Abstract
An integrated procedure is presented whereby gas chromatography-ion trap mass spectrometry is used to determine chemical markers of gram-negative bacterial lipopolysaccharide (3-hydroxy fatty acids with 10 to 18 carbon atoms), gram-positive bacteria (branched-chain fatty acids with 15 and 17 carbon atoms), bacterial peptidoglycan (muramic acid), and fungal biomass (ergosterol) in samples of settled house dust. A hydrolysate of 13C-labeled cyanobacterial cells is used as an internal standard for the first three markers. These analyses require two dust samples, one for 3-OH fatty acids, branched-chain fatty acids, and muramic acid and another for ergosterol. The method may be used to characterize microbial communities in environmental samples.
Inhalation of airborne microorganisms in indoor environments has been associated with the development of respiratory disorders. Substances such as endotoxins (lipopolysaccharides [LPS]) (14, 17, 18, 28), peptidoglycan (9, 12), and various fungal components and products (3, 6, 21) are among the suspected causative agents. Culture-based methods are suitable for detection of culturable infectious agents and allow species identification; however, it is widely agreed that only a small fraction (0.1 to 10%) of the total microbial flora in an indoor environment is currently culturable (29). Direct microscopy is at best semiquantitative. Although the Limulus amebocyte lysate test is extremely sensitive to endotoxin and can detect glucans, it measures bioactivity rather than absolute amounts and its reproducibility and specificity have been questioned (5). Nucleic acid-based methods including PCR are very specific, and by using broad-range (universal) probes and primers sets based on 16S rDNA (20) and 18S rRNA (31), most bacteria and fungi present in a sample can be identified. To date, such universal probes and primers have not been used to characterize the microbiology of indoor environments; rather, PCR has been used to detect specific fungi and bacteria in such environments (4, 11, 22).
In our laboratory we have developed and applied gas chromatography-mass spectrometry (GC-MS) methods to determine biomarker molecules in complex matrices including organic dust. Microorganisms contain unique compounds not found elsewhere in nature, which can be used as chemical markers of larger, bioactive structures (7). Endotoxins (LPS) are major constituents of the outer membrane of gram-negative bacteria. A backbone of lipid A, the toxic component of the LPS molecule, carries in general 4 mol of unique 3-hydroxy fatty acids (3-OH FAs) (23-26). Certain branched-chain FAs are found in most gram-positive bacteria (13, 30). Muramic acid is a unique marker of peptidoglycan (2, 8, 9, 15), which is the cross-linked macromolecular structure responsible for the rigidity of bacterial cell walls and is sometimes referred to as “gram-positive bacterial endotoxin” (27). Ergosterol is a common fungal membrane lipid that is widely used as a marker of fungal biomass, although the concentration depends on the species and growth conditions (1, 6).
The aim of the present study was to develop an integrated analytical method to characterize the microbial flora of indoor environments, including both culturable and nonculturable microorganisms and cellular debris. GC-MS-MS, using ion trap technology, was applied to determine the 3-OH FAs, certain branched-chain FAs, muramic acid, and ergosterol, since this method has previously been shown to provide high detection selectivity, allowing the accurate determination of markers even when present at nanograms levels in chemically complex matrices (23).
MATERIALS AND METHODS
Chemicals, columns, and dust samples.
Ergosterol and dehydrocholesterol were purchased from ICN Biomedicals (Aurora, Ohio), lyophilized cells of 13C-labeled cyanobacteria (blue-green algae) were purchased from Isotec (Miamisburg, Ohio), and methyl esters of 3-OH FAs with 10-, 12-, 14-, 16-, and 18-carbon chain lengths and of nonhydroxylated straight - and branched-chain FAs with 16- and 17-carbon chain lengths were purchased from Larodan Lipids (Malmö, Sweden). Solvents and reagents were of analytical grade and were used without further purification. The following solid-phase extraction columns were used: silica gel columns (100 mg) purchased from Varian (Middelburg, The Netherlands), and silica gel (25 mg) and propylsulfonic acid (PRS) (100 mg) columns purchased from International Sorbent Technology (Hengoed, United Kingdom). Five house dust samples collected on a bedroom shelf, a bed, a sitting room floor, a water-damaged garage (shelf), and a basement (shelf) in a single household were used as examples in the experiments. An additional dust sample divided into subsamples was used to evaluate the reproducibility of the method. Samples were collected on cellulose filters using a vacuum cleaner equipped with filter holder (ALK). According to the manufacturer, these filters retain 74% of particles 0.3 to 0.5 μm in diameter, 81% of particles 0.5 to 1.0 μm in diameter, and 95% of particles 1 to 10 μm in diameter. Approximately 2 to 4 m2 of bed, shelves, and carpet was vacuumed individually. All samples were sieved (particle diameter, <400 μm), and the fine dust fraction was used for analysis.
Sample preparation.
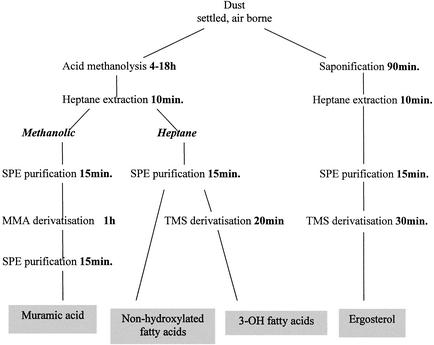
In the described procedure (Fig. 1), two portions (1 to 5 mg) of each dust sample were used, one for analyzing the levels of FAs and muramic acid and the other for analyzing ergosterol levels.
FIG. 1.
Integrated procedure for chemical marker analysis of environmental samples. SPE, solid-phase extraction; MMA, methyl ester O-methyl acetate.
(i) FAs and muramic acid.
Samples, in Teflon-lined glass test tubes, were heated in 1 ml of 2 M methanolic HCl at 85°C overnight. Subsequently, 30 μl of a methanolysate of 13C-labeled cyanobacterial cells (corresponding to 30 μg of cyanobacteria) was added, and the mixture was extracted with 1.5 ml of water-n-heptane (1:2, vol/vol). The heptane (upper) layer was used for analysis of FAs, whereas the aqueous (lower) layer was used for analysis of muramic acid as described below. Cyanobacterial 13C-labeled C16:0, 3-OH C16:0, and muramic acid were used as internal standards in the samples for non-hydroxylated FAs, 3-OH FAs, and muramic acid, respectively.
To determine the FAs, the heptane (upper) layer was evaporated under a stream of nitrogen at room temperature, redissolved in 1 ml of heptane-dichloromethane (1:1, vol/vol), and purified using a disposable silica gel column (100 mg). Prior to use, the silica gel column was washed twice with 1 ml of diethyl ether and twice with 1 ml of heptane-dichloromethane; the methyl ester-containing mixture was then added. Heptane-dichloromethane (2 ml) was added to the column to elute the nonhydroxylated FAs. The eluate was then collected in a separate test tube, evaporated, and redissolved in 100 μl of heptane for analysis. Diethyl ether (2 ml) was then added to the column to elute the hydroxy FA esters; the eluate was evaporated at room temperature. Trimethylsilyl (TMS) derivatives of the hydroxy FA esters were prepared by adding N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) (50 μl) and pyridine (5 μl) followed by heating for 20 min at 80°C. Heptane (50 μl) was then added. The preparations were analyzed following storage at 4°C overnight.
To determine muramic acid, methanol (1 ml) was added to the aqueous (lower) phase and the mixture was subjected to purification on a PRS column. Prior to use, the column was washed with 1 ml of water-methanol (1:1, vol/vol), and then the methanolysate was added followed by 1 ml of methanol. The entire elute was evaporated to dryness under nitrogen and further dried under vacuum in a desiccator (2 h). Acetylation was accomplished by heating the preparations in a mixture of pyridine (100 μl) and acetic anhydride (100 μl) at 60°C for 1 h. The reaction mixture was evaporated, dissolved in 2 ml of dichloromethane, and subsequently washed, first with a 0.05 M aqueous HCl solution (1 ml) and thereafter with water (1 ml). The samples were then evaporated to dryness, dissolved in 200 μl of chloroform, and purified using a small disposable silica gel column (25 mg). The column was washed with 400 μl of methanol and then 400 μl of chloroform prior to use, and the sample was applied. The same amounts first of chloroform and then methanol were added to elute the sample. The combined chloroform-methanol phases were evaporated, dissolved in 100 μl of chloroform, and analyzed.
(ii) Ergosterol.
Samples, in Teflon-lined glass test tubes, were heated in 3 ml of 10% methanolic KOH at 80°C for 90 min. A 5-μl volume of internal standard (dehydrocholesterol at 20 ng/μl) was added to each tube. Samples were partitioned with 2 ml of heptane-water (1:1, vol/vol), the heptane fraction was recovered, and a further 1 ml of heptane was added to and recovered from the reaction mixture. The combined heptane phases were evaporated to dryness under a stream of nitrogen at room temperature. The dried samples were subsequently dissolved in 1 ml of heptane-dichloromethane (1:1, vol/vol) and purified using a disposable silica gel column as described above for the 3-OH FA esters. The diethyl ether was evaporated, and TMS derivatization was performed by heating in BSTFA (50 μl)-pyridine (5 μl) at 60°C for 30 min. Heptane (50 μl) was added to each preparation, and the preparations were stored overnight at room temperature prior to analysis.
Reproducibility. (i) FAs and muramic acid.
Five 3-mg house dust subsamples were heated, each in 1 ml of 2 M methanolic HCl, at 85°C overnight. A 1-mg portion of the 13C-labeled cyanobacteria was heated analogously, evaporated, and dissolved in 1.0 ml of methanol; a 30-μl portion of this solution was added to each dust methanolysate as an internal standard. The mixtures were then subjected to the extraction and derivatization procedures described above. The final preparations were analyzed by GC-MS-MS.
(ii) Ergosterol.
Five 4-mg house dust subsamples were heated, each in 1 ml of 10% methanolic KOH, at 80°C for 90 min. An internal standard (5 μl of dehydrocholesterol at 20 ng/μl) was added to each hydrolysate. Further sample preparation was carried out as described above, followed by GC-MS-MS analysis.
Quantification. (i) 3-OH FAs.
A 1-mg portion of the 13C-labeled cyanobacteria was heated in 1 ml of 2 M methanolic HCl at 85°C overnight, evaporated to dryness, and dissolved in 1 ml of methanol. Of this solution, 30-μl portions were added to test tubes containing 0, 12.5, 25, 50, 100, or 200 ng of the reference 3-OH C16:0 FA methyl ester. Samples were further treated as described above prior to GC-MS-MS analysis.
(ii) Nonhydroxylated FAs.
Portions (30 μl) of a 13C-labeled cyanobacterial methanolysate prepared as described above were added to test tubes containing 0, 50, 100, 200, 400, 800, or 1,600 ng of the reference (nonhydroxylated) C16:0 FA methyl ester. The amount of 13C-labeled C16:0 FA in the cyanobacteria was calculated using GC-MS analysis in the scan mode, monitoring ions m/z 74 for the standard C16:0 FA and m/z 76 for the 13C-labeled cyanobacterial C16:0 FA. In a second series of experiments, 30-μl portions of 13C-labeled cyanobacteria were added to test tubes containing 0 to 1,600 ng of the reference C17:0, iso-C17:0, and anteiso-C17:0 FA methyl esters. Calibration graphs were constructed by performing GC-MS-MS analyses under optimized fragmentation conditions (see below). The results were extrapolated for quantification of C15:0, iso-C15:0, and anteiso-C15:0 fatty acids.
GC-MS-MS.
A Saturn 2000 ion trap GC-MS instrument (Varian, Palo Alto, Calif.) equipped with a fused-silica capillary column (CP-Sil 8 CB low bleed, 0.25 μm film thickness, 30 m by 0.25 mm inner diameter) (Chrompack, Middelburg, The Netherlands) was used in the present investigation. Volumes of 2 μl were injected in the splitless mode with a helium head column pressure of 69 kPa, using a Combi Pal autosampler (CTC Analytics AG, Zwingen, Switzerland). The temperature of the column was programmed from 90 to 280°C at 20°C/min when analyzing the FAs and muramic acid and from 170 to 290°C at 20°C/min when analyzing ergosterol; the temperature of injector was 280°C, and that of the transfer line (between the GC and MS systems) was 290°C. The ion trap temperature varied between 160 and 220°C. All analyses were made in the electron impact (EI) mode (23).
Mass spectra of the methyl ester/TMS 3-OH FA derivatives show abundant ions of m/z (M − 15), due to loss of a CH3 group, and m/z 175, due to cleavage of the C-3/C-4 linkage. The derivatized acids were measured by monitoring m/z 131 (a production of m/z 175) in GC-MS-MS (23). The amount (moles) of LPS in each sample was calculated by dividing the number of moles of the 3-OH C10 to C16 FAs by 4. EI spectra of branched-chain FA methyl esters show abundant ions of m/z (M − 43) (19). The acids were measured by monitoring m/z 101 (a product of m/z 213 for C15:0 FAs and a product of m/z 241 for C17:0 FAs). The fragmentation scheme for the muramic acid derivative has been described in detail elsewhere (2). Fragmentation of m/z 187 leads to high intensity of the product ion m/z 145, which was therefore monitored (2). The EI mass spectrum of the ergosterol TMS derivative is dominated by ions of m/z 363 (M − 105, loss of the trimethylsilanol group and one methyl group) and m/z 337 (M − 131, loss of the trimethylsilanol group and the C-1/C-3 fragment). The derivative was measured by monitoring m/z 157 (a product ion of m/z 363) (23).
RESULTS
Analytical aspects. (i) Ion trap temperature.
The ion trap temperature was found to strongly affect the chromatograms. Optimal peak shape was achieved at 220°C for the muramic acid and ergosterol derivatives, whereas 180°C was optimal for the FA derivatives (data not shown). All results hereafter reported were achieved using the optimized ion trap temperatures.
(ii) Nonhydroxylated FAs.
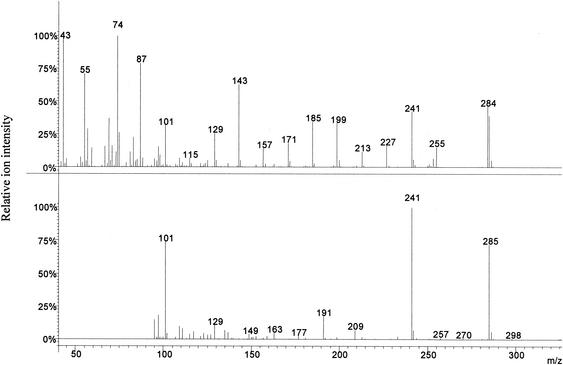
The mass spectra of the nonhydroxylated FAs with the same molecular weight were very similar. The high-mass regions gave distinctive ions of m/z 213 for the C15:0 and m/z 241 for the C17:0 (Fig. 2) FAs (M − 43). These ions were therefore selected for fragmentation in MS-MS (23 V C15:0/25 V C17:0, nonresonant mode, excitation storage level 48 m/z, isolation window 2 m/z), which resulted in fragment ions of m/z 101 used for quantification [loss of CH(CH3)- CH2-COOCH3)] (10, 32) of both groups of acids. The presence of ions of m/z 256 (results not shown) and m/z 285 (Fig. 2) in the MS-MS spectra of the C15:0 and C17:0 FA esters, respectively, may be explained by proton donor reactions. The internal standard 13C-labeled cyanobacterial C16:0 FA was analyzed in nonresonant mode (excitation storage level 48 m/z, isolation window 3 m/z); the ion of m/z 286 (parent ion, no fragmentation) was used in the quantifications.
FIG. 2.
Full-scan spectrum of the nonhydroxylated methyl ester of C17:0 (top) and the MS-MS spectrum of the same derivative following fragmentation of m/z 241 (bottom) to produce ions of m/z 101 used for monitoring in the MS-MS mode.
Calibration graphs showed good linearity within the studied range and followed the equations (y = area ratio of acid and internal standard, x = amount [nanograms] of acid): y = 0.0012x + 0.313 (C16:0; r = 0.995); y = 0.00084x (C17:0; r = 0.989); y = 0.00105x (iso-C17:0; r = 0.992); and y = 0.00044x (anteiso-C17:0; r = 0.999). The amount of 13C-labeled C16:0 in the cyanobacteria was estimated at 19.1 ng/μg (dry mass). The calibration results for C17:0, iso-C17:0, and anteiso-C17:0 fatty acids were extrapolated for quantification of the corresponding C15:0 acids.
(iii) Reproducibility.
The mean values and standard deviations calculated per milligram of dust sample were as follows: LPS, 0.026 ± 0.002 nmol; iso-C15:0, 0.125 ± 0.015 nmol; anteiso-C15:0, 0.187 ± 0.018 nmol; C15:0, 1.588 ± 0.072 nmol; iso-C17:0, 0.128 ± 0.014 nmol; anteiso-C17:0, 0.093 ± 0.009 nmol; C17:0, 0.695 ± 0.054 nmol; muramic acid, 22.124 ± 0.881 ng; and ergosterol, 2.115 ± 0.154 ng.
Comparison of dust samples.
All dust samples contained detectable amounts of 3-OH FAs, branched-chain FAs, muramic acid and ergosterol. The concentrations of the substances in the dust samples were as follows: LPS, 0.010 to 0.094 nmol/mg of dust; iso-C15:0, 0.008 to 0.318 nmol/mg of dust; anteiso-C15:0, 0.005 to 0.164 nmol/mg of dust; C15:0, 0.052 to 1.889 nmol/mg of dust; iso-C17:0, 0.004 to 0.203 nmol/mg of dust; anteiso-C17:0, 0.007 to 0.123 nmol/mg of dust; C17:0, 0.019 to 0.728 nmol/mg of dust; muramic acid, 7.2 to 134.9 ng/mg of dust: ergosterol, 0.72 to 43.6 ng/mg of dust (Table 1).
TABLE 1.
Composition of 3-OH FAs, nonhydroxylated FAs, muramic acid, and ergosterol in five different house dust samples
| Sample origin | Amt (nmol/mg of dust) of:
|
Amt (ng/mg of dust) of:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-OH C10 | 3-OH C12 | 3-OH C14 | 3-OH C16 | 3-OH C18 | LPS | iso-C15 | anteiso-C15 | C15 | iso-C17 | anteiso-C17 | C17 | MuAca | Erga | |
| Shelf | 0.0034 | 0.0255 | 0.0280 | 0.0835 | 0.0412 | 0.0351 | 0.3175 | 0.1642 | 1.8889 | 0.2031 | 0.1227 | 0.7282 | 34.1246 | 0.7243 |
| Bed | 0.0045 | 0.0094 | 0.0120 | 0.0410 | 0.0260 | 0.0167 | 0.1832 | 0.0599 | 0.5908 | 0.1337 | 0.0842 | 0.3507 | 11.3298 | 1.0637 |
| Floor | 0.0041 | 0.0094 | 0.0211 | 0.0327 | 0.0289 | 0.0168 | 0.0525 | 0.0145 | 0.3189 | 0.0409 | 0.0255 | 0.1553 | 7.1619 | 15.9993 |
| Garage | 0.0527 | 0.1118 | 0.1190 | 0.0911 | 0.0974 | 0.0937 | 0.0746 | 0.0317 | 0.1619 | 0.0507 | 0.0280 | 0.0691 | 134.8584 | 43.5851 |
| Basement | 0.0051 | 0.0114 | 0.0129 | 0.0088 | 0.0095 | 0.0096 | 0.0082 | 0.0046 | 0.0520 | 0.0043 | 0.0070 | 0.0193 | 22.7356 | 18.0565 |
MuA, muramic acid; Erg, ergosterol.
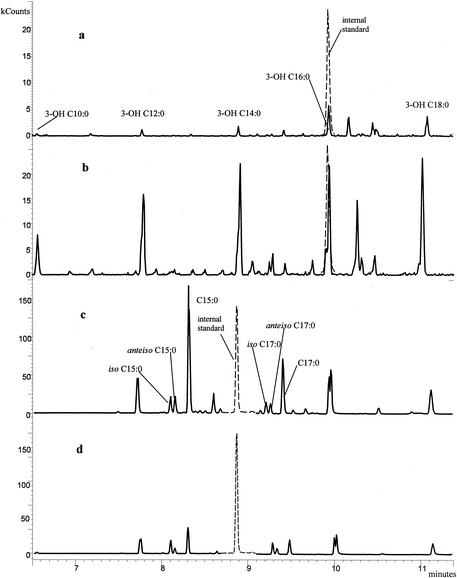
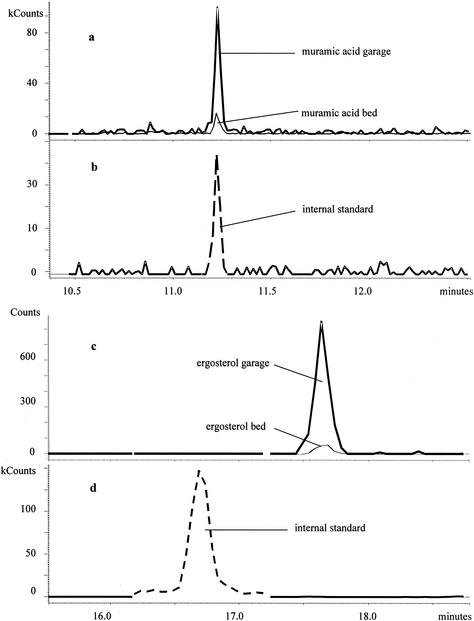
Comparative chromatograms of bed and garage dust samples are illustrated in Fig. 3 and Fig. 4. The two samples were collected from the same villa. The garage dust sample contained much more ergosterol, muramic acid, and 3-OH FAs than did the bed dust sample but had notably less of the nonhydroxylated FAs. This may suggest that the garage microflora was dominated by fungi and gram-negative bacteria. The bed dust sample contained larger relative amounts of the straight-chain C15:0 and C17:0 FAs compared to the branched-chain FAs than did the garage dust sample (Fig. 3), and 3-OH C16:0 dominated over the other 3-OH FAs more strongly in bed dust than in garage dust. These results indicate differences in bacterial populations.
FIG. 3.
(a and b) 3-OH FA profiles of dust samples from a bed (a) and a garage (b). (c and d) Nonhydroxylated FA profiles of dust samples from the bed (c) and the garage (d). The internal standards are shown as dashed peaks.
FIG. 4.
(a and b) Muramic acid profiles of dust samples from a bed and a garage without (a) and with (b) the internal standard; ergosterol profiles of dust samples from the bed and the garage without (c) and with (d) the internal standard.
DISCUSSION
Microorganisms are ubiquitous in our environment, including indoor air, and do not necessarily constitute a health hazard. The concentration at which contamination becomes a threat to health is unknown and may actually vary greatly with each individual. Intrusion of water into a building clearly contributes to microbial growth and is associated with adverse health effects. However, there is a lack of epidemiological data to clearly link disease states with the growth of microorganisms in damp buildings. One reason could be the lack of standardized methods to quantify and characterize the microbial flora in indoor environments.
Our investigation has resulted in the development of a chemical-analytical approach to characterize microbial communities in indoor environments. This approach provides information on both viable and nonviable microorganisms in a sample, e.g., of airborne and settled dust or building material (26). Microbial chemical markers are determined by using GC-MS with 3-OH FAs used as markers for endotoxin and gram-negative bacteria, muramic acid for bacterial peptidoglycan, and ergosterol for fungal biomass. Levels of ergosterol in dust correlate with cultivable fungi (24) and levels of 3-OH FAs correlate with endotoxin as determined by Limulus methods, particularly when only 3-OH FAs of 10-, 12-, and 14-carbon chain lengths are considered (24, 25). Air concentrations both of ergosterol and 3-OH FAs were found to increase in a building after water damage and slowly decrease during the drying period (16). GC-MS-MS is recommended for achieving optimal analytical performance. In this technique, ions formed initially in the mass spectrometer's ion source are subjected to further fragmentation and the fragment (daughter) ions are monitored. This leads to a very high degree of detection specificity (23, 25).
In the present study, we describe an integrated strategy for applying chemical marker analysis that also includes branched-chain FAs of 15- and 17-carbon chain lengths as markers mainly of gram-positive bacteria. Only two dust samples (1 to 5 mg each) are required to perform all analyses. As previously reported (2, 8), 13C-labeled muramic acid from the cyanobacteria is used as an internal standard for muramic acid in the samples. In addition, in the described procedure, 13C-labeled C16:0 in the same cyanobacteria is used as an internal standard for nonhydroxylated FAs (including branched-chain acids) and 13C-labeled 3-OH C16:0 is used as an internal standard for 3-OH FAs. This greatly simplifies sample preparation and should also result in improved reproducibility.
The temperature of the ion trap affected the detector signal. This was most evident for the muramic acid derivative. Thus, increasing the ion trap temperature from 200°C, as previously used (2), to 220°C improved peak shape and enhanced the detection sensitivity. We also introduced the use of a PRS column in the sample preparation procedure for muramic acid to remove dust particles and eliminate traces of lipids that might be present despite the heptane extraction. These improvements facilitated the derivatization, reduced the contamination of the GC-MS-MS system, and resulted in an overall better performance for our method in determining muramic acid in environmental samples (data not shown).
The described method may be used to characterize bacteriological and fungal profiles of environmental samples and includes both culturable and nonculturable microbes and cellular debris. In further studies, we will relate such microbial patterns to “healthy” and “sick” indoor environments. One question that will be addressed shortly is how water damage of houses affects the microbial patterns of indoor air.
Acknowledgments
This project was supported by The Knowledge Foundation and Skanska Teknik AB through the research school The Building and Its Indoor Environment and by FORMAS.
REFERENCES
- 1.Axelsson, B. O., A. Saraf, and L. Larsson. 1995. Determination of ergosterol in organic dust by gas chromatography-mass spectrometry. J. Chromatogr. Ser. B 666:77-84. [DOI] [PubMed] [Google Scholar]
- 2.Bal, K., and L. Larsson. 2000. New and simple procedure for the determination of muramic acid in chemically complex environments by gas chromatography-ion trap tandem mass spectrometry. J. Chromatogr. Ser. B 738:57-65. [DOI] [PubMed]
- 3.Brunekreef, B. 1992. Damp housing and adult respiratory symptoms. Allergy 47:498-502. [DOI] [PubMed] [Google Scholar]
- 4.Buttner, M. P., P. Cruz-Perez, and L. D. Stetzenbach. 2001. Enhanced detection of surface-associated bacteria in indoor environments by quantitative PCR. Appl. Environ. Microbiol. 67:2564-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun, D. T., V. Chew, K. Bartlett, T. Gordon, R. R. Jacobs, B. M. Larsson, L. Larsson, D. M. Lewis, J. Liesivuori, O. Michel, D. K. Milton, R. Rylander, P. S. Thorne, E. M. White, and M. E. Brown. 2000. Preliminary report on the results of the second phase of a round- robin endotoxin assay study using cotton dust. Appl. Occup. Environ. Hyg. 15:152-157. [DOI] [PubMed] [Google Scholar]
- 6.Dales, R. E., D. Miller, and J. White. 1999. Testing the association between residential fungus and health using ergosterol measures and cough recordings. Mycopathologia 147:21-27. [DOI] [PubMed] [Google Scholar]
- 7.Dillon, K., P. Heinsohn, and D. Miller. 1996. Field guide for the determination of biological contaminants in environmental samples. AIHA Publications, Fairfax, Va.
- 8.Fox, A., M. Krahmer, and D. Harrelson. 1996. Monitoring muramic acid in air (after alditol acetate derivatization) using a gas chromatograph-ion trap tandem mass spectrometer. J. Microbiol. Methods 27:129-138.
- 9.Fox, A., R. Rosario, and L. Larsson. 1993. Monitoring of bacterial sugars and hydroxy fatty acids in dust from air conditioners by gas chromatography- mass spectrometry. Appl. Environ. Microbiol. 59:4354-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross, M. L. 1994. Tandem mass spectrometric strategies for determining structure of biologically interesting molecules. Acc. Chem. Res. 27:361-369. [Google Scholar]
- 11.Haugland, R. A., N. Brinkman, and S. J. Vesper. 2002. Evaluation of rapid DNA extraction methods for the quantitative detection of fungi using real-time PCR analysis. J. Microbiol. Methods 50:319-323. [DOI] [PubMed] [Google Scholar]
- 12.Hauschildt, S., and B. Kleine. 1995. Bacterial stimulators of macrophages. Int. Rev. Cytol. 161:263-331. [DOI] [PubMed] [Google Scholar]
- 13.Kaneda, T. 1991. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55:288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline, J. N., J. D. Cowden, G. W. Hunninghake, B. C. Schutte, J. L. Watt, C. L. Wohlford-Lenane, L. S. Powers, M. P. Jones, and D. A. Schwartz. 1999. Variable airway responsiveness to inhaled lipopolysaccharide. Am. J. Respir. Crit. Care Med. 160:297-303. [DOI] [PubMed] [Google Scholar]
- 15.Kozar, M., M. Krahmer, A. Fox, L. Larsson, and J. Alton. 2001. Lunar dust, a negative control for biomarker analyses in extraterrestrial samples? Geochim. Cosmochim. Acta 65:3307-3317. [Google Scholar]
- 16.Larsson, L., and P. F. Larsson. 2001. Analysis of chemical markers as a means of characterising airborne micro-organisms in indoor environments: a case study. Indoor Built Environ. 10:232-237. [Google Scholar]
- 17.Michel, O. 2001. Role of house-dust endotoxin exposure in aetiology of allergy and asthma. Mediators Inflamm. 10:301-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michel, O., R. Ginanni, J. Duchateau, F. Vertongen, B. Le Bon, and R. Sergysels. 1991. Domestic endotoxin exposure and clinical severity of asthma. Clin. Exp. Allergy 21:441-448. [DOI] [PubMed] [Google Scholar]
- 19.Murphy, R. C. 1993. Fatty acids, p. 71-130. In F. Snyder (ed.), Mass spectrometry of lipids. Plenum Press, New York, N.Y.
- 20.Nadkarni, M., F. Martin, N. Jacques, and N. Hunter. 2002. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257-266. [DOI] [PubMed] [Google Scholar]
- 21.Pepys, J. 1969. Hypersensitivity diseases of the lungs due to fungi and organic dusts. Monogr. Allergy 4:1-147. [PubMed] [Google Scholar]
- 22.Rintala, H., A. Nevalainen, and M. Suutari. 2002. Diversity of streptomycetes in water-damaged building materials based on 16S rDNA sequences. Lett. Appl. Microbiol. 34:439-443. [DOI] [PubMed] [Google Scholar]
- 23.Saraf, A., and L. Larsson. 1996. Use of gas chromatography-ion trap tandem mass spectrometry for the determination of chemical markers of microorganisms in organic dust. J. Mass Spectrom. 31:389-396. [Google Scholar]
- 24.Saraf, A., L. Larsson, H. Burge, and D. Milton. 1997. Quantification of ergosterol and 3-hydroxy fatty acids in settled house dust by gas chromatography-mass spectrometry: comparison with fungal culture and determination of endotoxin by a Limulus amebocyte lysate assay. Appl. Environ. Microbiol. 63:2554-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saraf, A., J. H. Park, D. K. Milton, and L. Larsson. 1999. Use of quadrupole GC-MS and ion trap GC-MS-MS for determining 3-hydroxy fatty acids in settled house dust: relation to endotoxin activity. J. Environ. Monit. 1:163-168. [DOI] [PubMed] [Google Scholar]
- 26.Szponar, B., and L. Larsson. 2001. Use of mass spectrometry for characterizing microbial communities in bioaerosols. Ann. Agric. Environ. Med. 8:111-117. [PubMed] [Google Scholar]
- 27.Verhoef, J., and E. Kalter. 1985. Endotoxic effects of peptidoglycan, p. 101-112. In J. Cate, H. R. Buller, A. Sturk, and J. Levin (ed.), Bacterial endotoxin: structure, biomedical significance, and detection with Limulus amebocyte lysate test. Allan R Liss, Inc., New York, N.Y.
- 28.Wan, G. H., and C. S. Li. 1999. Indoor endotoxin and glucan in association with airway inflammation and systemic symptoms. Arch. Environ. Health 54:172-179. [DOI] [PubMed] [Google Scholar]
- 29.White, D. C. 1983. Analysis of microorganisms in terms of quantity and activity in natural environments, p. 37-66. In J. H. Slater, R. Whittenbury, and J. W. T. Wimpenny (ed.), Society for General Microbiology symposium series, vol. 34. Microbes in their natural environments. Cambridge University Press, Cambridge, United Kingdom.
- 30.Zelles, L. 1997. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275-294. [DOI] [PubMed] [Google Scholar]
- 31.Zhou, G., W. Z. Whong, T. Ong, and B. Chen. 2000. Development of a fungus-specific PCR assay for detecting low-level fungi in an indoor environment. Mol. Cell. Probes 14:339-348. [DOI] [PubMed] [Google Scholar]
- 32.Zirrolli, J. A., and R. C. Murphy. 1993. Low-energy tandem mass spectrometry of the molecular ion derived from fatty acid methyl esters: a novel method for analysis of branched-chain fatty acids. J. Am. Soc. Mass Spectrom. 4:223-229. [DOI] [PubMed] [Google Scholar]