Abstract
Transconjugant lactococcal starters which produce both lantibiotics lacticin 3147 and lacticin 481 were generated via conjugation of large bacteriocin-encoding plasmids. A representative of one of the resultant strains proved more effective at killing Lactobacillus fermentum and inhibiting the growth of Listeria monocytogenes LO28H than either of the single bacteriocin-producing parental strains, demonstrating the potential of these transconjugants as protection cultures for food safety applications.
In the last 20 years, intensive research efforts into bacteriocins produced by members of the lactic acid bacteria have resulted in the discovery of many new bacteriocins which, based on their structure-function relationships, can be divided into a number of classes. Class I consists of small, complex, posttranslationally modified peptides which are characterized by the presence of modified thioether amino acids such as lanthionine, β-methyllanthionine, and α, β-unsaturated amino acids such as dehydroalanine and dehydrobutyrine and are usually referred to as lantibiotics (21, 25).
Undoubtedly, the most extensively studied of the lantibiotics is nisin, which has gained widespread application in the food industry. This Food and Drug Administration (FDA)-approved bacteriocin is produced by the GRAS (i.e., generally recognized as safe) organism Lactococcus lactis and is in approximately 50 countries exploited as a food additive, particularly in processed cheese, dairy products, and canned foods (2).
Like nisin, lacticin 481 is a single-peptide lantibiotic produced by some strains of L. lactis (12, 13, 15). It exhibits a medium spectrum of inhibition, mainly active against other lactic acid bacteria (LAB), but it is also active against Clostridium tyrobutyricum. An interesting feature recently associated with this bacteriocin is that it causes lysis of starter lactococcal cultures, which continue to grow, albeit at a slower growth rate (12). The bacteriocin thus has potential applications for the acceleration of cheese ripening as a direct result of starter lysis with concomitant enzyme release in the cheese matrix. The structure of lacticin 481 is known (26), and the six genes responsible for bacteriocin production, immunity, and transport have been shown to be plasmid encoded (10, 12, 14, 15, 16).
In contrast to both nisin and lacticin 481, lacticin 3147 is a two-component lantibiotic, requiring both structural proteins (LtnA1 and LtnA2) to give full biological activity (7). Like nisin, lacticin 3147 exhibits a broad inhibitory spectrum and is active against a wide range of gram-positive bacteria (19). This bacteriocin has a number of potential food and biomedical applications (17). For example, it has previously been shown to improve the safety of cottage cheese (8) and the quality of cheddar cheese (18). This bacteriocin also has potential in veterinary medicine and has been shown to reduce the incidence of mastitic infection in cattle when incorporated into teat seals (20, 24). The genes encoding bacteriocin production and immunity are well characterized and are encoded on a 60-kb conjugative plasmid, pMRC01, which can be easily transferred between strains (1, 11).
A potential problem associated with bacteriocin use as biopreservatives in foods is the development of resistant populations of problematic bacteria. Consequently, a number of studies have examined the efficacy of bacteriocin combinations for pathogen inhibition, for example, sakacin A and nisin A (23) and pediocin PA-1 and nisin (4). In such instances, it is bacteriocin preparations, either purified or synthetic, that generally have been evaluated. An alternative, more economic method of introducing bacteriocins to foods could be the use of cultures that produce multiple bacteriocins. In this respect, researchers have examined the heterologous coproduction of bacteriocins in L. lactis, including enterocin A and pediocin PA-1 (6) and pediocin PA-1 and nisin (5). In both of these studies, coproduction of the bacteriocins did not improve the inhibitory activity of the strains.
Generation of double lantibiotic-producing strains.
The purpose of this study was to stack two lantibiotics in a single lactococcal strain in a food-grade manner. This initially involved performing a number of conjugations by the method described by Coakley et al. (1), using various combinations of nisin, lacticin 3147, and lacticin 481 producers as both donors and recipients. All the strains used in this study are listed in Table 1. The plasmid pCBG104, coding for lacticin 481 production and immunity, was successfully transferred into L. lactis DPC3147 at an efficiency of mobilization of 8.0 × 10−3 transconjugants/donor cell. The plasmid pMRC01, encoding lacticin 3147 production and immunity, was also successfully transferred from MG1363pMRC01 into L. lactis 481 and L. lactis DPC5552 at efficiencies of mobilization of 2.4 × 10−2 and 4.8 × 10−2 transconjugants/donor cell, respectively. The putative transconjugants were able to utilize lactose and were immune to both lacticin 3147 and lacticin 481. A number of transconjugants were selected from these conjugal matings and referred to as L. lactis strains 481pMRC01 and DPC5552pMRC01. In contrast, despite numerous attempts, isolation of transconjugants that coproduced either lacticin 3147 or lacticin 481 with nisin could not be achieved through conjugation.
TABLE 1.
Bacterial strains used in this study
| Bacterial straina | Relevant phenotype and genotypeb | Source or referencec |
|---|---|---|
| L. lactis MG1614 | Lac− | 3 |
| L. lactis MG1363pMRC01 | Lac−Ltn+ | 1 |
| L. lactis MG1614pCBG104 | Lac−Lct+ | 10 |
| L. lactis DPC3147 | Lac+Ltn+ | 19 |
| L. lactis DPC5552 | Lac+Lct+ | 12 |
| L. lactis CNRZ481 | Lac+Lct+ | 13 |
| L. lactis DPC5552pMRC01 | Lac+Lct+Ltn+ | This study |
| L. lactis 481pMRC01 | Lac+Lct+Ltn+ | This study |
| L. lactis DPC3147pCBG104 | Lac+Lct+Ltn+ | This study |
| L. lactis HP | Lac+ | DPRC |
| L. lactis 496 | Lac+ nis+ | DPRC |
| Lactobacillus fermentum | DPRC | |
| Listeria monocytogenes LO28H | DPRC |
All strains were grown at 30°C with the exception of L. fermentum and L. monocytogenes, which were grown at 37°C.
Lac, lactose; Lct, lacticin 481 genetic determinants; Ltn, lacticin 3147 genetic determinants; nis, nisin genetic determinants.
DPRC, Dairy Products Research Centre, Moorepark, Fermoy, County Cork, Ireland.
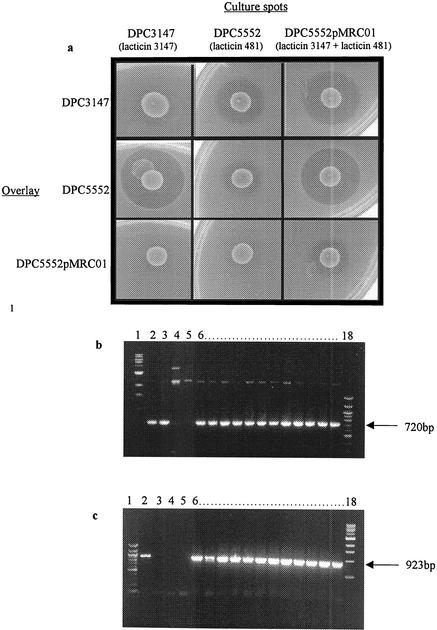
Evidence that these strains actually represented true transconjugants was obtained via PCR. Two PCRs were performed, the first of which was to indicate the presence of pMRC01 (the plasmid encoding lacticin 3147) and the second to indicate the presence of pCBG104 (the plasmid encoding lacticin 481). Primers for the first PCR were designed to amplify a 923-bp fragment which encompasses the lacticin 3147 immunity gene ltnI (9). Primers for the second PCR were designed to amplify a 720-bp fragment which encompasses the lacticin 481 structural gene lctA (14). The results demonstrated that all putative transconjugants contained both a 720-bp fragment including the lacticin 481 structural gene, lctA (Fig. 1b), and a 923-bp fragment encompassing the lacticin 3147 immunity gene, ltnI (Fig. 1c).
FIG. 1.
(a) Cross sensitivity study. DPC3147, DPC5552, and DPC5552pMRC01 were grown as culture spots (10 μl) on LM17 agar plates and overlaid with each of DPC3147, DPC5552, and DPC5552pMRC01. The appearance of zones indicates inhibition of the indicator due to bacteriocin production by the producer. (b) Identification of a portion of the lacticin 481 structural gene, lctA, by PCR using primers designed to amplify a 720-bp fragment of the gene. A linear 100-bp DNA ladder (lane 1) and a 1-kb DNA ladder (lane 18) were used as size markers. Lanes: 2, positive control (L. lactis DPC5552 genomic DNA as template); 3, positive control (L. lactis CNRZ481 genomic DNA as template); 4, negative control (L. lactis MG1614 genomic DNA as template); 5, negative control (L. lactis MGpMRC01 genomic DNA as template); 6 to 17, putative transconjugants. A band corresponding to a fragment of 720 bp is evident in lanes 2, 3, and 6 to 17, indicating that all these transconjugants contain the gene for lacticin 481 biosynthesis. (c) Identification of a portion of the lacticin 3147 immunity gene, ltnI, by PCR using primers designed to amplify a 923-bp fragment of the gene. A 1-kb DNA ladder (lane 1) and a linear 100-bp DNA ladder (lane 18) were used as size markers. Lanes: 2, positive control (L. lactis MGpMRC01 genomic DNA as template); 3, negative control (L. lactis MG1614 genomic DNA as template); 4, negative control (L. lactis DPC5552 genomic DNA as template); 5, negative control (L. lactis CNRZ481 genomic DNA as template); 6 to 17, putative transconjugants. A band corresponding to a fragment of 923 bp is evident in lanes 2 and 6 to 17, indicating that all these transconjugants contain the gene for lacticin 3147 immunity.
Of the twelve transconjugants generated from the conjugal matings, one was selected for further characterization, and this was designated DPC5552pMRC01. Cross sensitivity assays were performed with L. lactis DPC3147, a lacticin 3147 producer; L. lactis DPC5552, a lacticin 481 producer; and L. lactis DPC5552pMRC01, the double lantibiotic-producing transconjugant (Fig. 1a). The results demonstrate that the double lantibiotic-producing strain inhibits both parental strains DPC5552 and DPC3147 but is immune to the bacteriocins they produce. Interestingly, strains that produce lacticin 481 do exhibit some inhibition to themselves that is also seen with the double lantibiotic-producing strain.
Purification of lacticin 3147 and lacticin 481 from DPC5552pMRC01.
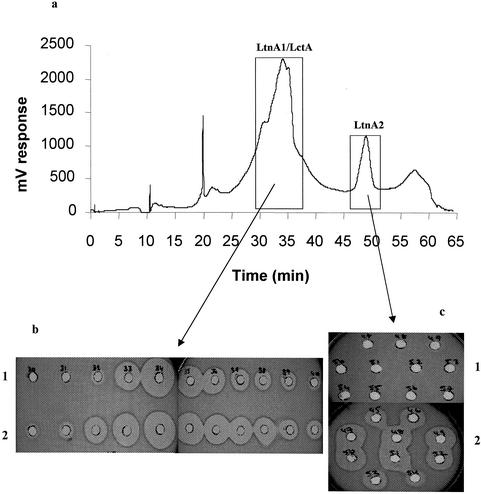
The purification protocols developed for the isolation of pure preparations of lacticin 3147 (22) and lacticin 481 (12) were modified slightly to facilitate isolation of both bacteriocins from the same culture supernatant. The modified protocol primarily involved application of 4L DPC5552pMRC01 culture supernatant to a column containing 50 g of XAD 16 beads. The column was washed with 30% (vol/vol) ethanol, and the bacteriocin was eluted in 70% (vol/vol) propan-2-ol. Following removal of the propan-2-ol by rotor evaporation, the bacteriocin preparation was applied to a C18 Varian column, from which it was subsequently eluted in 70% (vol/vol) propan-2-ol (pH 2). Following evaporation of the alcohol, 1-ml samples were subjected to repeated reversed-phase high-performance liquid chromatography (RP-HPLC). Verification that both bacteriocins maintained activity following each purification step was obtained by checking this preparation for activity against both DPC3147 and DPC5552 as indicator strains (using the DPC3147 strain as an indicator for lacticin 481 activity and vice versa). LtnA2 was easily separated from LtnA1 and LctA (lacticin 481 structural peptide), as it is significantly more hydrophobic and eluted at approximately 54% (vol/vol) propan-2-ol. In contrast, both LtnA1 and LctA coeluted at a propan-2-ol concentration of approximately 48% (vol/vol) and were indistinguishable chromatographically in a poorly defined peak (Fig. 2a). Concomitant with the separation of the two lacticin 3147 peptides on RP-HPLC, a significant loss of activity was observed when each was assayed individually against the indicator strain L. lactis HP, and full activity was restored only via complementation of fraction A1 with A2 and vice versa. Figure 2b reveals that fractions 33 to 36 gave substantial activity alone against L. lactis HP, and based on the fact that LtnA1 alone would not be expected to elicit such activity, this was ascribed to lacticin 481. Complementation of fractions 30 to 40 with purified A2 restored LtnA1 activity, as larger zones were observed (Fig. 2b). Therefore, we can conclude that these fractions contain LtnA1 and LctA, with the latter being predominant within these fractions. Complementation of fractions 45 to 54 with purified A1 restored LtnA2 activity in these fractions (Fig. 2c).
FIG. 2.
RP-HPLC analysis of LtnA1, LtnA2, and LctA. (a) Peptides were separated by HPLC, and eluted peaks were detected. (b and c) The corresponding fractions were then assayed for activity against L. lactis HP alone (1) and in combination with purified LtnA2 (2) (sections 1 and 2 of panel b, respectively) and alone (1) and in combination with purified LtnA1 (2) (sections 1 and 2 of panel c, respectively). Zones of clearing are indicative of active fractions.
Mass spectrometry analysis.
Three fractions were selected following RP-HPLC that were presumed to contain the two lacticin 3147 peptides and lacticin 481, and these fractions were subjected to mass spectrometry analysis. The profile obtained for the fraction presumed to contain lacticin 481 generated a peak of 2,900.21 Da, which is consistent with the mass of lacticin 481 (2,901 Da) (14). The profile obtained for the fraction presumed to contain LtnA2 generated a peak of 2,847 Da, which corresponds exactly to the mass of LtnA2 (22). The final fraction analyzed, which was expected to contain LtnA1, generated a signal representing 3,305 Da, consistent with the mass of LtnA1 (3,303 Da). Therefore, based on biological and biochemical analyses, we concluded that all three peptides are being produced by the double-producing strain.
Antimicrobial activity of the lacticin 3147-lacticin 481 producer.
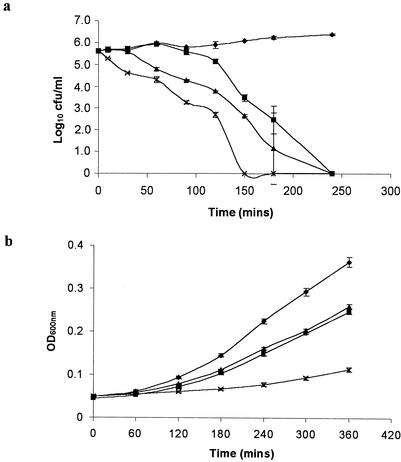
The antimicrobial potential of the double producer was assessed in two ways. In the first, live cultures of L. lactis DPC5552, L. lactis MG1363pMRC01, and L. lactis DPC5552pMRC01 were cocultured with relatively low numbers (∼105) of Lactobacillus fermentum over a 4-h incubation period. The results (Fig. 3a) demonstrate that the lacticin 481-producing culture, DPC5552, begins to inhibit Lactobacillus fermentum following 90 min of incubation and that complete kill is observed after 240 min. In the case of the lacticin 3147 producer, MG1363pMRC01, inhibition is observed after 30 min. However, in this instance, while the death rate of Lactobacillus fermentum is more rapid than in the presence of DPC5552, a complete kill is nevertheless observed only after 240 min. In contrast, the double lantibiotic-producing strain DPC5552pMRC01 exhibits an immediate inhibition of Lactobacillus fermentum. The death rate is more rapid than with either bacteriocin producer alone, and a complete kill is observed after 150 min.
FIG. 3.
(a) Growth kinetics of Lactobacillus fermentum alone (⧫) and when cocultured with L. lactis DPC5552, which produces lacticin 481 (▪); L. lactis MG1363pMRC01, which produces lacticin 3147 (▴); and L. lactis DPC5552pMRC01, which produces both lacticin 481 and lacticin 3147 (×). Error bars represent the standard deviations of duplicate experiments. (b) Growth kinetics of L. monocytogenes LO28H alone (⧫) and when treated with supernatant from L. lactis MG1363pMRC01 (□), L. lactis DPC5552 (▴), and L. lactis DPC5552pMRC01 (×). Error bars represent the standard deviations of triplicate experiments.
In a further experiment, Listeria monocytogenes LO28H was treated with supernatant from each of the bacteriocin-producing cultures, and its growth was monitored over a 6-h period. The results (Fig. 3b) demonstrate that supernatant from the double lantibiotic-producing strain has a more inhibitory effect on the growth rate of Listeria monocytogenes than either lacticin 3147 or lacticin 481 alone. Indeed, supernatant from the double bacteriocin producer reduced the growth rate of Listeria monocytogenes by almost fourfold (K values of ∼0.018 and ∼0.069 units of optical density [at 600 nm] per h, respectively). This would suggest that the bacteriocins act synergistically to mediate their inhibitory effect.
In summary, we have demonstrated that it is relatively straightforward to construct food-grade lactococcal strains which coproduce the lantibiotics lacticin 3147 and lacticin 481. This can be achieved either by conjugating the lacticin 3147 genetic determinants into a 481-producing recipient or vice versa. These strains may prove more efficacious for food applications than either parent when used either as a live cell protection culture or for the production of bacteriocin preparation for addition to food.
Acknowledgments
This work was supported by EU funds (FAIR CT98-4396), the Irish Government under the National Development Plan (2000-2006), and Science Foundation Ireland. Lisa O’Sullivan is in receipt of a Teagase Walsh fellowship.
We thank Paula O’Connor for technical assistance.
REFERENCES
- 1.Coakley, M., G. F. Fitzgerald, and R. P. Ross. 1997. Application and evaluation of the phage resistance and bacteriocin encoding the plasmid pMRC01 for the improvement of dairy starter cultures. Appl. Environ. Microbiol. 63:1434-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delves-Broughton, J. 1990. Nisin and its use as food preservative. Food Technol. 40:100-117. [Google Scholar]
- 3.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanlin, M. B., N. Kalchayanand, P. Ray, and B. Ray. 1993. Bacteriocins of lactic acid bacteria in combination have greater antibacterial activity. J. Food Prot. 56:252-255. [DOI] [PubMed] [Google Scholar]
- 5.Horn, N., M. I. Martinez, J. M. Martinez, P. E. Hernandez, M. J. Gasson, J. M. Rodriguez, and H. M. Dodd. 1999. Enhanced production of pediocin PA-1 and coproduction of nisin and pediocin PA-1 by Lactococcus lactis. Appl. Environ. Microbiol. 65:4443-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez, J. M., J. Kok, J. W. Sanders, and P. E. Hernandez. 2000. Heterologous coproduction of enterocin A and pediocin PA-1 by Lactococcus lactis: detection by specific peptide-directed antibodies. Appl. Environ. Microbiol. 66:3543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAuliffe, O., M. P. Ryan, R. P. Ross, C. Hill, P. Breeuwer, and T. Abee. 1998. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl. Environ. Microbiol. 64:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAuliffe, O., C. Hill, and R. P. Ross. 1999. Inhibition of Listeria monocytogenes in cottage cheese manufactured with a lacticin 3147-producing starter culture. J. Appl. Microbiol. 86:251-256. [DOI] [PubMed] [Google Scholar]
- 9.McAuliffe, O., C. Hill, and R. P. Ross. 2000. Identification and overexpression of ltnI, a novel gene which confers immunity to the two-component lantibiotic lacticin 3147. Microbiology 146:129-138. [DOI] [PubMed] [Google Scholar]
- 10.Mills, S., A. Coffey, L. O'Sullivan, D. Stokes, C. Hill, G. F. Fitzgerald, and R. P. Ross. 2001. Use of lacticin 481 to facilitate delivery of the bacteriophage resistant plasmid pCBG104 to cheese starters. J. Appl. Microbiol. 91:1-9. [DOI] [PubMed] [Google Scholar]
- 11.O'Sullivan, D., G. F. Fitzgerald, R. P. Ross, and C. Hill. 1998. Design of a phage-insensitive lactococcal dairy starter via sequential transfer of naturally occurring conjugative plasmids. Appl. Environ. Microbiol. 64:4618-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Sullivan, L., S. M. Morgan, R. P. Ross, and C. Hill. 2002. Elevated enzyme release from lactococcal starter cultures on exposure to the lantibiotic lacticin 481, produced by L. lactis DPC5552. J. Dairy Sci. 85:2130-2140. [DOI] [PubMed] [Google Scholar]
- 13.Piard, J.-C., P. M. Muriana, M. J. Desmazeaud, and T. R. Klaenhammer. 1992. Purification and partial characterization of lacticin 481, a lanthionine-containing bacteriocin produced by Lactococcus lactis subsp. lactis CNRZ481. Appl. Environ. Microbiol. 58:279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piard, J.-C., O. P. Kuipers, H. S. Rollema, M. J. Desmazeaud, and W. M. de Vos. 1993. Structure, organization and expression of the lct gene for Lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J. Biol. Chem. 268:16361-16368. [PubMed] [Google Scholar]
- 15.Rince, A., A. Dufour, S. Le Pogam, D. Thualt, C. M. Bourgeois, and J. P. Le Pennec. 1994. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis. Appl. Environ. Microbiol. 60:1652-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rince, A., A. Dufour, P. Uguen, J.-P. Le Pennec, and D. Haras. 1997. Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity. Appl. Environ. Microbiol. 63:4252-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross, R. P., M. Galvin, O. McAuliffe, S. M. Morgan, M. P. Ryan, D. P. Twomey, W. J. Meaney, and C. Hill. 1999. Developing applications for lactococcal bacteriocins. Antonie van Leeuwenhoek 76:337-346. [PubMed] [Google Scholar]
- 18.Ryan, M. P., M. C. Rea, C. Hill, and R. P. Ross. 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan, M. P. 1996. Characterization of a novel bacteriocin produced by a Lactococcus lactis subsp. lactis strain isolated from an Irish kefir grain. MSc thesis. University College Cork, Cork City, Ireland.
- 20.Ryan, M. P., J. Flynn, C. Hill, R. P. Ross, and W. J. Meaney. 1998. The natural food grade inhibitor, lacticin 3147, reduced the incidence of mastitis after experimental challenge with Streptococcus dysgalactiae in nonlactating dairy cows. J. Dairy Sci. 82:2625-2631. [DOI] [PubMed] [Google Scholar]
- 21.Ryan, M. P. 1999. Biochemical characterization of the novel two-component lantibiotic, lacticin 3147: exploitation for food and biomedical applications. PhD thesis. University College Cork, Cork City, Ireland.
- 22.Ryan, M. P., R. W. Jack, M. Josten, H. G. Sahl, G. Jung, R. P. Ross, and C. Hill. 1999. Extensive post-translational modification, including serine to D-alanine conversion, in the two-component lantibiotic, lacticin 3147. J. Biol. Chem. 274:37544-37550. [DOI] [PubMed] [Google Scholar]
- 23.Schillinger, U., R. Geisen, and W. H. Holzapfel. 1996. Potential of antagonistic microorganisms and bacteriocins for the biological preservation of foods. Trends Food Sci. Technol. 7:158-164. [Google Scholar]
- 24.Twomey, D. P., A. I. Wheelock, J. Flynn, W. J. Meaney, C. Hill, and R. P. Ross. 2000. Protection against Staphylococcus aureus mastitis in dairy cows using a bismuth-based teat seal containing the bacteriocin lacticin 3147. J. Dairy Sci. 83:1981-1988. [DOI] [PubMed] [Google Scholar]
- 25.Twomey, D., R. P. Ross, M. Ryan, B. Meaney, and C. Hill. 2002. Lantibiotics produced by lactic acid bacteria: structure, function and applications. Antonie van Leeuwenhoek 82:165-185. [PubMed] [Google Scholar]
- 26.van den Hooven, H. W., F. M. Lagerwerf, W. Heerma, J. Haverkamp, J.-C. Piard, C. W. Hilbers, R. J. Siezen, O. P. Kuipers, and H. S. Rollema. 1996. The structure of the lantibiotic lacticin 481 produced by Lactococcus lactis: location of the thioether bridges. FEBS Lett. 391:317-322. [DOI] [PubMed] [Google Scholar]