Abstract
Shewanella oneidensis couples anaerobic oxidation of lactate, formate, and pyruvate to the reduction of vanadium pentoxide (VV). The bacterium reduces VV (vanadate ion) to VIV (vanadyl ion) in an anaerobic atmosphere. The resulting vanadyl ion precipitates as a VIV-containing solid.
Vanadium is a transition metal which, at neutral pH, can exist in two oxidation states, VIV (vanadyl ion, cationic species VO2+), and VV (vanadate ion, anionic species, H2VO4−) (10, 11).
The environmental chemistry of vanadium is complex. Vanadium is an abundant element that has proven to be a valuable resource for different industrial applications such as vanadium alloys, oxidation catalysis in sulfuric acid manufacturing and automobile catalytic converters, photographic development, textile dyeing, and ceramic coloring. A number of bacteria are able to reduce metal compounds, most commonly iron and manganese, through anaerobic reduction. Some organisms are known to be able to reduce other metals such as arsenic, mercury, selenium, uranium, technetium, chromium, molybdenum, gold, silver, and copper (4, 8, 13). The microbial reduction of vanadate has also been reported (1, 14). To date, two Pseudomonas strains have been described to be capable of reducing vanadium (5, 15). In this study we demonstrate that the gram-negative facultative anaerobic nonfermenting bacterium Shewanella oneidensis (6) can also reduce vanadium VV.
Vanadium uptake on solid media.
Colonies of S. oneidensis were grown on Luria-Bertani 1% agar plates containing 5 mM vanadate at 28°C for 48 h. Plates were then exposed to hydrogen sulfide in a sealed container. Upon formation of metal sulfide, plates were examined for changes in the regions surrounding or inside bacterial colonies. A halo could be seen indicating possible sequestration of the metal. Darkening of the cells indicated accumulation or possible reduction of the metal (data not shown).
Vanadate detection.
To facilitate the detection and quantification of the reduction of vanadate to VIV, a vanadate detection assay was designed, based on a detection assay for chromium described by Sandel (12). Vanadate was thereby detected on the basis of its reaction with diphenylcarbazide (DPC) in acid. The assay solution was made by addition of 1% (wt/vol) DPC in acetone to an equal volume of 2 M H2SO4. A volume of 500 μl of diluted sample was added to the same volume of assay solution. Absorbance was measured at 320 nm after 15 min. A standard curve was made with vanadium pentoxide dilutions. Vanadyl ions do not react in this assay.
Anaerobic vanadium reduction.
S. oneidensis was grown aerobically on a rotary shaker (150 rpm) at 28°C in LB medium containing 2 or 10 mM V2O5 and anaerobically with a Coy anaerobic chamber (Coy Laboratories, Grass Lake, Mich.) in phosphate-buffered defined medium (7) containing 10 mM lactate as the electron donor and 2 or 10 mM V2O5 as the electron acceptor. An increase in lag phase could be detected in cultures grown with 10 mM but not with 2 mM V2O5 compared to cultures grown aerobically in pure LB medium or anaerobically in defined medium with fumaric acid as the electron acceptor (data not shown).
The color of the anaerobic cultures eventually became blue if vanadate was used, indicating the formation of the blue vanadyl ion. The formation of reduced vanadyl ion was confirmed by capillary electrophoresis. A capillary electrophoretic method was used for simultaneous detection of vanadate and vanadyl ion, essentially as described earlier (3), with a P/ACE 2100 (Beckman Instruments, Fullerton, Calif.) and a 50-μm fused silica capillary (Polymicro Technologies, Phoenix, Ariz.). The sample was diluted 50 times in assay solution and introduced by a 15-s hydrodynamic injection. Both the [P(VVMo11)O40]4− and the [P(VIVMo11)O40]5− complex were detected at 214 nm, confirming the presence of reduced vanadyl ion. For this experiment, 50 ml of an aerobically grown late-exponential-phase culture (2 × 108 cells/ml) was centrifuged, and cells were washed and resuspended in an equal volume of anaerobic bicarbonate buffer containing 5 mM V2O5 and 2 or 10 mM lactic acid. Vanadate reduction and vanadyl formation were monitored over time (Fig. 1). The results show the initial reduction of vanadate ion to vanadyl ion and subsequently the decrease in amount of vanadyl ion by reductive precipitation. The precipitation of vanadyl ion is in accordance with the low solubility of the ion at neutral pH (9).
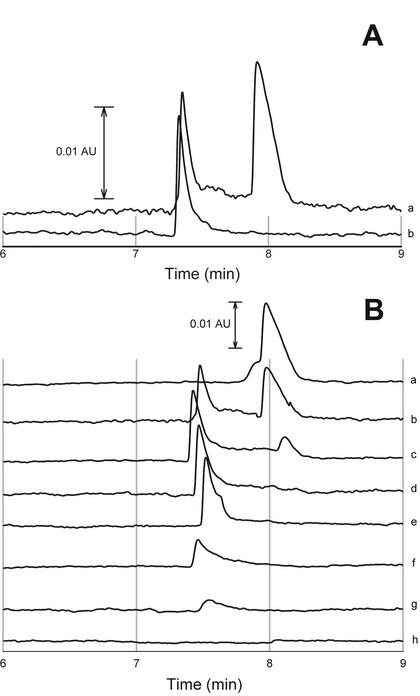
FIG. 1.
Electrophoretic determination of vanadium(V) and vanadium(IV). (A) Reference sample with 10 mM VV (right peak) and 10 mM VIV (left peak) (a) and analysis of precipitate composition (b). (B) Vanadium speciation during anaerobic reduction at different times: 0 min (a), 30 min (b), 45 min (c), 60 min (d), 90 min (e), 150 min (f), 1 day (g), and 1 week (h).
Vanadate accumulation.
To determine the accumulation of vanadate by the cells, a culture was grown aerobically on a rotary shaker (200 rpm) at 28°C in LB medium containing 2 or 10 mM V2O5. No significant reduction or precipitation was detected. The cultures had a density of 1.5 × 107 cells/ml and 1 × 107 cells/ml, respectively. Cells were harvested at 4,000 rpm for 15 min, washed and resuspended in Tris-EDTA buffer, and lysed by sonication. The vanadate concentration was determined with the DPC assay. Of the total vanadate initially present in the medium, 2% (standard error of the mean 6%) or 9% (standard error of the mean 5%) was detected in the cells grown with 2 and 10 mM V2O5, respectively.
Effect of different buffers.
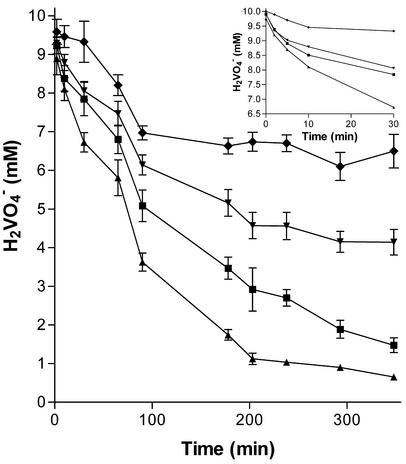
The effect of different buffers on anaerobic vanadium reduction was tested. Previous to all anaerobic experiments, sterile solutions were placed overnight in an anaerobic glove box (Coy Laboratories). Cultures were grown aerobically on a rotary shaker (150 rpm) at 28°C in LB medium to the late exponential growth phase (2 × 108 cells/ml) before being washed in appropriate buffer in anaerobic conditions and resuspended in equal volumes of either 20 mM Na2HPO4, pH 7, 20 mM Tris, pH 7, 20 mM HEPES, pH 7, or 20 mM NaHCO3, pH 7, containing 10 mM lactic acid and 5 mM V2O5. Vanadate reduction was monitored over time with the DPC assay. The buffer affected both the initial rate of reduction and the overall extent of reduction. Phosphate was revealed to be the most inhibitory, followed by Tris and HEPES. Carbonate-buffered solutions supported the highest reduction rates (Fig. 2).
FIG. 2.
Change in vanadate concentrations over time in the nongrowth mode for anaerobic cultures containing 5 mM V2O5 and 10 mM lactic acid in phosphate (⧫), Tris (▾), HEPES (▪), or carbonate (▴) buffer.
Effect of different electron donors.
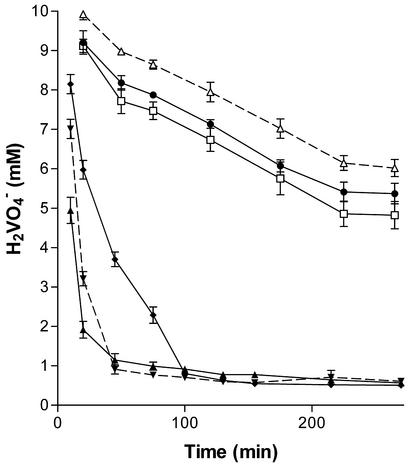
To examine the effect of different carbon sources and electron donor compounds on vanadate reduction, formic, pyruvic, citric, and fumaric acids were tested in addition to lactate. In each experiment, 50 ml of aerobically grown late-exponential-phase cultures (2 × 108 cells/ml) were centrifuged; the cells were washed, weighed, and resuspended in equal volumes of anaerobic bicarbonate buffer containing 5 mM V2O5 and 10 mM lactic, formic, pyruvic, fumaric, or citric acid. Vanadate reduction rates were monitored over time. While only a limited reduction of vanadate was measured in cultures containing fumaric and citric acid compared to cultures containing no electron donor, the cultures containing formic, lactic, and pyruvic acid significantly reduced vanadate (Fig. 3). Citric and fumaric acid cannot be used as electron donors by the organism and hence they do not support vanadate reduction.
FIG. 3.
Change in vanadate concentrations over time in the nongrowth mode of cultures containing 5 mM V2O5 and 10 mM formic (▴), lactic (▾), pyruvic (⧫), fumaric (•), or citric (□) acid. The control experiment contained 5 mM V2O5 (▵).
Vanadium reduction is a biochemical process.
Proof was found to eliminate the possibility that vanadate reduction was the result of a chemical or spontaneous process catalyzed by a biological component or product of the cell. In each experiment, 50 ml of an overnight-grown culture (2 × 108 cells/ml) was centrifuged; the cells were washed, weighed, and resuspended in equal volumes of anaerobic bicarbonate buffer containing 10 mM lactate and 5 mM V2O5. To demonstrate that the reduction is a biological process, one set of cells was heat killed prior to inoculation. Over a period of 5 h, no reduction in vanadate concentration could be measured. The reference culture had an initial reduction rate of 12.4 mmol of VV per h per g of cells (wet weight). By heat killing the cells and subsequently assaying their vanadate reduction, we demonstrated that this reduction required intact and metabolically active S. oneidensis cells.
To assess whether vanadate reduction requires de novo protein synthesis, the ribosome inhibitor tetracycline was added at a final concentration of 5 μg/ml. This concentration is known to completely inhibit growth but does not affect cell viability. In the experiment, 50 ml of an overnight-grown culture was centrifuged; the cells were washed and resuspended in equal volumes of anaerobic bicarbonate buffer containing 5 μg of tetracycline per ml. After 5 min of incubation, 10 mM lactate and 5 mM V2O5 were added. Vanadate concentration was monitored over time with the DPC assay. No significant decrease in the reduction rate could be measured, indicating that the activity does not depend on the induction of specific proteins (results not shown). The vanadate reduction pathway thus seems to be constitutively present in the bacterium.
Vanadium-containing granular precipitate.
In the course of the experiments with anaerobic vanadate-reducing cultures, a significant formation of precipitate was detected. In an attempt to rule out precipitation of a compound from the medium, different media were tested. Growth on vanadate in Luria Bertani broth, carbonate buffer, HEPES buffer, Tris buffer, and, to a limited extent, phosphate buffer resulted in a vanadate-containing precipitate. The granules were revealed to be insoluble at neutral pH, insoluble in 10 N NaOH, and soluble in 2 M H2SO4. VV is known to be soluble in basic and acidic solutions (2), while VIV is soluble in acid solutions and is not oxidized to VV below pH 2 (9). The sediment was washed three times with water. The pellet was solubilized in 2 M H2SO4. The sample was centrifuged to remove cell debris and subsequently analyzed by capillary electrophoresis. Only VIV and no vanadate could be detected (Fig. 1A).
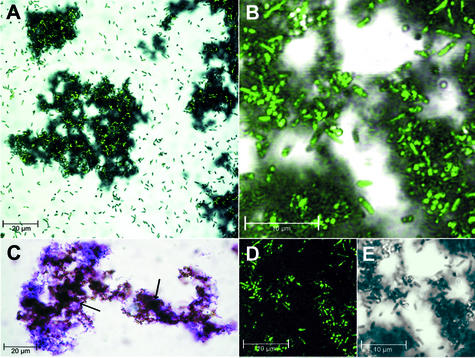
Further examination of the precipitate revealed large granules with a diameter of up to 100 μm after 5 days of growth. Precipitate formed by cultures grown with 10 mM lactic acid and 5 mM V2O5 was examined with differential interference contrast (DIC) microscopy at a magnification factor of 320× (Leica DMLB, Wetzlar, Germany). Microscopic examination combined with crystal violet staining showed that the granules were completely colonized with S. oneidensis (Fig. 4C). By confocal microscopy with a combination of DIC and HeNe laser excitation at 543 nm (Zeiss Axiovert 100 M; Carl Zeiss), an autofluorescence of the bacteria was detected (Fig. 4D and E), which allowed us to unambiguously localize the bacterium in the granule matrix. Only a small number of motile free-living cells were seen compared to the dense accumulations of sessile cells contained in the granule matrix (Fig. 4A and B).
FIG. 4.
Microscopic analysis of vanadium granules and bacterial localization. (A) Confocal microscopic image of a colonized granule (central) with autofluorescence of S. oneidensis superimposed in green (HeNe laser excitation at 543 nm). (B) Enlarged image of A, central part of the granule (split view in D and E indicating the association of autofluorescence with the presence of bacterial cells). (C) Differential interference contrast image of crystal violet-stained cells (purple) on vanadium granules (brown, indicated by arrows). (D) Confocal autofluorescence image. (E) Differential interference contrast image showing a perfect overlap with D.
In summary, we present evidence for a dissimilatory vanadate reduction process in S. oneidensis. The bacterium is able to reduce VV (vanadate ion) to VIV (vanadyl ion) under anaerobic conditions. We evaluated different electron donor compounds for their ability to sustain vanadium pentoxide reduction and showed that significant reduction can be attained with formic, lactic, and pyruvic acids under these conditions. A small but measurable accumulation of vanadium pentoxide was present inside the bacterial cells. Anaerobic reduction resulted in a granular precipitate containing predominantly VIV (vanadyl ion), which was revealed to be completely colonized by sessile S. oneidensis. Further studies will focus on identification and isolation of the components of the vanadate reduction pathway.
Acknowledgments
This work was supported by the Institute for the Promotion and Innovation of Science and Technology in Flanders (IWT) through STWW research grant 174IWT20.
REFERENCES
- 1.Bautista, E. M., and M. Alexande. 1972. Reduction of inorganic compounds by soil microorganisms. Soil Sci. Soc. Am. J. 36:918-920. [Google Scholar]
- 2.Greenwood, N. N., and A. Earnshaw. 1984. Chemistry of the elements, Pergamon Press, Oxford, p. 1147-1148.
- 3.Kitazumi, I., Y. Nakashima, and S. Himeno. 2001. Simultaneous electrophoretic determination of vanadium(V) and vanadium(IV) based on the complex formation with a Mo(VI)-P(V) reagent. J. Chromatogr. A 21:123-129. [DOI] [PubMed] [Google Scholar]
- 4.Lovley, D. R. 1993. Dissimilatory metal reduction. Annu. Rev. Microbiol. 47:263-290. [DOI] [PubMed] [Google Scholar]
- 5.Lyalikova, N. N., and N. A. Yurkova. 1992. Role of microorganisms in vanadium concentration and dispersion. Geomicrob. J. 10:15-26. [Google Scholar]
- 6.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 7.Myers, C. R., and K. H. Nealson. 1990. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J. Bacteriol. 172:6232-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niggemyer, A., S., S. Spring, E. Stackebrandt, and R. F. Rosenzweig. 2001. Isolation and characterization of a novel As(V)-reducing bacterium: implications for arsenic mobilization and the genus Desulfitobacterium. Appl. Environ. Microbiol. 67:5568-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel, B., S. J. Haswell, and R. Grzeskowiak. 1989. Flow-injection flame atomic-absorption spectrometry system for the pre-concentration of vanadium(V) and characterization of vanadium(IV) and vanadium(V) species. J. Anal. Atom. Spectrom. 4:195-198. [Google Scholar]
- 10.Rehder, D. 1991. The bioorganic chemistry of vanadium. Angew. Chem. 30:148-167. [Google Scholar]
- 11.Rehder, D. 1992. Structure and function of vanadium compounds in living organisms. Biometals 5:3-12. [DOI] [PubMed] [Google Scholar]
- 12.Sandell, E. B. 1959. Colorimetric determination of traces of metals, 3rd ed., p. 82. Interscience Publishers, New York, N.Y.
- 13.von Canstein, H., Y. Li, K. N. Timmis, W.-D. Deckwer, and L. Wagner-Döbler. 1999. Removal of mercury from chloralkali electrolysis wastewater by a mercury-resistant Pseudomonas putida strain. Appl. Environ. Microbiol. 65:5279-5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yurkova, N. A., and N. N. Lyalikova. 1990. New vanadate-reducing facultative chemolithotrophic bacteria. Microbiology 59:672-677. [Google Scholar]
- 15.Yurkova, N. A., and N. N. Lyalikova. 1993. Oxidation of molecular-hydrogen and carbon-monoxide by facultatively chemolithotrophic vanadate-reducing bacteria. Microbiology 62:367-370. [Google Scholar]