necrosis factor-α in human dilated cardiomyopathy. Circulation. 1999;99:3260–3265. doi: 10.1161/01.cir.99.25.3260. [DOI] [PubMed] [Google Scholar]
- Pinnock SB, Herbert J. Corticosterone differentially modulates expression of corticotropin releasing factor and argine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. Eur J Neurosci. 2001;13:576–584. doi: 10.1046/j.0953-816x.2000.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks GP, Clover LM, Bainbridge DR, Redman CW, Sargent IL. Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta. 2001;22:550–559. doi: 10.1053/plac.2001.0686. [DOI] [PubMed] [Google Scholar]
- Vince G, Shorter S, Starkey P, Humphreys J, Clover LM, Wilkins T, Sargent I, Redman C. Localization of tumour necrosis factor production in cells at the materno/fetal interface in human pregnancy. Clin Exp Immunol. 1992;88:174–180. doi: 10.1111/j.1365-2249.1992.tb03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor κ B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67:668–673. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, Hai T, Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- McGeehan GM, Becherer JD, Bast RCJ, Boyer CM, Champion B, Connolly KM, Conway JG, Furdon P, Karp S, Kidao S. Regulation of tumour necrosis factor-α processing by a metalloproteinase inhibitor. Nature. 1994;370:558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- Hurtado O, Lizasoain I, Fernandez-Tome P, Alvarez-Barrientos A, Leza JC, Lorenzo P, Moro MA. TACE/ADAM17-TNF-α pathway in rat cortical cultures after exposure to oxygen-glucose deprivation or glutamate. J Cereb Blood Flow Metab. 2002;22:576–585. doi: 10.1097/00004647-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- Xiao J, Garcia-Lloret M, Winkler-Lowen B, Miller R, Simpson K, Guilbert LJ. ICAM-1 mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts: implications for placental villitis. Am J Pathol. 1997;150:1845–1860. [PMC free article] [PubMed] [Google Scholar]
- Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002;39:155–160. doi: 10.1161/hy0102.100778. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Stengelin S, Gimbrone MAJ, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leucocyte emigration: the multi-step paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Krauss F, Kuhn W, Lakoma C, Augustin HG. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am J Obstet Gynecol. 1997;177:443–449. doi: 10.1016/s0002-9378(97)70213-8. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg JF, von Asmuth EJ, Jeunhomme TM, Buurman WA. IFN-γ regulates the expression of the adhesion molecule ELAM-1 and IL-6 production by human endothelial cells in vitro. J Immunol. 1990;145:2110–2114. [PubMed] [Google Scholar]
- Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol. 2000;156:321–331. doi: 10.1016/S0002-9440(10)64733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]