Abstract
Through use of commercially available DnaK proteins and anti-DnaK monoclonal antibodies, a competitive enzyme-linked immunosorbent assay was developed to quantify this heat shock protein in Escherichia coli ATCC 25922 subjected to various heating regimens. For a given process lethality (F7010 of 1, 3, and 5 min), the intracellular concentration of DnaK in E. coli varied with the heating temperature (50 or 55°C). In fact, the highest DnaK concentrations were found after treatments at the lower temperature (50°C) applied for a longer time. Residual DnaK after heating was found to be necessary for cell recovery, and additional DnaK was produced during the recovery process. Overall, higher intracellular concentrations of DnaK tended to enhance cell resistance to a subsequent lethal stress. Indeed, E. coli cells that had undergone a sublethal heat shock (105 min at 55°C, F7010 = 3 min) accompanied by a 12-h recovery (containing 76,786 ± 25,230 molecules/cell) resisted better than exponentially growing cells (38,500 ± 6,056 molecules/cell) when later heated to 60°C for 50 min (F7010 = 5 min). Results reported here suggest that using stress protein to determine cell adaptation and survival, rather than cell counts alone, may lead to more efficient heat treatment.
Heat treatment, or cooking, is an effective means of reducing the total number of microorganisms present in foods. Cell stress increases with the intensity of the heat treatment and leads to significant changes within the cells, including protein denaturation and degradation, and finally to cell death (8, 10, 22). In most countries, raw- and processed-meat cooking has traditionally been regulated on the basis of reaching a legally required temperature in the coldest zone of the product, prior to cooling (i.e., end point temperature concept). For example, formulated or brine-injected processed meats are generally cooked to an internal temperature of at least 70°C, whereas lower temperatures may be acceptable for uncut, whole-muscle products, such as roast beef (e.g., 64°C). These practices have proven useful in standard regulatory situations, but they do not give a true picture of the extent of bacterial kill achieved during cooking. The killing efficacy is dependent on the amount of energy provided to the meat and bacteria and is therefore influenced by the time-temperature history of the product during heating, which is generally referred to as the cooking cycle by meat processors.
In practical terms, the extent of bacterial kill (log10 CFU/gram) for a particular organism can be calculated by dividing the process lethality value FTrefz (in minutes) by the decimal reduction time DTref (in minutes), in which Tref is any chosen reference temperature and z is a temperature sensitivity index for the organism concerned with the calculation. Although this quantitative approach (classical thermobacteriology) has been used since the beginning of the 20th century to ensure commercial sterility in the canning industry, it could also be used to evaluate the safety of cooking processes. In fact, however, process lethality values have not yet replaced the much more limited end point temperature concept in monitoring of meat cooking, due in part to the lack of reliable, scientifically measured DTref and z values for pathogens or indicator organisms in the appropriate food matrices. Quantitative assessment of heat treatments, however, is gaining more and more recognition, and some efforts are being made to collect all measured data and to make them available to all scientists and regulators (17).
Classical thermobacteriology also has some limitations. It was elaborated before the discovery of bacterial sensitivity and resistance and/or adaptation to stress, in particular heat stress. In several species, prior exposure to a sublethal heat treatment was shown to increase bacterial resistance to a subsequent and severer treatment (3, 11, 12, 14), and this knowledge challenges the predictions of classical bacteriology during heating at low-to-moderate temperatures (50 to 65°C).
The heat shock response is particularly well documented in Escherichia coli. Until now, over 30 proteins associated with the physiological response to heat have been identified, including GroES, GroEL, and DnaK chaperone proteins involved in the folding, repair, and degradation of proteins. Expression of the heat shock genes is under the control of an alternative sigma factor, σ32 (RpoH) [22]). The rpoH mRNA is already present in the cell under optimal growth temperature, but its secondary structure prevents translation. Upon heat stress, the transcript loses its secondary structure and the translation start site becomes available for the translation machinery. Cells can therefore react rapidly to heat stress (23). A second transcription factor, σ24, is also involved in the heat shock response when the temperature reaches 50°C. This transcription factor is the product of the rpoE gene, which recognizes one of the four promoters of the rpoH gene (σ32) at high temperatures, whereas the other promoters are virtually inactive (23). At high temperatures, σ24 promotes rpoH transcription and synthesis of heat shock proteins (HSP) is maintained.
DnaK plays an important role in the regulation of the heat shock response, because its association with σ32 at optimal growth temperature prevents the formation of the RNA polymerase-σ32 complex (5, 7). Hence, HSP expression is determined by a homeostatic balance between DnaK bound to denatured proteins and DnaK interacting with σ32 (1, 5, 6). Because DnaK binds preferentially with denatured proteins upon heat stress, σ32 is free to bind to the RNA polymerase core protein. The specific transcription of heat shock genes begins, which in turn increases the concentration of HSP, including DnaK. When cells return to an optimal growth temperature, the concentration of denatured proteins decreases and free DnaK interacts with σ32, thereby stopping the transcription of HSP genes (9, 20). During exponential growth, DnaK represents 1% of the total proteins (6), while it may increase up to 13% when cells are grown at 30°C and then exposed to 42°C (8).
Considering the key role of DnaK in the heat shock response and the modulation of its concentration upon heat stress, a competitive enzyme-linked immunosorbent assay (ELISA) was developed to measure intracellular concentrations of DnaK in E. coli. The consequences of DnaK production on the survival of the organism after observation of various sets of heating conditions and adaptation and/or recuperation scenarios were evaluated.
(Part of this work was presented at a poster session of the 100th general meeting of the American Society for Microbiology, Los Angeles, Calif., 21 to 25 May 2000.)
MATERIALS AND METHODS
Bacterial cultures and growth conditions.
Stock cultures of E. coli strains ATCC 25922 and Epicuran Coli BL21 (Stratagene, La Jolla, Calif.) were stored at −80°C in brain heart infusion (BHI; Difco Laboratories, Detroit, Mich.) broth supplemented with 20% glycerol. Prior to experimental use, the frozen cells were subcultured (1% [vol/vol]) daily for a minimum of 2 days and a maximum of 7 days in BHI broth. Cell suspensions were incubated overnight at 37°C.
Determination of intracellular DnaK by competitive ELISA.
Cells from a 200-ml culture were collected by centrifugation (13,000 × g, 15 min). The pellet was washed and was resuspended in 50 ml of 0.1 M NaHPO4 buffer at pH 7.0. Sonication (Sonic Dismembrator model 300; Artek Systems Corp., Farmingdale, N.Y.) was performed at a frequency of 16.8 kilocycles/s for a total of 30 min. One minute of sonication was alternated with 1 min of rest on ice in order to prevent protein denaturation. The sonicated suspension was centrifuged (13,000 × g, 15 min), and the DnaK concentration was determined immediately by a competitive ELISA as previously described (4). Briefly, the microplates (Plate MaxiSorp surfaces; Nunc-Immuno, Roskilde, Denmark) used for the ELISA were coated with 100 μl of purified DnaK (0.25 μg/ml; StressGen Biotechnologies Corp., Victoria, British Columbia, Canada) per well. Stock DnaK solution for well coating (1 mg/ml) was prepared in 0.1 M NaHCO3 at pH 9.6. The microplate contents were incubated at 4°C overnight. They were then washed four times with a phosphate-buffered saline (PBS) supplemented with 0.05% Tween 20. A PBS-1% bovine serum albumin solution (PBS-BSA; 200 μl per well) was used as a blocking agent. Plate contents were incubated for 1 h at room temperature (22°C) and were then washed four times with the PBS-0.05% Tween solution. For the competition step, a mouse anti-DnaK monoclonal antibody (1 mg/ml; StressGen Biotechnologies Corp.) was diluted to 1:10,000 in PBS-1% BSA-0.05% Tween 20. A volume of 150 μl of anti-DnaK and 150 μl of the test sample were mixed in a microtube, which was agitated for 1 h at room temperature (22°C) before addition of 100 μl to the coated wells. The plate contents were then incubated for 2 h at 37°C and were washed four times with PBS-0.05% Tween 20 solution. One hundred microliter of sheep antimouse immunoglobulin G-peroxidase conjugate (5 mg/ml; Medicorp Inc., Montréal, Québec, Canada) per well was used as a secondary antibody probe (1:50,000 dilution in PBS-1% BSA-0.05% Tween 20). The plate contents were then incubated at 37°C for 90 min and were washed four times with PBS-0.05% Tween solution. The substrate, tetramethylbenzene (Medicorp Inc.), was added for colorimetric reaction. Absorbance at 370 nm was recorded with a microplate reader (PowerWaveX; Bio-Tek Instruments, Inc., Winooski, Vt.) according to the manufacturer's specifications. The standard curve was performed with purified DnaK diluted in a PBS-1% BSA-0.05% Tween 20 solution (detection limit, 1,500 molecules/cell).
The accuracy of the competitive ELISA was evaluated by comparing results obtained with values reported in the literature for specific conditions. A 1% overnight culture of E. coli was used to inoculate an Erlenmeyer flask (500 ml) containing 200 ml of BHI broth. Flask contents were incubated at 30, 37, or 42°C. Upon reaching the exponential growth phase (optical density at 600 nm [OD600] = 0.5; ∼8 log10 CFU/ml), the cells were immediately collected and the intracellular concentration of DnaK was determined as described above.
Heat treatment.
Cell exposure to the various heat treatments was performed by immersion of an exponentially growing culture (200 ml in BHI broth at 37°C; OD600 = 0.5) in a shaking water bath. After treatment, the cell suspension was shaken and cooled in an ice water bath until the temperature dropped to 37°C. The temperature was recorded through time by using a scanning thermocouple (Model 92 8000-10; Digi-Sense, Barnant Co., Barrington, Ill.).
Process lethality values (FTrefz [in minutes]) were calculated as 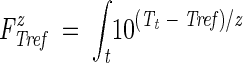 dt, in which t is the time (in minutes), Tt is the temperature at time t (in degrees Celsius), Tref is a selected reference temperature (in degrees Celsius), and z is the thermal sensitivity value (in degrees Celsius) of the selected reference organism. The temperature of 70°C was chosen as reference because it represents, for most meat products, the core temperature that must be reached at the end of cooking in order to meet North American food safety regulations. Also, Enterococcus faecalis was selected as the reference organism because of its documented higher thermal resistance, with a thermal sensitivity value (z) of 10°C, when heated in cured meats (16). The process lethality values corresponding to the investigated heating cycles were reported as F7010 (Table 1).
dt, in which t is the time (in minutes), Tt is the temperature at time t (in degrees Celsius), Tref is a selected reference temperature (in degrees Celsius), and z is the thermal sensitivity value (in degrees Celsius) of the selected reference organism. The temperature of 70°C was chosen as reference because it represents, for most meat products, the core temperature that must be reached at the end of cooking in order to meet North American food safety regulations. Also, Enterococcus faecalis was selected as the reference organism because of its documented higher thermal resistance, with a thermal sensitivity value (z) of 10°C, when heated in cured meats (16). The process lethality values corresponding to the investigated heating cycles were reported as F7010 (Table 1).
TABLE 1.
Actual time needed to reach process lethality values (F7010) of 0.5, 1, 3, and 5 min for the different heating cyclesa
| F7010 (min) | Time needed (min) at:
|
|||
|---|---|---|---|---|
| 50°C | 55°C | 60°C | 70°C | |
| 0.5 | 50 | 15 | NEb | NE |
| 1.0 | 105 | 35 | NE | NE |
| 3.0 | 285 | 105 | NE | NE |
| 5.0 | 480 | 150 | 50 | 10 |
Time was calculated from the beginning of the temperature increase (37°C) to the end of the cooling period (back to 37°C).
Not evaluated.
To study the recovery of E. coli after the heat shock, treatments were carried out at 55°C (F7010 of 3 and 5 min) as described above. Upon reaching 37°C during the cooling period, the bacterial suspension was incubated for 12 and 24 h at 37°C to enable viable cells to recover and multiply. In addition, heat-induced adaptation of cells prior to a lethal heat treatment at 60°C (F7010 of 5 min) was evaluated by comparing the survival of exponentially growing cells (37°C) to that of cells that were heated to 55°C (F7010 of 3 min) and were then incubated 12 h at 37°C for recovery.
Bacterial enumeration.
Cell enumeration was performed by plating serial dilutions (1:10) onto BHI agar. Plate contents were incubated at 37°C for 24 h, unless otherwise specified. Cell enumeration by fluorescence microscopy was carried out by using the LIVE/DEAD BacLight kit (Molecular Probes, Inc., Eugene, Oreg.) according to the manufacturer's specifications.
Determination of total proteins.
To determine the protein concentration, a 500-μl aliquot of the sonicated cell suspension was centrifuged (13,000 × g, 15 min). The protein quantification was carried out on the supernatant with the bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.) according to the manufacturer's specifications. Absorbance was read at 570 nm with a spectrophotometer (Lambda Reader; Perkin-Elmer, Wilton, Conn.).
Statistical analysis.
The effects of temperature and process lethality value on intracellular DnaK concentration and cell counts were tested with a two-way analysis of variance by using the General Linear Model (GLM) procedure of the SAS system, version 8.02 (19). When the interaction term was significant (P < 0.05), the main effect of a factor was tested by using a one-way analysis of variance performed for each level of the other factor. The identification of significant differences between levels of a factor was obtained by the Duncan multiple-comparison test. For all response variables, analysis was done in duplicates and the experiments were repeated three times.
RESULTS
Validation of the ELISA method.
According to the literature (8), the temperatures known as benchmarks for DnaK expression are 30, 37 and 42°C. The DnaK/total protein ratio (number of nanograms/number of nanograms) in E. coli measured at these three temperatures by the competitive ELISA in this study gave values that are similar to those reported in the literature (Table 2) (8), suggesting that the present ELISA quantification method is valid. However, DnaK concentrations, expressed as number of molecules/cell, were different from previously reported values. At 37°C, the measured DnaK concentration reached 38,500 ± 6,056 molecules/cell, whereas the literature reported a concentration of 5,000 molecules/cell (15). A study was thus carried out to determine the influence of the E. coli strain and growth medium on the expression of DnaK. The DnaK concentrations of E. coli ATCC 25922 in BHI and Luria broth were 38,500 ± 6,056 and 40,100 ± 6,643 molecules/cell, respectively, indicating that the culture media had little effect on the DnaK concentration within the cell. However, when the two E. coli strains were tested in the BHI broth, the DnaK concentration was almost two times lower for Epicurian Coli BL21 (22,000 ± 4,451 molecules/cell) than for strain ATCC 25922 (38,500 ± 6,056 molecules/cell), suggesting that the DnaK concentration varies from strain to strain.
TABLE 2.
Intracellular concentrations of DnaK in E. coli ATCC 25922, after incubation in BHI broth at various temperaturesa
| Temp (°C) | No. of DnaK molecules/cellb | Ratio of DnaK to total proteinsc (%)
|
|
|---|---|---|---|
| Exptl value | Value cited in literatured | ||
| 30 | 18,767 ± 3,044 | 0.63 ± 0.11 | 0.66 |
| 37 | 38,500 ± 6,056 | 0.98 ± 0.07 | 1.00 |
| 42 | 103,867 ± 13,735 | 1.60 ± 0.32 | 1.42 |
Cells were grown until an OD600 of 0.5 was reached. The experiments were repeated three times.
The concentrations per cell were calculated with the number of cells present before the treatment.
Total proteins were evaluated after sonification.
Values are cited by Herendeen et al. (8).
Intracellular DnaK concentration after heat treatment.
To verify that no DnaK was lost in the supernatant during heat treatment the DnaK concentration was evaluated after each step prior to sonication. No DnaK was lost at any step when the supernatant was tested after incubation at 37°C or treatment at 50°C for 105 min (F7010 = 1 min), 55°C for 35 min (F7010 = 1 min), and 60°C for 50 min (F7010 = 5 min). Hence, if any DnaK were lost in the supernatant, prior to sonication, it would be below detection level (<1,500 molecules/cell).
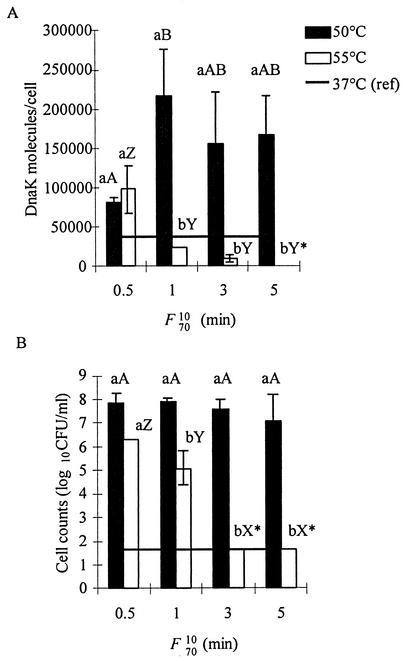
The statistical analysis revealed a significant interaction between the effects of temperature and process lethality values on the intracellular concentration of DnaK (P = 0.0026) (Fig. 1A) and on the counts of surviving cells (P = 0.0002) (Fig. 1B). This indicates that, in the range of temperatures studied (50 to 55°C), the effects of the process on bacteria were not only dependent on the process intensity, as expressed by the classical process lethality value (F7010), but were also influenced by temperature. Whereas intracellular DnaK was not detected in strain ATCC 25922 after treatments at 60 and 70°C, yielding F7010 values of 5 min (data not shown), higher DnaK concentrations were detected after less severe treatments (F7010 = 0.5 min) at 50 and 55°C (Fig. 1A). Under these conditions, DnaK contents were found to be about two times (81,968 ± 6,347 molecules/cell at 50°C) and three times (105,131 ± 38,733 molecules/cell at 55°C) higher than the value of 38,500 ± 6,056 molecules/cell measured in cells grown at 37°C. Intracellular DnaK contents remained high (155,773 ± 64,976 to 217,445 ± 59,010 molecules/cell) when further heating at 50°C was carried out (up to an F7010 of 5 min), and no significant cell inactivation was observed (7.15 to 7.96 log10 CFU/ml) (Fig. 1B). DnaK concentrations obtained for treatments at 50°C were relatively similar (P = 0.063). Although P (P = 0.063) was higher than 0.05, the Duncan multiple-comparison test was performed to identify potential differences between two pasteurization values. The only significant difference detected was between the process lethality values of 0.5 (81,968 ± 6,347 molecules/cell) and 1 (217,445 ± 59,010 molecules/cell) min.
FIG. 1.
Intracellular DnaK concentration (A) and surviving-cell counts (B) of E. coli ATCC 25922 cells exposed to heat treatment of various intensities (F7010). The concentrations per cell were calculated with the number of cells present before the treatment. (A) Horizontal line indicates the DnaK level found in cells during exponential growth (OD600 = 0.5) at 37°C (38,500 molecules/cell). (B) Horizontal line indicates the cell count detection limit. The experiment was repeated three times. Bars represent the mean plus or minus standard deviation. For a specific process lethality value, results with a similar lowercase letter are not significantly different. Capital letters are used for a specific temperature, i.e., AB for 50°C and XYZ for 55°C. Results below the detection level are indicated by an asterisk.
In contrast, significant differences in intracellular DnaK concentrations were found at 55°C (P = 0.0023). Intracellular DnaK concentrations decreased to levels below the 37°C value when heating at 55°C was increased to F7010 values exceeding 1 min (23,959 ± 677, 9,565 ± 5,317, and <1,500 molecules/cell for F7010 values of 1, 3, and 5 min, respectively). DnaK concentrations at a process lethality value of 0.5 min were significantly higher (P < 0.05) than were concentrations obtained at process lethality values of 1, 3, and 5 min. However, no differences were observed between process lethality values of 1, 3, and 5 (Fig. 1A). Moreover, the number of surviving cells decreased from approximately 6.2 log10 CFU/ml to undetectable levels (<1.7 log10 CFU/ml). In fact, surviving-cell counts were different (P < 0.0001) among all process lethality values tested, except between F7010 values of 3 and 5 min (Fig. 1B), where counts were below detection levels.
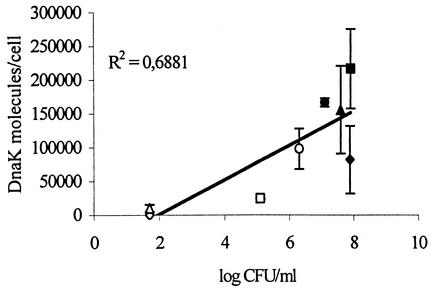
Overall, the intracellular DnaK concentration in E. coli strain ATCC 25922 was highly correlated (P < 0.0001) to the number of surviving cells, but the relationship was only poorly described (R2 = 0.6881) by a linear regression (Fig. 2). Furthermore, for a given process lethality value, DnaK concentrations and surviving-cell counts obtained at 50°C were significantly higher (P ≤ 0.05) than were the concentrations and counts obtained at 55°C, except at F7010 of 0.5, where they were essentially the same (Fig. 1A).
FIG. 2.
Correlation between the concentration of intracellular DnaK and surviving-cell counts. R2 is the linear regression. Closed and opened figures are results from treatments performed at 50 and 55°C, respectively. ○, □, ▵ and ◊ indicate F7010 values of 0.5, 1, 3, and 5 min, respectively.
Heat-induced production of DnaK and its role in resistance to subsequent heating.
In order to evaluate the effects of DnaK on subsequent cell recovery in strain ATCC 25922, overnight cultures in BHI broth, incubated at 37°C, were submitted to heating at 55°C until an F7010 value of 3 or 5 min was reached. The intracellular DnaK concentrations and the numbers of surviving cells were determined immediately following the heat treatment and after 12 and 24 h of subsequent incubation at 37°C.
Immediately following heat treatment at F7010 = 3 min, where the DnaK concentration was 12,347 ± 2,363 DnaK molecules/cell, the number of surviving cells was lower than the plating technique detection level (0.7 log10 CFU/ml), although 79 viable cells out of 1,000 cells (about 8%) were detected with the LIVE/DEAD BacLight differential coloration kit (Table 3). After further incubation at 37°C, a substantial growth was observed, yielding 4.57 and 8.29 log10 CFU/ml after 12 and 24 h, respectively, as evinced by a strong increase in the proportion of live versus dead cells. Concurrently, DnaK contents increased to levels that were much higher (56,443 to 76,786 molecules/cell) than the level normally found in cells grown at 37°C (38,500 molecules/cell). In contrast, no surviving cells and no residual DnaK were found in cultures exposed to a treatment of F7010 = 5 min, whether determination was made immediately following the treatment or after incubation at 37°C for 12 or 24 h (Table 3). Furthermore, no viable cells were detected by fluorescence microscopy after 7 days of incubation at 37°C (data not shown), suggesting that the presence of DnaK was instrumental to post-heating cell recovery.
TABLE 3.
Intracellular DnaK concentration and cell counts of E. coli ATCC 25922 cells after 0, 12, and 24 h of recovery following heat treatmentsa
| Timeb (h) | Result at:
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
F7010 = 3 min (55°C, 105 min)
|
F7010 = 5 min (55°C, 150 min)
|
|||||||
| DnaK concn (no. of molecules/cell) | Cell count
|
DnaK concn (no. of molecules/cell) | Cell count
|
|||||
| Agar platesc (log10 CFU/ml) | Fluorescence
|
Agar plates (log10 CFU/ml) | Fluorescence
|
|||||
| Live cells | Dead cells | Live cells | Dead cells | |||||
| 0 | 12,347 ± 2,363 | <0.7 | 79 | 921 | <1,500 | <0.7 | 0 | 1,000 |
| 12 | 76,786 ± 25,230 | 4.57 | 90 | 910 | <1,500 | <0.7 | 0 | 1,000 |
| 24 | 56,443 ± 20,417 | 8.29 | 577 | 423 | <1,500 | <0.7 | 0 | 1,000 |
The experiment was repeated three and two times for F7010 values of 3 and 5 min, respectively.
Recuperation time at 37°C after heat treatment.
A volume of 200 μl spread on five plates for a detection limit of <0.7 log10 CFU/ml.
An overnight culture of strain ATCC 25922 in BHI broth, incubated at 37°C (containing 38,500 DnaK molecules/cell), did not survive a treatment (F7010 = 5 min) at 60°C (Table 4), and no growth was observed after a 24-h resuscitation time at 37°C. In contrast, cells that were subjected to a sublethal treatment at 55°C (F7010 = 3 min) and were further incubated at 37°C for 12 h (containing 76,786 DnaK molecules/cell), prior to the 60°C lethal treatment, could grow back to high numbers (7.36 log10 CFU/ml) after 24 h of resuscitation at 37°C, even though they were not detectable immediately after a treatment at 60°C (F7010 = 5 min). Hence, a higher intracellular concentration of DnaK appears to provide a better protection against a subsequent lethal treatment in E. coli.
TABLE 4.
Survival of E. coli ATCC 25922 cells to a lethal treatment following prior exposure to a sublethal treatmenta
| Cell category | Cell count (log10 CFU/ml) for:
|
||||
|---|---|---|---|---|---|
| Exponentially growing cellsc | Sublethal treatmentb
|
Lethal treatment
|
|||
| After treatment (55°C; 105 min; F7010 = 3 min) | After recuperation (37°C [12 h]) | After treatment (60°C; 50 min; F7010 = 5 min) | After recuperation (37°C [24 h]) | ||
| Cells exposed to sublethal heating | 8.03 | <0.7 | 7.06 | <0.7 | 7.36 |
| Control cells (not exposed to sublethal heating)d | 8.20 | NAe | NA | <0.7 | <0.7 |
Lethal treatment was F7010 = 5 min, performed at 60°C. The experiment was repeated three times.
Heating conditions that provide a DnaK concentration of 12,347 ± 2,363 and 76,786 ± 25,230 molecules/cell after treatment and 12 h of recuperation at 37°C, respectively (Table 3).
Incubation of cells at 37°C until an OD600 of 0.5 was reached.
Optimal growth conditions (37°C; OD600 = 0.5) that provide a DnaK concentration of 38,500 ± 6,056 molecules/cell (Table 2).
NA, not applicable.
DISCUSSION
Efficacy of the ELISA technique developed to quantify DnaK.
On the one hand, similar DnaK concentrations were obtained in two broth media, suggesting that the growth medium does not influence the intracellular DnaK concentration in E. coli. On the other hand, the DnaK/total protein ratios (number of nanograms/number of nanograms) obtained with the ELISA technique described here were very similar to those reported in the literature for temperatures of 30, 37, and 42°C (Table 2) (8), but the DnaK molecule-to-cell ratios measured here in two E. coli strains were much higher than the value of 5,000 molecules/cell reported by Neidhardt and VanBogelen (6, 15). Strain differences may account for the observed variation. In the later case, the E. coli strain used was a prototroph defective in B-type DNA restriction enzyme, whereas ATCC 25922 is a clinical isolate typically used as a control organism for testing of bacterial sensitivity to antibiotics (13). The higher DnaK molecule-to-cell ratios reported in the present study could also be explained by a higher sensitivity of the detection method employed here than that of radioactive markers (8), sodium dodecyl sulfate-polyacrylamide gel electrophoresis, or immunoblotting (21). The detection limit of our ELISA technique (1,500 molecules/cell) enabled us to show differences between the various sets of heating conditions (Tables 2 to 4 and Fig. 1 and 2).
Efficacy of heat treatments to control E. coli ATCC 25922.
The presence of DnaK in E. coli ATCC 25922 heated at 50 and 55°C suggests that cells can still react and adapt in order to carry out protein synthesis and metabolic activities. Indeed, intracellular DnaK concentrations for all treatments performed at 50°C and for treatment at 55°C with a process lethality value of 0.5 min were higher than the concentration found in cells grown at 37°C (Fig. 1A). As described by Herendeen et al. (8), coordination of metabolic functions for growth at 23 to 37°C is modulated by the specific activity of enzymes rather than their amount. Outside this range, growth is restricted and the concentrations of several proteins will vary accordingly. Proteins generally involved in transcription and translation are found at reduced levels under restrictive growth conditions (8). Those proteins involved in the repair and elimination of denatured proteins, for example, are at higher concentrations (1). According to Neidhardt et al. (14), an almost exclusive synthesis of proteins associated with the heat shock response occurred at 50°C or higher until protein synthesis was compromised. At that point, cells could no longer produce HSP to ensure their survival and cell death occurred. Under the conditions used, treatments at a temperature equal to or higher than 60°C seemed sufficient to control E. coli ATCC 25922, as no cells or DnaK was detected.
The presence of HSP has a profound effect on monitoring cooking. According to classical thermobacteriology (i.e., based on the thermal death time concept of Bigelow and Esty [2]), the bactericidal effect of different heat treatments is similar when the process lethality values (FTrefz) are identical, independent of temperatures reached during the treatment. Computation of the lethality value generally starts at 50 to 55°C, because no significant bactericidal effect is expected below these temperatures. The contribution of the time spent at a heating temperature of 50 to 55°C to the overall killing effect evaluated by the process lethality value is negligible in a typical industrial meat process cooking operation, such as in a smokehouse (70°C end point temperature). The situation is different, however, when product safety or bacterial stability is based on a combination of mild heating with other processing or storage hurdles (minimally processed foods) or in prolonged, low-temperature “sous vide” cooking. The product may then stay at temperatures below 60°C for extended periods, and the contribution of heating at low temperatures to the overall killing effect may be significant. In this context, the findings reported here (i.e., that cell survival and intracellular DnaK concentrations were higher when bacteria were heated at 50°C than at 55°C, even when process lethality values were identical) suggest that the use of classical thermobacteriology and the computation of process lethality values are only meaningful in ranges of temperatures above the cell adaptation zone.
Because the correlation between intracellular DnaK concentration and cell counts is weakly linear, one parameter cannot be used directly to predict the level of the other without a more sophisticated mathematical model. However, the presence of DnaK was an indication that some cells were still viable and were able to recover under optimum growth conditions, as demonstrated in the recovery experiment (Table 3). Furthermore, the absence of growth on agar or by differential staining, after a recovery period of 24 h or more under optimum growth conditions, corresponded with the absence of DnaK after treatment. Hence, with use of cell enumeration on agar plates alone, the efficacy of heat treatment may be overestimated if cells are too injured or too stressed to be culturable and to form visible colonies. In this study, cellular enumeration of E. coli by using the differential detection via fluorescence microscopy was more appropriate to evaluate the viability of cells subjected to heat treatment, since viable cells were already detectable immediately after the treatment and not only after recuperation at optimum growth temperature, as was the case for the agar plate method. Indeed, while no cells were counted on the agar plates, fluorescence microscopy observation indicated that 8% of the population was viable for a treatment of 55°C at a process lethality value of 3 min (Table 3).
E. coli adaptation and resistance to various heat treatments.
Previous studies on the stress response in E. coli revealed an increase in intracellular DnaK concentration during a change in incubation temperature from 30 to 50°C (14). This increase in DnaK concentration was one of the first indications that DnaK is involved in the cellular heat shock response. Afterwards, the role of DnaK in the repair and reactivation of proteins damaged following a heat shock was established (18). So, the more stressful that a heat shock is, the more important the damage is, which in turn calls for more proteins that are capable of repairing denatured proteins so as to maintain vital functions. The results obtained for cell counts and intracellular DnaK concentrations (Fig. 1) show that a prolonged exposure to low temperature tends to be more favorable for DnaK synthesis at a higher intracellular concentration than is a high temperature applied for a shorter time (Table 1). E. coli seems to resist greater process lethality values (e.g., 1, 3, and 5 min) at a temperature of 50°C than at 55°C, because cells can produce more stress proteins required for their survival (Fig. 1).
According to Arsène et al. (1), raising the temperature from 30 to 42°C causes a rapid induction (15-fold) of HSP synthesis, which is followed by an adaptation period where the synthesis decreases to a new steady-state level. In the recuperation experiment, a DnaK concentration of cells treated at 55°C for a process lethality value of 3 min markedly increased after 12 and 24 h of incubation at 37°C compared to a DnaK concentration measured under optimum growth conditions (Table 3). Concurrently, cell counts reached levels of 4 and 8 log10 CFU/ml after 12 and 24 h of incubation, respectively. These results indicate that maintaining a higher DnaK concentration while cells are growing at optimal conditions could be advantageous to surviving a subsequent stress. Furthermore, the heat shock information is passed on to following generations when cells are incubated under optimum growth conditions. As the cell counts increased, the intracellular concentration of DnaK remained greater than the concentration obtained for unstressed cells grown under optimum conditions. The selective development of a more resistant subpopulation is also a possible explanation. In order to evaluate the occurrence and the importance of such spontaneous resistant mutants, intracellular DnaK concentrations would have to be compared among a certain number of survival cells after growth under optimum conditions and on several subcultures.
Greater heat resistance by a prior exposure to a sublethal heat stress has been reported for E. coli (14) and other gram-negative (11, 12) and gram-positive (3) bacteria. In Salmonella enterica serovar Typhimurium, this elevated heat resistance was shown to persist for at least 10 h of exposure at preincubation temperatures of 42, 45, and 48°C for cells in a stationary phase (11). The protective effect provided by a greater intracellular DnaK concentration against a lethal heat treatment was demonstrated by the greater resistance of heat-shocked cells than of exponentially growing cells (Table 4). Indeed, the heat-shocked cells that were held at 37°C for 12 h, prior to the lethal stress, had an intracellular DnaK concentration two times higher than that of unshocked cells. Although cell concentrations obtained after heat treatment were below detection levels, cell counts increased in the heat-shocked cells, whereas the exponentially growing cells were still below detection levels after 24 h of recuperation at 37°C. These results confirm that intracellular DnaK is a good indication of the cell capacity to resist and adapt to heat stress. To our knowledge, this is the first study that uses a stress protein to assess the efficacy of heat treatment. The results demonstrate that using cell enumerations on agar plates alone can lead to an overestimation of the efficacy of heat treatment being studied, whereas the presence of stress protein determines the conditions beyond which the cell is no longer able to adapt and resist.
Acknowledgments
We thank Sylvain Moineau for fruitful discussions and Marie Dupuis for her technical support.
K.S. is the recipient of a Fondation des Gouverneurs graduate scholarship. This research was supported by the Program on Energy Research and Development of Energy and Natural Resources Canada and Agriculture and Agri-Food Canada, and the operating budget of L.S. and M.L. from Agriculture and Agri-Food Canada.
REFERENCES
- 1.Arsène, F., T. Tomoyasu, and B. Bukau. 2000. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55:3-9. [DOI] [PubMed] [Google Scholar]
- 2.Bigelow, W. D., and J. R. Esty. 1920. Thermal death point in relation to time of typical thermophilic organisms. J. Infect. Dis. 27:602. [Google Scholar]
- 3.Broadbent, J. R., C. J. Oberg, H. Wang, and L. Wei. 1997. Attributes of the heat shock response in three species of dairy Lactobacillus. Syst. Appl. Microbiol. 20:12-19. [Google Scholar]
- 4.Coligan, J. E., A. M. Kruibeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.). 1992. Current protocols in immunology. John Wiley & Sons, New York, N.Y.
- 5.Craig, E. A., and C. A. Gross. 1991. Is hsp70 the cellular thermometer? Trends Biochem. Sci. 16:135-139. [DOI] [PubMed] [Google Scholar]
- 6.Gamer, J., H. Bujard, and B. Bukau. 1992. Physical interaction between heat shock proteins DnaK, DnaJ and GrpE and the bacterial heat shock transcription factor σ32. Cell 69:833-842. [DOI] [PubMed] [Google Scholar]
- 7.Hartl, F. U., J. Martin, and W. Neupert. 1992. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu. Rev. Biophys. Biomol. Struct. 21:293-322. [DOI] [PubMed] [Google Scholar]
- 8.Herendeen, S. L., R. A. VanBogelen, and F. C. Neidhardt. 1979. Levels of major proteins of Escherichia coli during growth at different temperatures. J. Bacteriol. 139:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman, C., D. Thevenet, R. D'Ari, and P. Bouloc. 1995. Degradation of σ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. USA 92:3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemaux, P. G., S. L. Herendeen, P. L. Bloch, and F. C. Neidhardt. 1978. Transient rates of synthesis of individual polypeptides in Escherichia coli following temperature shifts. Cell 13:427-434. [DOI] [PubMed] [Google Scholar]
- 11.Mackey, B. M., and C. M. Derrick. 1986. Elevation of the heat resistance of Salmonella typhimurium by sublethal heat shock. J. Appl. Bacteriol. 61:389-393. [DOI] [PubMed] [Google Scholar]
- 12.Mackey, B. M., and C. M. Derrick. 1987. Changes in the heat resistance of Salmonella typhimurium during heating at rising temperatures. Lett. Appl. Microbiol. 4:13-16. [Google Scholar]
- 13.Neidhardt, F. C., P. L. Bloch, S. Pedersen, and S. Reeh. 1977. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J. Bacteriol. 129:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neidhardt, F. C., R. A. VanBogelen, and V. Vaughan. 1984. The genetics and regulation of heat shock proteins. Annu. Rev. Genet. 18:295-329. [DOI] [PubMed] [Google Scholar]
- 15.Neidhardt, F. C., and R. A. VanBogelen. 1987. Heat shock response, p. 1334-1345. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 16.Reichert, J. E., H. Bremke, and J. Baumgart. 1979. Zur Ermittlung des Erhitzungseffektes für Kochschinken (F-wert). Fleischerei 30:624-633. [Google Scholar]
- 17.Roberts, T. A., A. C. Baird-Parker, and R. B. Tompkins. 1996. Microorganisms in foods, vol. 5. Microbiological specifications of food pathogens. Blackie Academic & Professional Publishers, London, United Kingdom.
- 18.Rüdiger, S., A. Buchberger, and B. Bukau. 1997. Interaction of Hsp 70 chaperones with substrates. Nat. Struct. Biol. 4:342-349. [DOI] [PubMed] [Google Scholar]
- 19.SAS Institute, Inc. 1999. SAS System for Windows, 1999-2001, version 8.02. SAS Institute, Inc., Cary, N.C.
- 20.Tomoyasu, T., J. Gamer, B. Bukau, M. Kanemori, H. Mori, A. J. Rutman, A. B. Oppenheim, T. Yura, K. Yamanaka, H. Hiki, S. Higara, and T. Ogura. 1995. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor σ32. EMBO J. 14:2551-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomoyasu, T., T. Ogura, T. Tatsuta, and B. Bukau. 1998. Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in Escherichia coli. Mol. Microbiol. 30:567-581. [DOI] [PubMed] [Google Scholar]
- 22.Woodcock, E., and G. C. Grigg. 1972. Repair of thermally induced DNA breakage in Escherichia coli. Nat. New Biol. 237:76-79. [DOI] [PubMed] [Google Scholar]
- 23.Yura, T., M. Kanemori, and M. T. Morita. 2000. The heat shock response: regulation and function, p. 3-18. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.