Abstract
Tannins are plant-derived polyphenols with antimicrobial effects. The mechanism of tannin toxicity towards Escherichia coli was determined by using an extract from Acacia mearnsii (Black wattle) as a source of condensed tannins (proanthocyanidins). E. coli growth was inhibited by tannins only when tannins were exposed to oxygen. Tannins auto-oxidize, and substantial hydrogen peroxide was generated when they were added to aerobic media. The addition of exogenous catalase permitted growth in tannin medium. E. coli mutants that lacked HPI, the major catalase, were especially sensitive to tannins, while oxyR mutants that constitutively overexpress antioxidant enzymes were resistant. A tannin-resistant mutant was isolated in which a promoter-region point mutation increased the level of HPI by 10-fold. Our results indicate that wattle condensed tannins are toxic to E. coli in aerobic medium primarily because they generate H2O2. The oxidative stress response helps E. coli strains to overcome their inhibitory effect.
Tannins are polyphenolic compounds, produced by a wide variety of plants, that can be separated into two structural groups. Hydrolysable tannins consist of a central polyol, e.g., glucose, surrounded by several gallic acid units. More complex elaggitannins are derived from pentagalloylglucose by oxidative reactions (19). Condensed tannins, or proanthocyanidins, are the most common type of tannin found in forage and browse and consist of polymers of flavonol units (24). Tannins may protect plants from herbivory, increase resistance against pathogens, or protect tissues such as wood against decay (23). Their antimicrobial activity has been the focus of research in many fields: food science, wood science, soil science, plant pathology, pharmacology, and human and animal nutrition (23). The complexity and diversity of tannin structures, as well as the presence of mixtures of phenolic compounds in plants, complicate the study of tannins.
The mechanism(s) by which tannins inhibit bacteria have not been clearly elucidated, but the biological activity of tannins is probably determined to a great extent by the molar content and spatial configuration of the ortho-phenolic hydroxyl groups (24). Studies with green tea catechins, which are monomeric polyphenolics, report that gram-positive bacteria are more sensitive to their bactericidal effect than gram-negative bacteria (12). These catechins have been shown to disrupt membrane integrity, as they cause leakage from liposomes (12). Their activity was lower in the presence of negatively charged lipids, indicating that the higher resistance of gram-negative bacteria can be explained to some extent by the presence of negatively charged lipopolysaccharides (12). Work on green tea catechins by other authors confirms that membrane damage occurred and that flavonoids such as catechins and epigallocatechin gallate insert or interact in the outer polar zone of lipid bilayers in liposomes (9, 13, 21, 29, 32).
However, dihydroxy phenolic groups can form stable complexes with many metal ions. In the presence of a nonlethal concentration of copper(II), catechins were shown to be bactericidal to gram-negative Escherichia coli, resulting in damage to the cytoplasmic membrane (10). The bactericidal activity of catechin-Cu(II) complexes was lower against the gram-positive Staphylococcus aureus (10). In this case, the mechanism of action is proposed to be due to recycling redox reactions between catechin-complexed Cu(II) and Cu(I) that are caused by molecular oxygen, generating hydrogen peroxide on the cell surface (11). The possible reason for a lower activity against gram-positive cells may be that the catechin-copper ion complex does not bind to S. aureus cells, but it appears to be attracted to the negative charge of E. coli lipopolysaccharide, generating hydrogen peroxide locally on the cell surface (10). There are other indications that growth of E. coli may be inhibited by tannins through complexation with metal ions. Growth of E. coli ATCC 25922 on low-iron plates with tannic acid, a hydrolyzable tannin, occurred only around wells containing an iron solution (4). Another experiment indicated that growth of E. coli ATCC 25922 on tannic acid-containing medium was restored after iron addition (4). Increased iron sequestration may therefore prevent inhibition by tannins.
E. coli cells possess antioxidant enzymes which are induced in response to oxidative stress. The oxyR gene positively induces the expression of at least nine hydrogen peroxide-inducible proteins, including hydroperoxidase I (HPI) (7). HPI is an OxyR-inducible catalase encoded by the katG gene expressed during exponential growth, whereas HPII is encoded by katE and induced by RpoS in stationary phase (6). Inducing the H2O2 stress response increased the survival rate of E. coli incubated with catechins in the presence of Cu(II) (11). It seems possible, then, that E. coli could resist the toxicity of tannins by engaging high-affinity iron acquisition systems and/or by activating its oxidative stress response. There is variation among E. coli strains in sensitivity to green tea catechins, as 8 of 20 clinical E. coli isolates were found to be sensitive to the effect of green tea catechins (34).
The present study was done to determine if more complex condensed tannins have an effect on Escherichia coli similar to that of green tea catechins. A commercially available extract from Acacia mearnsii (Black wattle) used in the tanning industry was used as a source of condensed tannins. The tannins present in this extract range from 300 to 3,000 atomic mass units, with an average numerical mass of approximately 1,250 atomic mass units (33). Our approach was to employ an E. coli strain that was sensitive to the effect of tannins and isolate spontaneous tolerant mutants. In this way, we could detect the mutation that confers the resistance and deduce the nature of the stress caused by tannins. This paper reports our findings that auto-oxidation of tannins resulting in hydrogen peroxide production inhibits growth of E. coli. An increase in the oxidative stress response in E. coli can decrease the inhibitory tannin effect.
MATERIALS AND METHODS
Strains.
E. coli strains used in this study are listed in Table 1.
TABLE 1.
E. coli strains used in this study
| Strain | Phenotype or genotype | Source or reference |
|---|---|---|
| BW13711a | Δ(lac)X74 | 17 |
| WTT1 | Wattle tannin-tolerant mutant of BW13711t | This study |
| TA4131b | Wild-type K12 strain | 3 |
| TA4110b | oxyR2 (hydrogen peroxide-resistant mutant) | 3 |
| JI361 | katG17::Tn10 | 5 |
| BW6165 | argE::Tn10 ara-41 lacY xyl-7 mel-2 Hfr PO120 | E. coli Genetic Stock Center |
| GS022 | Φ (katG_::lacZ) araD1139 Δ(argF-lac)169 flhD5301 fruA25 relA1 rpsL150 rbsR22 deoC1 λRS45 | 5 |
| XS01 | katG17::Tn10 | P1 (JI361) × BW13711 |
| XS02 | katG::Tn10 | P1 (JI361) × WTT1 |
| XS06 | argE::Tn10; tannin-tolerant | P1 (BW6165) × WTT1 |
Supplied by W. Metcalf, University of Illinois, Urbana, Ill.
Supplied by G. Storz, National Institute of Health, Bethesda, Md.
Media.
Experiments were performed on MOPS medium (20) with glucose (0.4%) as the carbon source and iron and trace elements provided by trace element solution SL-10 (DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany). Medium without glucose was used as a diluent. Medium prepared anaerobically was gassed with CO2 and dispensed in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, Mich.) under an atmosphere of 95% CO2 and 5% H2. In some cases, a cysteine-sulfide solution as a reducing agent was added to anaerobic medium (final concentrations of l-cysteine · HCl and Na2S · 9H2O were 0.025%). Wattle tannin extract (WTE) medium, prepared as above, contained an aqueous extract from wattle bark (Acacia mearnsii). The extract contained 65.6% phenols as tannic acid equivalents and was donated by Wickett and Craig of America, Inc. (Curwensville, Pa.). In some experiments, tannins were purified from this extract. Tannin extracts were added as filter-sterilized solutions to heat-sterilized basal medium. Agar plates were poured less than 24 h before inoculation, and liquid medium was prepared fresh prior to inoculation.
Culture conditions and measurement of growth rate.
Aerobic growth was on agar plates or in broth in tubes rotated on a Roto-Torque heavy-duty rotator (Cole-Parmer, Vernon Hills, Ill.). Anaerobic growth was in sterile anaerobic Balch tubes or on agar plates incubated in the anaerobic chamber. Cultures were incubated at 37°C.
Growth rates were monitored by measuring a change in optical density at 415 nm in sterile 96-well plates (Costar; Corning Inc. Life Sciences, Acton, Mass.) with an automated microplate reader (Bio-Tek instruments, Winooski, Vt.) run with Deltasoft 3 software (BioMetallics, Inc., Princeton, N.J.). For the 96-well-plate experiments, overnight cultures of E. coli were diluted 10−4 in MOPS diluent, and a 10-μl inoculum was inoculated into 200 μl of medium in triplicate.
Purification of condensed tannins.
Tannins were purified from wattle based on the method to purify condensed tannin from commercially available quebracho extract (2) modified by Hagerman (8). The WTE was fractionated into three parts, namely, (i) an ethanol fraction with low-molecular-weight phenols and other impurities, (ii) an ethyl acetate fraction with low-molecular-weight polyphenols (monomers, dimers and trimers), and (iii) an aqueous fraction with high-molecular-weight polyphenols.
Tannin analyses.
WTE fractions were separated by thin-layer chromatography in a solvent with 79% ethyl acetate, 11% methanol, and 10% water on silica gel plates (Sigma-Aldrich. St. Louis, Mo.). Condensed tannins were detected by spraying with vanillin-HCl reagent. Measurement of total phenolics in plant extracts was performed by the Folin-Denis method (26). Tannic acid (Fisher Scientific, Pittsburgh, Pa.) was used to generate the standard curve.
Peroxide assay.
The production rate of hydrogen peroxide (H2O2) in the growth media at room temperature was determined with an Amplex Red-horseradish peroxidase detection system (5).
Peroxidase assay.
Cell pellets of cultures at logarithmic growth were washed sequentially with cold 50 mM phosphate buffer (pH 7.8) and 10 mM phosphate buffer (pH 6.4). Cells were lysed by French press. Cell debris was removed by centrifugation at 13,000 × g for 20 min. Hydroperoxidase I (HPI) activity was assayed by the o-dianisidine method (16).
β-galactosidase assay.
Analysis of β-galactosidase was performed as described previously (5).
E. coli transduction.
Transduction of E. coli strains was done as described previously (18).
Sequencing of katG gene.
Total genomic DNA was extracted from three independent cultures with the Ultraclean soil DNA isolation kit (Mo Bio Laboratories, Inc., Solana Beach, Calif.) in accordance with the manufacturer's instructions. A 3,153-bp region of the genomic DNA which included the katG gene was amplified by PCR with primers 5′-GAAATGAGGGCGGGAAAATAAGGT-3′ (160 bp upstream of promoter region) and 5′-TGCGGCACCAGTAAAGCCACCACA-3′ (712 bp downstream of the stop codon). The PCR products were cloned into E. coli JM109-competent cells with the pGEM-T Easy Vector System I (Promega Corporation, Madison, Wis.) in accordance with the manufacturer's instructions. Clones were sequenced at the W. M. Keck Center for Comparative and Functional Genomics, Biotechnology Center, University of Illinois. The promoter regions were sequenced in both directions. The promoter region sequences together with the katG promoter region from E. coli K12 retrieved from GenBank (National Center for Biotechnology Information, Bethesda, Md.) were aligned using Clustal X (1).
RESULTS
Sensitivity of E. coli BW13711 to WTE under aerobic conditions.
An overnight culture was serially diluted in MOPS diluent and plated on MOPS and 0.1% WTE medium to determine if E. coli BW13711 was sensitive to tannins in the WTE. The colony count on MOPS medium was 1 × 109 CFU/ml, but no growth was obtained on 0.1% WTE plates at higher than a 10−1 dilution. At a 10−1 dilution, clusters of colonies grew on 0.1% WTE plates. Colonies were picked from the edge of the clusters and streaked on 0.1% WTE medium. Only three of the seven colonies picked grew, and when these three were transferred onto fresh WTE-containing medium, only one, designated WTT1, produced isolated colonies on 0.1% WTE medium. The other two grew only on densely streaked areas of the plate. The clustering of colonies and density-dependent growth suggested that E. coli cells were scavenging a diffusible inhibitor, which they could inactivate when cells were present in large numbers.
The MIC of WTE under aerobic conditions was determined for BW13711 and WTT1 in liquid culture (Table 2). Due to density-dependent growth in the presence of tannins, the overnight cultures were diluted 10−4 before the addition of a 0.05% inoculum to the media. At a concentration of 0.075% WTE, BW13711 grew at a slower rate, and there was no growth on 0.1% WTE medium. However, WTT1 could still grow in 0.1% WTE medium, but no growth was detected in 0.15% WTE medium. WTT1 could therefore tolerate a higher level of WTE in liquid medium but was still susceptible to inhibition when WTE was present above 0.1%.
TABLE 2.
Growth rates of E. coli strains on WTE medium with differing concentrations of WTE under aerobic conditionsa
| WTE concn (%) | Growth (min−1) of E. coli strain:
|
|||
|---|---|---|---|---|
| BW13711 | WTT1 | TA4131 | TA4110 | |
| 0 | 0.011 ± 0.0007 | 0.010 ± 0.0005 | 0.016 ± 0.0014 | 0.014 ± 0.0007 |
| 0.05 | 0.009 ± 0.0002 | 0.009 ± 0.0003 | 0.013 ± 0.0001 | 0.011 ± 0.0006 |
| 0.075 | 0.008 ± 0.0002 | 0.008 ± 0.0003 | 0.013 ± 0.0004 | 0.010 ± 0.0002 |
| 0.1 | NG | 0.007 ± 0.0001 | NG | 0.010 ± 0.0003 |
| 0.15 | NG | NG | NG | 0.009 ± 0.0006 |
Strain WTT1 is wattle tannin tolerant; strain TA4110 is a constitutive oxyR mutant. Results are means ± standard deviations (n = 3). NG, no growth after 48 h of incubation at 37°C.
An experiment was performed to determine if the effect of WTE on E. coli BW13711 was bactericidal or bacteriostatic under aerobic conditions. An overnight culture was diluted and inoculated into MOPS diluent, 0.1 and 0.2% WTE diluent, and 0.1 and 0.2% WTE medium, and the cell viability was monitored at intervals over a 24-h period. In MOPS diluent, the number of cells increased slightly, indicating that there was some nutrient carryover from the original culture (Table 3). In both diluent and medium containing 0.1% WTE, the number of viable bacteria remained steady for the first 8 h but increased slightly after 24 h, indicating possible detoxification over time (Table 3). The effect of 0.1% WTE in MOPS medium on E. coli BW13711 did not result in a decrease in the number of cells. However, at 0.2% wattle tannin, there were less than 10 CFU/ml after 4 h, indicating that the effect of wattle tannin in the medium is bactericidal.
TABLE 3.
Counts of viable E. coli BW13711 cells after incubation at 37°C under aerobic conditions to determine bacteriocidal or bacteriostatic effect of condensed tanninsa
| Time (h) | Cells (CFU/ml) in:
|
||
|---|---|---|---|
| MOPS diluent | 0.1% WTE diluent | 0.1% WTE medium | |
| 0 | 3.6 ± 0.08 | 3.6 ± 0.23 | 3.9 ± 0.09 |
| 4 | 4.2 ± 0.22 | 3.6 ± 0.41 | 3.3 ± 0.19 |
| 8 | 4.9 ± 0.16 | 3.1 ± 0.35 | 3.2 ± 0.07 |
| 24 | 5.4 ± 0.06 | 4.7 ± 0.54 | 5.3 ± 0.22 |
Results are means ± standard deviations (n = 3).
Since the initial experiments were carried out with a crude WTE, it was necessary to determine whether the toxicity was due to condensed tannins or other components of the extract. The WTE (65.6% phenols as tannic acid equivalents) was fractionated into three parts, with a 67% extraction efficiency of the tannins. The ethanol fraction contained 60.2% phenols (43% of the original phenolic material), the ethyl acetate fraction contained 102.4% phenols (34%), and the aqueous extract contained 91% phenols (23%). Condensed tannins were present in all three extracts as determined with the vanillin-HCl method on thin-layer chromatography plates. Sensitivity to the various fractions was determined by monitoring growth rates of E. coli BW13711 and WTT1 in medium containing 0.1% of each fraction. Both BW13711 and WTT1 could grow in medium containing the ethanol fraction, but neither grew on the ethyl acetate or aqueous extract-containing media, indicating that sensitivity is due to the tannins present in the WTE.
Condensed tannins oxidize in the presence of oxygen (27), so an experiment was conducted to determine whether auto-oxidation of tannin influences growth of E. coli BW13711. Growth rates were determined with fresh medium and stored refrigerated medium, which was oxidized as judged by a color change. E. coli BW13711 could grow at normal growth rates in oxidized tannin-containing medium but did not grow in fresh tannin-containing medium, suggesting that E. coli BW13711 is inhibited by an unstable auto-oxidation product produced in the medium.
Sensitivity of E. coli BW13711 to wattle tannin in anaerobic conditions.
To determine if E. coli BW13711 and WTT1 were sensitive to tannins under anaerobic conditions, a plate count experiment was performed. Dilutions of overnight cultures of BW13711 and WTT1 were plated on MOPS and 0.1% WTE medium. One set of media was made up aerobically and incubated aerobically. Another set was made up aerobically and incubated anaerobically. The third set was made up anaerobically, and cysteine-sulfide solution was added to the medium as a reducing agent. The fourth set was anaerobic medium without cysteine-sulfide solution to exclude any possible effect that cysteine or sulfide may have on the tannin. Growth of E. coli BW13711 under aerobic and anaerobic conditions was inhibited when WTE media was prepared aerobically; however, anaerobic growth of E. coli BW13711 was not inhibited in WTE medium prepared under anaerobic conditions (Table 4). This confirmed that it is a product of tannin auto-oxidation which inhibits E. coli BW13711. The tolerance of WTT1 to these auto-oxidation products is dependent on a gene expressed during growth under aerobic conditions, as WTT1 did not grow on media prepared aerobically but incubated anaerobically (Table 4).
TABLE 4.
Counts (log10 CFU/ml) of viable cells in overnight cultures of E. coli strains BW13711 and WTT1 on MOPS and 0.1% WTE medium prepared and incubated in the presence or absence of oxygena
| Presence of oxygen during preparation/incubationb | BW13711
|
WTT1
|
||
|---|---|---|---|---|
| MOPS | 0.1% WTE | MOPS | 0.1% WTE | |
| +/+ | 9.0 ± 0.11 | <6 | 8.8 ± 0.05 | 8.1 ± 0.43 |
| +/− | 8.7 ± 0.14 | <6 | 9.0 ± 0.29 | <6 |
| −/−c | 9.3 ± 0.06 | 9.1 ± 0.05 | 9.2 ± 0.07 | 8.8 ± 0.14 |
| −/− | 9.3 ± 0.05 | 9.0 ± 0.54 | 9.1 ± 0.11 | 9.1 ± 0.20 |
Results are means ± standard deviations (n = 3).
+, oxygen present (aerobic conditions); −, oxygen absent (anaerobic conditions).
Cysteine-sulfide solution added to reduce medium.
Oxidative stress caused by auto-oxidation of wattle tannins.
Oxidation of neighboring hydroxyl groups on the flavonol B ring could result in production of hydrogen peroxide (27). We hypothesized that hydrogen peroxide is being produced during auto-oxidation of wattle tannins and that this is responsible for the inhibitory effect on E. coli under aerobic conditions. Hydrogen peroxide was detected in 0.1% WTE medium; the rate of production, measured over 45 min after addition of the WTE, was determined to be 0.033 μM H2O2/min (standard deviation, 0.006). The rate of production in MOPS medium without the addition of tannins was 0.005 μM H2O2/min. Adding diethylenetriamine pentacetic acid, an ion chelator, to the medium resulted in a decreased accumulation rate of H2O2 (0.021 μM H2O2/min; standard deviation, 0.003). This indicated that metal ions enhanced, but were not essential for, H2O2 production. Auto-oxidation of wattle tannins in aqueous conditions therefore resulted in H2O2 production, which was higher in the presence of metal ions. We therefore hypothesized that H2O2 inhibits growth of E. coli BW13711 in WTE medium under aerobic conditions. The addition of 1% catalase (2% wt/vol) (Boehringer Mannheim) to the dilution series of an overnight culture of E. coli BW13711 before plating on 0.1% WTE medium resulted in colony counts of 9.1 ± 0.18 log10 CFU/ml compared to 9.2 ± 0.04 log10 CFU/ml on MOPS medium. The colony count on 0.1% WTE medium without catalase was <6 log10 CFU/ml. This experiment indicates that H2O2 was inhibiting growth.
To determine whether WTT1 could tolerate higher levels of H2O2 than the parent strain, MOPS medium containing various concentrations of H2O2 was inoculated. After 48 h, WTT1 had grown on both MOPS medium alone and medium containing 20 and 40 nM H2O2. Two of three wells inoculated with BW13711 were turbid in medium containing 20 nM H2O2, but no growth was observed at 40 nM H2O2. Peroxidase activity of cells in MOPS medium harvested at log phase was 0.004 U/mg of protein (standard deviation, 0.004) for BW13711 and 0.055 U/mg (standard deviation, 0.011) for WTT1. Constitutive peroxidase activity was more than 10-fold higher in the tannin-tolerant mutant, E. coli WTT1.
A constitutive OxyR mutant strain TA4110 was shown to tolerate higher levels of WTE medium than either its isogenic parent strain TA4131 or WTT1 (Table 2), again demonstrating that an increase in the oxidative stress response allows E. coli strains to overcome the inhibitory effect of wattle tannins under aerobic conditions. An experiment to confirm the importance of HPI in tannin tolerance was done by knocking out the katG gene in E. coli BW13711 and WTT1. The katG17::Tn10 null allele was transduced from JI361, and the sensitivity of the resulting transductants (XS01 and XS02) to WTE was determined. The transductants did not grow in liquid medium containing 0.05% WTE (results not shown), indicating that HPI catalase is essential for growth in the presence of wattle tannin auto-oxidation products.
Genetic analysis of the tannin-tolerant strain, WTT1.
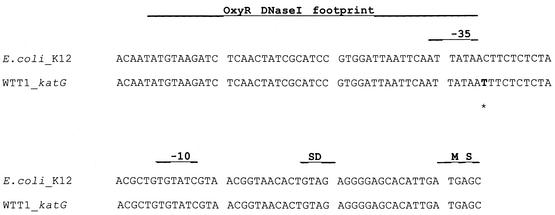
Experiments were performed to identify the mutation that causes WTT1 to overproduce HPI catalase. Our hypothesis was that either the regulatory gene oxyR or the katG gene itself was mutated. To test this hypothesis, we introduced the wild-type genes into WTT1 to determine if peroxidase activity decreased. WTT1 was transduced with P1 virus which had been grown on strain BW6165 (argE86::Tn10). The argE gene is at 89.4 min, oxyR at 89.5 min, and katG at 89.0 min. According to cotransduction frequencies, oxyR will be cotransduced with the selected argE86::Tn10 allele at an 85% frequency and katG will be cotransduced at a frequency of 50%. Eight of 17 (47%) argE::Tn10-transduced colonies screened lost the ability to overproduce peroxidase. This indicated that the mutation was in the katG gene. To ensure that the mutation was not in the oxyR gene, a transductant that contained the argE::Tn10 allele but still overproduced HPI was transduced into strain GS022. GS022 contains wild-type chromosomal oxyR and katG alleles, but it has a lambda bacteriophage bearing a katG::lacZ fusion integrated at the lambda attachment site. Four of 17 (24%) transductants of GS022 overproduced HPI catalase activity by 10-fold. However, these strains did not show any increase in expression of the katG::lacZ fusion, which would have been the case if oxyR had been activated. This indicated that the mutation must be in katG allele itself. To verify this, the promoter region of the katG gene in WTT1 was sequenced, and a C-to-T transversion was detected 1 bp beyond the putative −35 region (Fig. 1).
FIG. 1.
Sequence alignment of E. coli katG promoter regions from the GenBank database and the sequence from E. coli WTT1. Potential −35, −10, and Shine-Dalgarno sequences are indicated (31). The in vitro OxyR DNase I footprint and the sites bound by OxyR are indicated (30).
DISCUSSION
Our results indicate that oxidative modification of wattle tannins resulting in hydrogen peroxide production is responsible for E. coli BW13711 sensitivity under aerobic conditions. Further, an increase in the oxidative stress response allows E. coli strains to overcome the inhibitory effect.
Nonenzymatic auto-oxidation of neighboring hydroxyl groups of the flavonol B ring would result in formation of H2O2 (27). These results with complex condensed tannins corroborate results with monomeric polyphenols in which recycling redox reactions between copper(II) and (I) complexed to catechins resulted in bactericidal hydrogen peroxide production (11). In our experiments, the effect on E. coli BW13711 appeared to be bacteriostatic rather than bactericidal at 0.1% wattle tannins, but the H2O2 accumulation rate was lower in our study [0.033 compared to 0.69 μM H2O2/min with 100 μM (−)-epigallocatechin and 1 μM Cu(II)]. At higher tannin concentration (0.2% wattle tannins), the effect on E. coli BW13711 was bactericidal. In the presence of a chelator, there was reduction in tannin-produced H2O2, indicating that metal ions assist the production of H2O2 but are not necessary. Fe2+and Cu2+ have been shown to cause an increase in the degradation rate and an increase in browning during oxidative modification of the flavonoids quercetin and rutin (quercetin 3-O-rhamnosylglucoside) (14). In our experiments, both iron (7.5 μM) and copper (0.0117 μM) were present in WTE medium and may have formed complexes with the tannins.
The production of hydrogen peroxide would vary for different polyphenolic compounds. In the experiments performed by Makris and Rossiter (14), oxidative modification with and without metal ions was more pronounced for quercetin than for rutin. In unpublished experiments, we determined that E. coli BW13711 was not sensitive to 0.1% quebracho extract, even though the total phenolic content as tannic acid equivalents of the quebracho extract was higher than that of the WTE (88.7 compared to 65.6%). In comparative studies, it was shown that four tannins differ in the proportion of copper precipitated, with quebracho tannin giving the lowest yield of copper precipitation (15). These authors did not determine copper precipitation by wattle tannin. If metal ion precipitation by wattle tannin is greater than that of quebracho tannin, it may explain why E. coli BW13711 was not sensitive to 0.1% quebracho extract. The hydrogen peroxide accumulation rate in 0.1% quebracho tannin medium was one-fourth that of the accumulation in 0.1% WTE medium (data not shown).
The oxidative stress response is necessary for E. coli to overcome the inhibitory effect of condensed tannins in the medium. Mutants that lacked the HPI gene, an inducible catalase gene, were more sensitive to the effect of tannins. Mutants overexpressing antioxidant enzymes were less sensitive to the effect of tannins. The wattle tannin-tolerant strain, WTT1, isolated in our experiments had a more-than-10-fold increase in HPI catalase activity. A point mutation 1 base beyond the −35 region of the promoter is responsible for the increase in peroxidase activity in WTT1. Overproducing HPI mutants described in the literature were also indicated to have mutations in the katG promoter based on cotransduction frequencies (7). It has been shown that transcription activation at OxyR-dependent promoters is dependent on protein-protein contact between OxyR and the RNA polymerase α (28). The mutation may allow for the polymerase to bind in the absence of OxyR. Another possibility is that OxyR binds inappropriately to the mutant promoter region under reducing conditions and may induce the protein. OxyR has been shown to make four intermediate-strength contacts along the region to which it binds (30). In some genes, OxyR can function as both an activator and a repressor by binding to different contact points in the promoter region, with reduced OxyR having an elongated DNase I footprint. Reduced OxyR does not bind to the katG promoter region (30), but the mutation may allow for inappropriate binding of reduced OxyR and activation of the catalase.
Hydrogen peroxide is an important molecule contributing to oxidative damage in cells. In environments in which tannins produce hydrogen peroxide as a product of auto-oxidation, organisms will be inhibited. This may have an effect in soil systems where tannins will be present in decaying plant biomass. Even in gastrointestinal systems, hydrogen peroxide may be produced during mastication of plant material and in the rumen, as studies have measured levels of up to 1,630 nmol of O2/liter (25). Hydrogen peroxide is generated in beverages such as coffee and tea due to oxidation of polyphenolic components, and it has been suggested to be the main cause of coffee-induced mutagenesis (22). In many microbial species, the OxyR protein induces antioxidant defense genes in response to hydrogen peroxide. Interestingly, even in fully aerobic media, endogenous H2O2 formation appears insufficient to activate the OxyR system (5). Thus the OxyR regulon must have evolved to defend bacteria against external H2O2 sources, which have not been described. Tannins might be one such external H2O2 source.
Acknowledgments
This research and development was supported in part by funds provided by the USDA Forest Service and by the USDA Cooperative State Research, Education and Extension Service, to the International Arid Lands Consortium (IALC), which was established in 1990 as a means to promote research, demonstrations, and training applied to development, management, restoration, and reclamation of arid and semiarid lands in North America, the Middle East, and elsewhere in the world.
A. H. Smith expresses her gratitude to the Agricultural Research Council, South Africa, for support during her studies at the University of Illinois.
REFERENCES
- 1.Aiyar, A. 2000. The use of CLUSTAL W and CLUSTAL X for multiple sequence alignment. Methods Mol. Biol. 132:221-241. [DOI] [PubMed] [Google Scholar]
- 2.Asquith, T. N., and L. G. Butler. 1985. Use of dye-labeled protein as spectrophotometric assay for protein precipitants such as tannin. J. Chem. Ecol. 11:1535-1544. [DOI] [PubMed] [Google Scholar]
- 3.Christman, M. F., R. W. Morgan, F. S. Jacobson, and B. N. Ames. 1985. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41:753-762. [DOI] [PubMed] [Google Scholar]
- 4.Chung, K. T., Z. Lu, and M. W. Chou. 1998. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 36:1053-1060. [DOI] [PubMed] [Google Scholar]
- 5.Costa Seaver, L., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa Seaver, L., and J. A. Imlay. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183:7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg, J. T., and B. Demple. 1988. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 7:2611-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagerman, A. E. 2002. Tannin chemistry: Hagerman Laboratory Methods. [Online.] http://www.users.muohio.edu/hagermae/.
- 9.Hashimoto, T., S. Kumazawa, F. Nanjo, Y. Hara, and T. Nakayama. 1999. Interaction of tea catechins with lipid bilayers investigated with liposome systems. Biosci. Biotechnol. Biochem. 63:2252-2255. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino, N., T. Kimura, F. Hayakawa, A. Yamaji, and T. Ando. 2000. Bactericidal activity of catechin-copper (II) complexes against Staphylococcus aureus compared with Escherichia coli. Lett. Appl. Microbiol. 31:213-217. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino, N., T. Kimura, A. Yamaji, and T. Ando. 1999. Damage to the cytoplasmic membrane of Escherichia coli by catechin-copper (II) complexes. Free Radic. Biol. Med. 27:1245-1250. [DOI] [PubMed] [Google Scholar]
- 12.Ikigai, H., T. Nakae, Y. Hara, and T. Shimamura. 1993. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1147:132-136. [DOI] [PubMed] [Google Scholar]
- 13.Kitano, K., K. Y. Nam, S. Kimura, H. Fujiki, and Y. Imanishi. 1997. Sealing effects of (−)-epigallocatechin gallate on protein kinase C and protein phosphatase 2A. Biophys. Chem. 65:157-164. [DOI] [PubMed] [Google Scholar]
- 14.Makris, D., and J. Rossiter. 2000. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 48:3830-3838. [DOI] [PubMed] [Google Scholar]
- 15.McDonald, M., I. Mila, and A. Scalbert. 1996. Precipitation of metal ions by plant polyphenols: optimal conditions and origin of precipitation. J. Agric. Food Chem. 44:599-606. [Google Scholar]
- 16.Messner, K. R., and J. A. Imlay. 1999. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 274:10119-10128. [DOI] [PubMed] [Google Scholar]
- 17.Metcalf, W. W., P. M. Steed, and B. L. Wanner. 1990. Identification of phosphate starvation-inducible genes in Escherichia coli K-12 by DNA sequence analysis of psi::lacZ(Mu d1) transcriptional fusions. J. Bacteriol. 172:3191-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Mueller-Harvey, I. 2001. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 91:3-20. [Google Scholar]
- 20.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratty, A. K., J. Sunamoto, and N. P. Das. 1988. Interaction of flavonoids with 1,1-diphenyl-2-picrylhydrazyl free radical, liposomal membranes and soybean lipoxygenase-1. Biochem. Pharmacol. 37:989-995. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Laguna, J., and C. Pueyo. 1999. Hydrogen peroxide and coffee induce G:C→T:A transversions in the lacI gene of catalase-defective Escherichia coli. Mutagenesis 14:95-102. [DOI] [PubMed] [Google Scholar]
- 23.Scalbert, A. 1991. Antimicrobial properties of tannins. Phytochemistry 30:3875-3883. [Google Scholar]
- 24.Schofield, P., D. M. Mbugua, and A. N. Pell. 2001. Analysis of condensed tannins: a review. Anim. Feed Sci. Technol. 91:21-40. [Google Scholar]
- 25.Scott, R. I., N. Yarlett, K. Hillman, T. N. Williams, A. G. Williams, and D. Lloyd. 1983. The presence of oxygen in rumen liquor and its effects on methanogenesis. J. Appl. Bacteriol. 55:143-149. [Google Scholar]
- 26.Seigler, D. S., S. Seilheimer, J. Keesy, and H. F. Huang. 1986. Tannins from four common Acacia species of Texas and northeastern Mexico. Econ. Bot. 40:220-232. [Google Scholar]
- 27.Singleton, V. L. 1987. Oxygen with phenols and related reactions in musts, wines, and model systems: observations with practical implications. Am. J. Enol. Vitic. 38:69-77. [Google Scholar]
- 28.Tao, K., C. Zou, N. Fujita, and A. Ishihama. 1995. Mapping of the OxyR protein contact site in the C-terminal region of RNA polymerase α subunit. J. Bacteriol. 177:6740-6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terao, J., M. Piskula, and Q. Yao. 1994. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 308:278-284. [DOI] [PubMed] [Google Scholar]
- 30.Toledano, M. B., I. Kullik, F. Trinh, P. T. Baird, T. D. Schneider, and G. Storz. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78:897-909. [DOI] [PubMed] [Google Scholar]
- 31.Triggs-Raine, B. L., B. W. Doble, M. R. Mulvey, P. A. Sorby, and P. C. Loewen. 1988. Nucleotide sequence of katG, encoding catalase HPI of Escherichia coli. J. Bacteriol. 170:4415-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuchiya, H. 1999. Effects of green tea catechins on membrane fluidity. Pharmacology 59:34-44. [DOI] [PubMed] [Google Scholar]
- 33.Viviers, P. M., J. J. Botha, D. Ferreira, and D. G. Roux. 1983. Synthesis of condensed tannins. Part 7. Angular [4,6:4,8]-prorobinetinidin triflavonoids from black wattle (“Mimosa”) bark extract. J. Chem. Soc. Perkin Trans. I:17-22. [Google Scholar]
- 34.Yam, T. S., S. Shah, and J. M. Hamilton-Miller. 1997. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol. Lett. 152:169-174. [DOI] [PubMed] [Google Scholar]