Abstract
Aminotransferases, which catalyze the last step of biosynthesis of most amino acids and the first step of their catabolism, may be involved in the growth of Lactococcus lactis in milk. Previously, we isolated two aminotransferases from L. lactis, AraT and BcaT, which are responsible for the transamination of aromatic amino acids, branched-chain amino acids, and methionine. In this study, we demonstrated that double inactivation of AraT and BcaT strongly reduced the growth of L. lactis in milk. Supplementation of milk with amino acids and keto acids that are substrates of both aminotransferases did not improve the growth of the double mutant. On the contrary, supplementation of milk with isoleucine or a dipeptide containing isoleucine almost totally inhibited the growth of the double mutant, while it did not affect or only slightly affected the growth of the wild-type strain. These results suggest that AraT and BcaT play a major role in the growth of L. lactis in milk by degrading the intracellular excess isoleucine, which is responsible for the growth inhibition. The growth inhibition by isoleucine is likely to be due to CodY repression of the proteolytic system, which is necessary for maximal growth of L. lactis in milk, since the growth of the CodY mutant was not affected by addition of isoleucine to milk. Moreover, we demonstrated that AraT and BcaT are part of the CodY regulon and therefore are regulated by nutritional factors, such as the carbohydrate and nitrogen sources.
Lactococcus lactis is widely used as a starter in the cheese industry, and consequently, all the enzymatic activities involved in its growth in milk and in the formation of aroma compounds are of major interest. Several studies have shown that the proteolytic system of L. lactis, especially the extracellular proteinase (PrtP) and the oligopeptide transport system (Opp), is essential for rapid growth of L. lactis in milk (6, 18, 21). Indeed, dairy strains are auxotrophic for a number of amino acids, generally including leucine, valine, isoleucine, histidine, and methionine, and the free amino acids in milk are not sufficient to support the growth of L. lactis at high cell densities. However, the proteolytic system does not provide all the amino acids necessary for growth in milk, and biosynthesis of certain amino acids, such as aspartic acid, is also necessary (8, 34, 35).
Amino acid transamination, catalyzed by aminotransferases, is a reversible reaction and is therefore responsible for both the first step of amino acid catabolism and the last step of amino acid biosynthesis. Consequently, aminotransferases may be involved in the growth of L. lactis in milk either by providing the amino acids released at low concentrations by the proteolytic system or by degrading some amino acids, such as branched-chain amino acids, that accumulate in cells and mediate the repression of major components of the L. lactis proteolytic system (17). In L. lactis, repression of the proteolytic system occurs via the pleiotropic transcriptional repressor CodY, which senses the intracellular pool of branched-chain amino acids (17). In other gram-positive bacteria (e.g., Bacillus subtilis), the nutritional regulator CodY also regulates several other genes involved in nitrogen metabolism, such as the genes involved in amino acid biosynthesis and catabolism (10). Therefore, CodY may be involved in aminotransferase regulation. Finally, the aminotransferases of lactic acid bacteria are involved in aroma formation in cheese by initiating the conversion of amino acids to aroma compounds (37).
In previous work, we purified and characterized two major aminotransferases, AraT and BcaT from L. lactis, which are responsible for the transamination of aromatic and branched-chain amino acids and methionine, the major precursors of aroma compounds (36, 38). Because we used single mutants, we could not conclude whether other aminotransferases active with these amino acids were present. Indeed, inactivation of AraT did not totally prevent degradation of aromatic amino acids which did not appear to be substrates for BcaT (36). Similarly, the bcaT mutant retained low residual activity with isoleucine and valine, although these amino acids were not identified as substrates for AraT (38). Moreover, Rijnen et al. (25) showed that a glutamate phenylpyruvate aminotransferase activity was induced by suppressing phenylalanine in the growth medium. This activity allowed an araT mutant to grow in chemically defined medium without phenylalanine, after a delay. Finally, in addition to araT and bcaT several other genes in the genome of L. lactis have been shown to exhibit high homology with genes that encode aminotransferases (3).
In this study, using a double araT-bcaT mutant, we verified that AraT and BcaT are the only two aminotransferases involved in the metabolism of aromatic and branched-chain amino acids in L. lactis. Moreover, we demonstrated that these enzymes play a major role in the growth of L. lactis in milk by regulating the intracellular pool of isoleucine and that they are part of the CodY regulon.
MATERIALS AND METHODS
Chemicals.
Amino acids, keto acids, inhibitors, pyridoxal 5′-phosphate, and erythromycin were obtained from Sigma Chemical Co. (St. Louis, Mo.). Radiolabeled amino acids were obtained from Amersham Pharmacia Biotechnology (Freiburg, Germany).
Bacterial strains, plasmids, and culture conditions.
Strains and plasmids used in this study are listed in Table1. Escherichia coli strains were grown aerobically in Luria-Bertani medium at 37°C with or without 1.5% agar (27). L. lactis strains were grown at 30°C either in M17 medium supplemented with 0.5% (wt/vol) lactose or glucose (30) or in chemically defined media (CDM) (28). Normal CDM contain 19 amino acids (all amino acids except aspartic acid) and glucose as the sugar. Different modified CDM were used. In CDM−Ile, the concentration of isoleucine was reduced from 200 to 2 mg per liter. In CDM−Phe, phenylalanine was absent. In CDM+casein, the amino acids were replaced by casein at the concentration found in milk (3.2%, wt/vol). The casein used was prepared by precipitation (at pH 4.6) of milk reconstituted as described below. In CDM+Casitone, free amino acids were replaced by Casitone (Sigma-Aldrich) at a concentration of 1.5% (wt/vol). In lac-CDM, glucose was replaced by lactose (0.25%, wt/vol). For growth experiments, the growth rates of the L. lactis strains in different minimal media were measured with a Bioscreen C analyzer (Labsystems, Helsinki, Finland) and a Biolink software program. A total of 300 μl of medium was inoculated with 6 μl of cells harvested from a culture in CDM in the late exponential phase of growth and washed twice with β-glycerophosphate. The optical density at 450 nm (OD450) was measured every 10 min for 30 h. The results are means of at least four independent experiments.
Growth experiments in buffered 75 mM β-glycerophosphate milk were done at 30°C and monitored as previously described (26). Milk was reconstituted from NILAC low-heat spray powder (NIZO, Ede, The Netherlands) at a concentration of 10% (wt/vol) in distilled sterilized water at 30°C. It was inoculated at an initial OD480 of 0.05 with a preculture in milk. When specified, milk was supplemented with certain amino acids at the concentrations used in CDM, with certain α-keto acids at the same concentrations, with dipeptides at a concentration of 1 mM, or with Casitone at a concentration of 1.5% (wt/vol). Bacterial growth was monitored by measuring the OD480 after clarification of milk by 10-fold dilution in 5 mM EDTA (pH 12) (32). The growth rate was defined as the maximal slope of the semilogarithmic graph of growth, as determined by measuring the optical density. As specified below, growth rates were compared by variance analysis.
When necessary, erythromycin (5 μg ml−1 for L. lactis and 150 μg ml−1 for E. coli) or ampicillin (50 μg ml−1 for E. coli) was added to the culture medium.
DNA techniques.
All DNA manipulations were performed as described by Sambrook et al. (27). DNA restriction and modification enzymes were purchased from GIBCO-BRL (Cergy Pontoise, France), Eurogentec (Seraing, Belgium), or Boehringer (Mannheim, Germany) and used as recommended by the suppliers. L. lactis and E. coli electrocompetent cells were prepared and transformed by using standard techniques (19, 27).
Plasmid DNA was prepared with a plasmid purification kit from Qiagen Inc. (Chatsworth, Calif.) for E. coli and by the method of O'Sullivan and Klaenhammer for L. lactis (24).
PCR amplification was performed with a Perkin-Elmer DNA thermal cycler 480 or 2400 by using Taq DNA polymerase (Appligene, Illkirch, France) as previously described (25). Oligonucleotides were synthesized by Eurogentec.
L. lactis MG1363 and JIM7596 were electroporated with the total plasmid DNA isolated from L. lactis NCDO763. Clones transformed with the lactose/protease plasmid were selected by plating on fast strain differencing agar (20). The positive clones were checked for their plasmid contents. TIL672 and TIL856 contained only the protease/lactose plasmid.
Northern hybridization.
Total RNA of cells collected in the late exponential phase of growth was prepared as previously described for B. subtilis (13). After extraction and treatment with phenol-chloroform, the RNA was precipitated with ethanol, and 10 μg of glyoxalated RNA was electrophoresed through a 1% agarose gel containing 10 mM sodium iodoacetic acid in 10 mM sodium phosphate buffer (pH 7). DNA probes were prepared with PCR-amplified fragments of the araT and bcaT genes (700 bp for bcaT and 400 bp for araT) by using an ECL kit (Amersham, Little Chalfont, Buckinghamshire, United Kingdom). Simultaneous hybridization with both probes was performed as described by the supplier. Equal loading of the gel slots and the integrity of the RNA were checked by bromophenol blue staining of the blots that were to be used for hybridization. Hybridization experiments were carried out three times with independently isolated RNA.
Construction of negative mutants.
Construction of the araT mutant (TIL313) has been described previously (25).
The food-grade bcaT mutant, TIL357, was obtained by deletion of a central part of the gene by gene replacement by using the thermosensitive pG+host 9 plasmid (2). To do this, pTIL252, which contained a 2.5-kb insert with the bcaT gene, was digested with KpnI and BsmI, which eliminated a 430-bp fragment of the bcaT gene in the middle of the 2.5-kb insert. After the ends were made blunt with T4 DNA polymerase, the plasmid was religated in a diluted solution (500 ng ml−1), which resulted in pTIL255, which had a 430-bp deletion in the bcaT gene. The resulting 2.1-kb insert of pTIL255 was then removed from the pGEM-T vector by digestion with ApaI and PstI and ligated with ApaI-PstI-restricted pG+host 9, resulting in pTIL257. bcaT gene replacement was performed by the method of Biswas et al. (2). Briefly, the pTIL257 plasmid was introduced into TIL46 by electroporation. The cells were grown overnight at 28°C in M17 medium containing lactose in the presence of erythromycin and then diluted 1,000-fold in the same medium and grown at 28°C for 2 h. The cultures were then shifted to 37.5°C overnight. Integrants were isolated on M17-erythromycin plates at 37°C and cultivated in M17 medium containing lactose and erythromycin for 1 day before each culture was diluted 1:106 in M17 medium without antibiotic and cooled to 28°C. The overnight saturated culture was plated at various concentrations and incubated at 37°C with or without erythromycin selection. Colonies in which gene replacement had occurred were erythromycin sensitive.
The double araT-bcaT mutant, TIL358, was constructed from TIL357 in which the araT gene was disrupted by a single crossover with the integrative vector pTag containing a 1-kb fragment of araT (pTIL212), as previously described for the araT mutant (25).
Aminotransferase activities.
The aminotransferase activities in extracts of cells grown in different media to the late exponential phase were determined as previously described (25, 38). Cell extracts were prepared as previously described (25) except for cell disruption, which was performed in 0.2 M Tris-HCl buffer (pH 8). The cell extracts were then diluted in such a way that after 15 min of reaction no more than 10% of the substrate was used. Data are reported below as means of the results for triplicate cultures.
Amino acid catabolism.
The catabolism of amino acids by whole cells of L. lactis subsp. cremoris TIL46 and mutants was studied by using radiolabeled amino acids as tracers according to a previously described protocol (26, 38). Briefly, each reaction mixture contained 100 mM Tris-HCl buffer (pH 8), unlabeled amino acid at a concentration of 2 mM, 0.05 μM tritiated amino acid, and 10 mM α-ketoglutarate. A quantity of cells grown in CDM corresponding to an OD480 of 10 was added to 500 μl of the reaction mixture. After incubation at 37°C for 0, 10, 20, and 40 h, the reaction mixtures were analyzed by reverse-phase high-performance liquid chromatography with both UV (214-nm) detection and radioactivity detection. Data are reported below as means of the results of duplicate reactions.
Protein concentration determination.
Protein concentrations were determined by the micromethod of Bradford (5) by using bovine serum albumin fraction as the standard according to the instructions of the supplier (Pierce Chemical Company, Rockford, Ill.).
RESULTS
Effect of inactivation of AraT and BcaT on the aminotransferase activities of the strains grown in CDM.
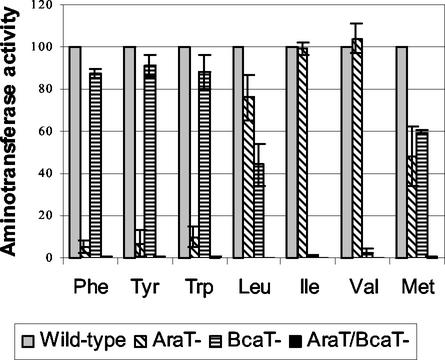
The aminotransferase activities of the wild-type and mutant strains were determined with cell extracts by measuring the glutamate produced in a reaction medium containing α-ketoglutarate and every one of the amino acids. Figure 1 shows the aminotransferase activities of the mutant strains expressed as percentages of the activities of the wild-type strain. It is clear that the double araT-bcaT mutant did not exhibit any residual activity towards aromatic and branched-chain amino acids or towards methionine, indicating that no other aminotransferase is active with these amino acids in L. lactis. Consequently, we assumed that the residual activity of the araT mutant is due to BcaT and that the residual activity of the bcaT mutant is due to AraT. Therefore, although we did not detect any activity towards aromatic amino acids with purified BcaT (36), BcaT is responsible for 5 to 10% of the activity towards these amino acids, in addition to its major activity with the branched-chain amino acids and methionine. In the same way, AraT is also capable of transaminating Ile and Val weakly, in addition to its high levels of activity with aromatic amino acids, leucine, and methionine.
FIG. 1.
Effect of inactivation of the aminotransferase genes araT and bcaT of L. lactis wild-type strain TIL46 on aminotransferase activities of the strain. The activities are expressed as percentages of the activities of the wild-type strain (Wild-type) and were determined by using phenylalanine (Phe), tyrosine (Tyr), tryptophan (Trp), leucine (Leu), isoleucine (Ile), valine (Val), and methionine (Met) as substrates and α-ketoglutarate as the cosubstrate. AraT−, the derivative araT mutant TIL313; BcaT−, the derivative bcaT mutant TIL357; AraT−/BcaT−, the derivative double araT bcaT mutant TIL358.
Effect of double inactivation of AraT and BcaT on amino acid catabolism.
The double mutant could not degrade aromatic and branched-chain amino acids or methionine when resting cells were incubated with each amino acid for 40 h at 37°C at pH 8 in the presence or in the absence of α-ketoglutarate (Table 2). This result strongly suggests that L. lactis does not possess other enzymes capable of degrading these amino acids.
TABLE 2.
Percentages of amino acid degradation by the wild-type strain and the double araT bcaT mutant after incubation of resting cells with each amino acid in the presence of α-ketoglutarate for 40 h at pH 8
| Strain | % Degradation of:
|
|||||
|---|---|---|---|---|---|---|
| Isoleucine | Valine | Leucine | Methionine | Phenylalanine | Tryptophan | |
| Wild type | 36.1 ± 0.8 | 48.7 ± 3.5 | 84.5 ± 5.6 | 65.6 ± 11 | 42.6 ± 14.8 | 30.5 ± 8 |
| araT bcaT mutant | 3.1 ± 3 | 4.2 ± 3.6 | 3 ± 2.6 | 0 | 9.2 ± 9.8 | 0.5 ± 0.7 |
Effect of inactivation of AraT and BcaT on the growth of L. lactis in CDM and in milk.
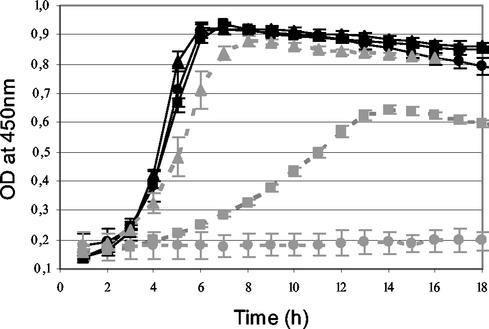
The growth of L. lactis in complete CDM was not affected by inactivation of AraT and BcaT separately or together (Fig. 2). In contrast, growth in CDM without phenylalanine was delayed and decreased by AraT inactivation and was totally inhibited by inactivation of both AraT and BcaT. These results indicate that AraT is highly involved in the biosynthesis of Phe but that BcaT is also capable of synthesizing this amino acid slowly after a latency period and that no other aminotransferase is involved in the biosynthesis of Phe.
FIG. 2.
Growth curves for wild-type strain TIL46 (triangles), the derivative araT mutant TIL313 (squares), and the double araT bcaT mutant TIL358 (circles) in CDM (black solid lines) and in CDM without phenylalanine (grey dotted lines).
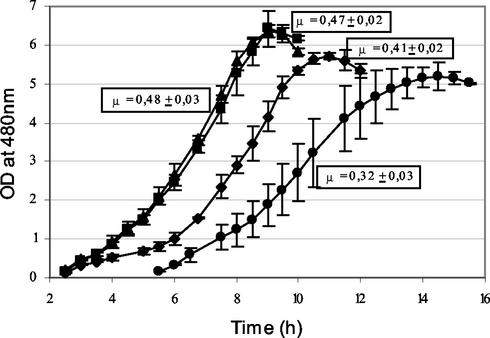
Growth of L. lactis in milk also was not affected by AraT inactivation, while it was delayed and slightly reduced by BcaT inactivation and reduced a great deal by inactivation of both aminotransferases together (Fig. 3). Indeed, disruption of both genes significantly reduced the optimal growth rate (P = 0.99), delayed growth, and reduced the final absorbance of the culture.
FIG. 3.
Growth curves for wild-type strain TIL46 (▴), the derivative araT mutant TIL313 (▪), the derivative bcaT mutant TIL357 (♦), and the double araT bcaT mutant TIL358 (•) in milk. The growth rate (μ) was calculated by determining the maximum slope of the semilogarithmic graph of growth obtained by measuring the optical density.
Aminotransferases may be involved in the growth of L. lactis either through their role in amino acid catabolism or through their role in amino acid biosynthesis. Indeed, biosynthesis of certain amino acids may be necessary for growth in milk, or catabolism of certain amino acids may be essential because accumulation of these amino acids in the cells induces repression of enzyme biosynthesis or because catabolism provides metabolites required for growth. To test these different hypotheses, we tried to complement milk with the different amino acid substrates (either as free forms or as dipeptide forms) of both aminotransferases and with the corresponding α-keto acids.
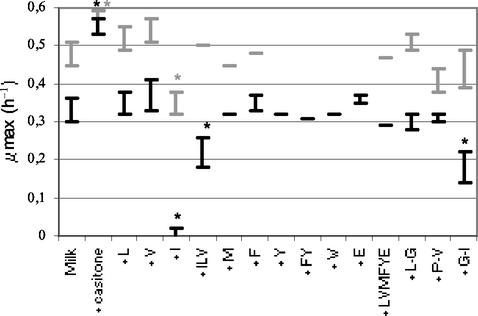
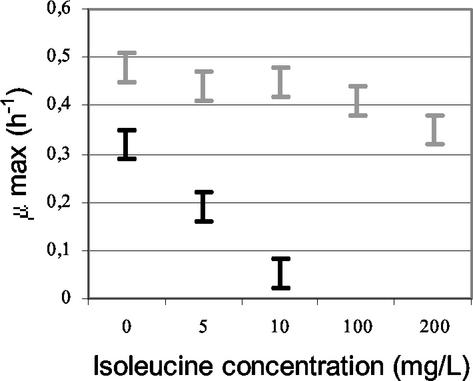
Addition of Casitone to milk led to similar rapid growth of the wild-type strain and the double mutant (Fig. 4). This result suggests that the function affected by the inactivation was related to the amino acid supply. Indeed, Casitone, which is a casein hydrolysate, is a source of easily assimilated amino acids. However, adding the amino acid substrates of AraT and BcaT (Phe, Ile, Leu, Tyr, Trp, Val, Met) to milk did not significantly increase the maximal growth rate of the double mutant (Fig. 4). On the contrary, addition of free isoleucine, even at a low concentration (5 mg/liter), or addition of a dipeptide containing isoleucine greatly reduced the growth rate of the double mutant (Fig. 4 and 5) and increased the latency time (to around 6 h more). The growth of the wild-type strain was also reduced and delayed by addition of isoleucine to the milk (Fig. 4,) but only when the Ile concentration was higher than 100 mg per liter of milk (Fig. 5). Moreover, growth of the wild-type strain was only delayed by 4 h by addition of a dipeptide containing isoleucine. These results suggest that the aminotransferases play a major role by degrading the intracellular excess isoleucine, which is responsible for growth inhibition of the strain.
FIG. 4.
Growth rates (μmax) (with standard deviations) of wild-type strain TIL46 (grey bars) and the derivative double araT bcaT mutant TIL358 (black bars) in milk and in milk supplemented with different nitrogen sources. Most data were obtained from at least two independent experiments; the exceptions are the data indicated by lines, which were obtained from only one experiment. A variance analysis was performed for the two strains separately. An asterisk indicates that there was a significant change in the growth rate (P = 0.99). The compounds added to the milk are indicated on the x axis. Casitone was added at a concentration of 1.5%, free amino acids were added either separately or together at the concentrations found in CDM (around 2 mM), and dipeptides were added at a concentration of 1 mM. L, leucine; V, valine; I, isoleucine; M, methionine; F, phenylalanine; Y, tyrosine; W, tryptophan; E, glutamic acid; L-G, leucine-glycine dipeptide; P-V, proline-valine dipeptide; G-I, glycine-isoleucine dipeptide.
FIG. 5.
Growth rates (μ max) (with standard deviations) obtained for wild-type strain TIL46 (grey bars) and the derivative double araT bcaT mutant TIL358 (black bars) in milk and in milk supplemented with isoleucine at different concentrations.
Addition of α-keto acids produced by aminotransferases from branched-chain amino acids did not affect the growth of the double mutant (results not shown), indicating that the effect of aminotransferase inactivation on growth in milk was not due to the absence of amino acid degradation products.
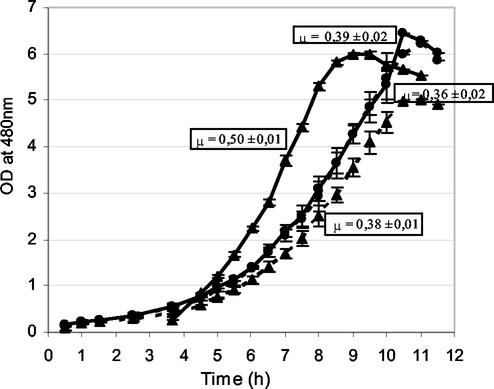
Since CodY is a repressor of the proteolytic system of L. lactis, which plays a major role in growth, we suspected that CodY might be involved in the growth inhibition by isoleucine. To verify this hypothesis, we compared the effects of isoleucine addition to milk on the growth of L. lactis TIL672 (Table 1) and the derivative CodY mutant TIL856 (Table 1 and Fig. 6). The wild-type strain grew faster in milk than the CodY mutant grew. However, addition of isoleucine to the milk (at a concentration of 200 mg/liter) significantly reduced the growth of the wild-type strain (P = 0.99), while it did not affect the growth of the CodY mutant, confirming that the growth inhibition by isoleucine is due to CodY regulation.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant features | Reference or source |
|---|---|---|
| Strains | ||
| L. lactis subsp. cremoris NCDO763 | Wild-type strain derived from NCDO712, PrtP+ Lac+ | National Collection of Food Bacteria (Shinfield, Reading, England) |
| TIL46 | NCDO763 cured of its 2-kb plasmid | |
| TIL313 | Emr TIL46 araT mutant | 25 |
| TIL357 | TIL46 food-grade bcaT mutant | This study |
| TIL358 | Emr TIL46 araT bcaT mutant | This study |
| L. lactis subsp. cremoris MG1363 | Plasmid- and phage-free derivative of NCDO712, Lac− PrtP− | 11 |
| TIL672 | MG1363 containing pTIL672, Lac+ PrtP+ | This study |
| JIM7596 | Emr, MG1363 codY | 17 |
| TIL856 | Emr, JIM7596 codY containing pTIL672, PrtP+ Lac+ | This study |
| Escherichia coli TG1 | 12 | |
| Plasmids | ||
| pTIL212 | Ampr Emr, 1-kb fragment of araT in pTag, 6 kb | 24 |
| pG+host9 | Emr ori thermosensitive, 3.8 kb | 2 |
| pTIL252 | Ampr, 2.5-kb TIL46 DNA fragment containing bcaT in pGEM-T, 5.5 kb | 36 |
| pTIL255 | Emr PTIL252 with a deletion of 430 bp in the bcaT gene, 5.1 kb | This study |
| pTIL257 | Emr, pG+host9 with 2.1-kb insert containing bcaT with deletion of 430 bp (from pTIL255), 5.9 kb | This study |
| pTIL672 | Lac+ PrtP+ plasmid from L. lactis NCDO763, 55 kb | This study |
FIG. 6.
Growth curves for wild-type strain TIL672 (▴) and the derivative CodY mutant TIL856 (•) in milk (solid lines) and in milk supplemented with isoleucine at a concentration of 200 mg/liter (dotted line). The curves obtained with the CodY mutant in both media are superimposed. μ, growth rate.
Regulation of AraT and BcaT by components of the growth medium.
We observed previously that the AraT activity was only slightly affected by the growth medium (25), while the BcaT activity was strongly affected by the nitrogen source and by isoleucine starvation (36). In order to increase our knowledge concerning the effectors of AraT and BcaT regulation, we tested other growth media. In particular, we tested the effect of the carbohydrate source by using CDM with lactose or glucose. We also tested different nitrogen sources (casein, free amino acids, and Casitone) whose amino acids are more or less rapidly assimilated by L. lactis. Finally, we tested the effect of starvation for Ile and Phe, which are major amino acid substrates for each aminotransferase, by using CDM without Phe or with a minimal concentration of isoleucine (minimal concentration for growth).
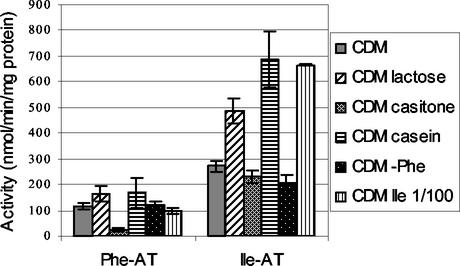
Since 90 to 95% of the Phe aminotransferase activity is due to AraT and 95 to 100% of the Ile aminotransferase activity is due to BcaT, we used these activities to monitor the activity of AraT and BcaT in wild-type strain TIL46 grown in the various media (Fig. 7).
FIG. 7.
Phenylalanine and isoleucine aminotransferase activities (Phe-AT and Ile-AT, respectively) in cells of L. lactis TIL46 grown in different media to the late exponential phase. CDM, CDM with glucose; CDM lactose, CDM with lactose instead of glucose; CDM casitone, CDM with Casitone instead of free amino acids; CDM casein, CDM with casein instead of free amino acids; CDM−Phe, CDM without phenylalanine; CDM Ile 1/100, CDM with a 100-fold-lower isoleucine concentration.
The carbohydrate source had a great effect on the BcaT activity and, to a lesser extent, on the AraT activity. Indeed, the activities of the cells grown in the medium containing lactose were higher than the activities of the cells grown in the medium containing glucose, suggesting that repression was induced by the presence of glucose in the medium.
The nitrogen source also affected the AraT and BcaT activities, but there were some differences in the intensity of the effect on AraT and BcaT activities. When the activities of cells grown in CDM containing free amino acids were used as references, the aminotransferase activities were reduced by the presence of Casitone (especially the AraT activity) and increased by the presence of casein (especially the BcaT activity). These results suggest that AraT and BcaT activities are regulated by the intracellular pool of free amino acids since amino acids from Casitone are more rapidly assimilated than free amino acids, while amino acids from casein are less rapidly assimilated than free amino acids.
Finally, we observed that isoleucine or phenylalanine starvation regulated BcaT activity. Ile starvation increased the BcaT activity twofold in the wild-type strain, while Phe starvation only increased this activity in the araT mutant (501 ± 23 versus 257 ± 21 nmol min−1 mg of protein−1). Indeed, we did not observe an increase in BcaT activity in the wild-type strain when Phe was suppressed from CDM, probably because AraT allows the biosynthesis of phenylalanine, which is consequently present in the cells.
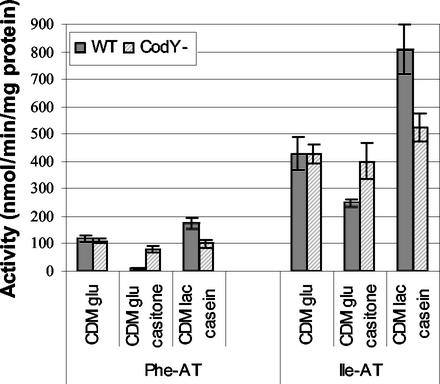
CodY appeared to be a good candidate for a regulator of AraT and BcaT activities since most of the identified effectors of AraT and BcaT regulation are effectors of CodY regulation and CodY is known to be a regulator of nitrogen metabolism in gram-positive bacteria. In order to verify that CodY is involved in AraT and BcaT regulation, we studied the effect of inactivating CodY in L. lactis on the aminotransferase activities of the organism grown in media in which we observed significant variations in activity, including CDM, CDM with casein and lactose, and CDM with Casitone (Fig. 8). To do this, we used strain TIL672 (MG1363 prtP+ lac+) and the derivative CodY mutant TIL856. Repression of BcaT activity by Casitone in the growth medium and an increase in AraT activity when casein was used were clearer in strain TIL672 than in strain TIL46. Inactivation of CodY almost totally suppressed the modifications of activities observed with the wild-type strain, both in the medium containing casein and in the medium containing Casitone. Indeed, the AraT and BcaT activities of the CodY mutant were not affected by the growth medium and were similar to the activities of the wild-type strain grown in CDM. These results suggest that there was CodY-dependent induction of BcaT and AraT in the medium containing casein and lactose and that there was CodY-dependent repression of AraT and BcaT in the medium containing Casitone.
FIG. 8.
Phenylalanine and isoleucine aminotransferase activities (Phe-AT and Ile-AT, respectively) in cells of L. lactis TIL672 (WT) and the derivative CodY mutant TIL856 (CodY−) grown in different media to the late exponential phase. CDM glu, CDM with glucose; CDM glu casitone, CDM with glucose and with Casitone instead of free amino acids; CDM lac casein, CDM with lactose and casein instead of free amino acids.
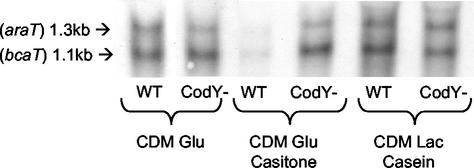
Finally, to verify that AraT and BcaT are regulated by CodY at the level of transcription, we examined by using Northern hybridization araT and bcaT transcription in wild-type strain TIL672 and the derivative CodY mutant TIL856, under various growth conditions. Figure 9 shows that in the wild-type strain the level of transcription of both araT and bcaT was much lower in the growth medium containing Casitone and was a bit higher in the medium containing casein and lactose than in the normal CDM. Figure 9 also shows that both repression and induction of araT and bcaT expression by the growth media were suppressed by CodY inactivation.
FIG. 9.
Northern hybridization with araT and bcaT probes of total RNA extracted from cells of L. lactis TIL672 (WT) and the derivative CodY mutant TIL856 (CodY−) grown in different media to the late exponential phase. CDM glu, CDM with glucose; CDM Glu Casitone, CDM with glucose and with Casitone instead of free amino acids; CDM Lac Casein, CDM with lactose and casein instead of free amino acids.
DISCUSSION
Physiological role of AraT and BcaT during growth.
In previous studies we showed that AraT and BcaT initiate the catabolism of aromatic amino acids, branched-chain amino acids, and methionine in L. lactis (36, 38). In the present work, we constructed and used the double araT bcaT mutant to demonstrate that only these two aminotransferases are involved in the metabolism of aromatic amino acids and branched-chain amino acids and that no other enzyme is capable of performing the last step of biosynthesis or the first step of catabolism.
AraT is mainly involved in the metabolism of aromatic amino acids, Leu, and Met, but it can also participate in the metabolism of Ile and Val. In the same way, BcaT, which is mainly involved in the metabolism of Ile, Val, and Leu, can participate in the metabolism of aromatic amino acids. Therefore, only inactivation of both enzymes together completely blocked the metabolism of all aromatic amino acids and branched-chain amino acids.
Because of their essential role in the metabolism of aromatic amino acids and branched-chain amino acids, AraT and BcaT are intimately involved in the growth of L. lactis in milk and in some minimal media. Inactivation of both AraT and BcaT completely inhibited the growth of L. lactis in CDM without Phe and greatly reduced it in milk.
During growth in the medium lacking Phe, AraT is mainly responsible for the production of Phe, but when AraT is inactivated, BcaT can replace it by providing the Phe necessary for growth. However, growth of the araT mutant in this medium required a latency period corresponding to induction of BcaT activity. Indeed, the BcaT activity in cells of the araT mutant grown in CDM without Phe was twofold higher than that in cells grown in CDM. This induction of BcaT activity in the absence of Phe occurred only in the araT mutant, since in the wild-type strain AraT rapidly produces Phe, which seems to be an effector of bcaT expression.
The role of BcaT and, to a lesser extent, the role of AraT in the growth of L. lactis in milk are probably mainly to regulate the intracellular pool of free branched-chain amino acids, especially the pool of Ile. During the first phase of growth in milk (until the OD480 is 0.5 to 1), L. lactis uses the free amino acids and small peptides that constitute the nonprotein fraction of milk. The nonprotein fraction contains about equal amounts of Ile and Leu (about 7 mg liter−1) and twice as much Val (about 14 mg liter−1), while protein biosynthesis in L. lactis requires twice as much Leu as Ile and Val (31). Therefore, excess Ile and excess Val are probably supplied to the cells for protein biosynthesis during the first growth phase. In the wild-type strain, the aminotransferases can degrade the amino acids supplied in excess amounts, but inactivation of AraT and BcaT completely eliminates the catabolism of branched-chain amino acids. Consequently, Ile and Val probably accumulate in the cells of the double mutant during the first phase of growth in milk. Intracellular accumulation of branched-chain amino acids is known to be a signal that controls several regulation systems involved in nutrient supply. Ile and Leu have been shown to be effectors of transcriptional repression of branched-chain amino acid biosynthesis in L. lactis (14-16), but since our strain is auxotrophic for branched-chain amino acids, regulation of branched-chain amino acid biosynthesis is probably not the cause of the growth inhibition in milk containing Ile and Leu. Leu (or other branched-chain amino acids) is also the main signal of leucine response regulatory protein (Lrp)-dependent regulation in E. coli (9, 22), but no homologues of the Lrp protein have been identified in L. lactis (4). Finally, intracellular accumulation of Ile, Leu, and Val was recently shown to be an effector of the CodY-dependent repression of the major components of the proteolytic system of L. lactis (17). This proteolytic system, including the PrtP proteinase, the Opp transporter, and the PepN, PepC, and PepO1 peptidases, is highly involved in the growth of L. lactis and especially in the second phase of growth that corresponds to the maximal growth rate (23, 31, 33). Therefore, repression of this proteolytic system is likely to be responsible for the decrease in the maximal growth rate of the double araT bcaT mutant and, to a lesser extent, of the single bcaT mutant. Indeed, Guédon et al. showed that expression of the OppD gene in different media containing branched-chain amino acids either as free forms or as dipeptide forms was fivefold lower in the araT bcaT mutant than in the wild-type strain (17). Moreover, we confirmed that CodY repression is actually involved in the growth inhibition by isoleucine since growth of the CodY mutant in milk was not affected by isoleucine addition. However, the mechanism of this repression in milk has not been totally elucidated, since we observed only Ile as possible signal while leucine and valine were also demonstrated to be effectors of CodY regulation in L. lactis MG1363. Moreover, we cannot explain why the wild-type strain grows faster in milk than the CodY mutant grows; one possibility is that CodY-dependent induction of the proteolytic system occurs in the wild-type strain growing in milk, and another possibility is that CodY represses a function harmful to growth in milk.
AraT and BcaT are part of the CodY regulon.
In the present work we demonstrated that expression of AraT and expression of BcaT in L. lactis are regulated at the level of transcription by nutritional factors via the CodY regulator.
CodY is well known as a pleiotropic transcriptional repressor in gram-positive bacteria, such as B. subtilis and L. lactis. In B. subtilis, it negatively regulates about 10 genes involved in nitrogen metabolism, acetate metabolism, and peptide transport (10). In contrast to some regulators, such as Lrp, which can be either an activator or a repressor of transcription (7), CodY has only been described as a negative regulator of gene expression. However, in Streptococcus pyogenes, CodY has been proposed to be a good candidate for upregulation of virulence and proteolysis genes induced by valine and isoleucine starvation (17, 29). It is worth noting that the proteolysis genes that are transcriptionally upregulated during Ile and Val starvation include genes of the oligopeptide (opp) and dipeptide (dpp) permease systems and the peptidase gene (pepB), which are homologues of L. lactis CodY-regulated genes. Our results suggest that CodY may be responsible for the positive control of bcaT expression, unless the positive regulation is the result of repression of a repressor or the result of an indirect effect of CodY regulation.
Until now, among all the amino acids, only the branched-chain amino acids had been identified as major effectors of CodY regulation in L. lactis (17). Our results suggest that one of the branched-chain amino acids, Ile, plays a special role and that other amino acids, such as Phe, probably also are effectors of CodY regulation. Further work is required to definitively identify the amino acid effectors of CodY regulation in L. lactis.
In conclusion, the present study demonstrated the beneficial role of AraT and BcaT during growth of L. lactis in milk. BcaT and AraT also play a major role in flavor formation in cheese by initiating the conversion of several amino acids to aroma compounds. By regulating the intracellular concentration of branched-chain amino acids, they could also influence the formation of other aroma compounds, such as diacetyl and acetoin, since branched-chain amino acids regulate α-acetolactate decarboxylase activity, which is involved in the formation of these compounds (1, 16).
Acknowledgments
This work was supported by contract FAIR CT 97-3173 of the Commission of the European Communities.
We thank C. Delorme and E. Guédon for the gift of L. lactis JIM7596 and for helpful discussions, as well as M. Nardi and C. Hervé for transforming the MG1363 and JIM7596 strains with the PrtP+ Lac+ plasmid. We also thank Véronique Monnet for critical reading of the manuscript. We are indebted to Annick Lacombe (INRA Translation Unit, Jouy-en-Josas, France) for revising the English.
REFERENCES
- 1.Aymes, F., C. Monnet, and G. Corrieu. 1999. Effect of α-acetolactate decarboxylase inactivation on α-acetolactate and diacetyl production by L. lactis subsp. lactis biovar diacetylactis. J. Biosci. Bioeng. 87:87-92. [DOI] [PubMed] [Google Scholar]
- 2.Biswas, I., A. Gruss, D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolotin, A., S. Mauger, K. Malarme, S. D. Ehrlich, and A. Sorokin. 1999. Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Leeuwenhoek 76:27-76. [PubMed] [Google Scholar]
- 4.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Bruinenberg, P. G., P. Vos, and W. M. de Vos. 1992. Proteinase overproduction in Lactococcus lactis strains: regulation and effect on growth and acidification in milk. Appl. Environ. Microbiol. 58:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvo, J. M., and R. G. Matthews. 1994. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol. Rev. 58:466-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley, E. G., and J. L. Steele. 2001. Lactococcus lactis LM0230 contains a single aminotransferase involved in aspartate biosynthesis, which is essential for growth in milk. Microbiology 147:215-224. [DOI] [PubMed] [Google Scholar]
- 9.Ernsting, B. R., M. R. Atkinson, A. J. Ninfa, and R. G. Matthews. 1992. Characterization of the regulon controlled by the leucine responsive regulatory protein in Escherichia coli. J. Bacteriol. 174:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher, S. H. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la difference! Mol. Microbiol. 32:223-232. [DOI] [PubMed] [Google Scholar]
- 11.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson, T. J. 1984. Studies on the Eppstein Barr virus genome. University of Cambridge, Cambridge, United Kingdom.
- 13.Glatron, M. F., and G. Rapoport. 1972. Biosynthesis of the parasporal reclusion of Bacillus thuringiensis: half-life of its corresponding messenger RNA. Biochimie 54:1291-1301. [DOI] [PubMed] [Google Scholar]
- 14.Godon, J. J., M. C. Chopin, and S. D. Ehrlich. 1992. Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J. Bacteriol. 174:6580-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godon, J. J., C. Delorme, J. Bardowski, M.-C. Chopin, S. D. Ehrlich, and P. Renault. 1993. Gene inactivation in Lactococcus lactis. I. Branched chain amino acid biosynthesis. J. Bacteriol. 175:4383-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goupil-Feuillerat, N., G. Corthier, J. J. Godon, S. D. Ehrlich, and P. Renault. 2000. Transcriptional and translational regulation of alpha-acetolactate decarboxylase of Lactococcus lactis subsp. lactis. J. Bacteriol. 182:5399-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guédon, E., P. Serror, S. D. Ehrlich, P. Renault, and C. Delorme. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol. Microbiol. 40:1227-1239. [DOI] [PubMed] [Google Scholar]
- 18.Helinck, S., J. Richard, and V. Juillard. 1997. The effects of adding lactococcal proteinase on the growth rate of Lactococcus lactis in milk depend on the type of enzyme. Appl. Environ. Microbiol. 63:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huggins, A. M., and W. E. Sandine. 1984. Differentiation of fast and slow milk coagulating isolates in strains of streptococci. J. Dairy Sci. 67:1674-1679. [Google Scholar]
- 21.Juillard, V., D. Le Bars, E. R. S. Dunji, W. N. Konings, J.-C. Gripon, and J. Richard. 1995. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl. Environ. Microbiol. 61:3024-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, R. T., R. D'Ari, and E. B. Newman. 1992. Lambda placMu insertions in genes of the leucine regulon: extension of the regulon to genes not regulated by leucine. J. Bacteriol. 174:1948-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mierau, I., E. R. S. Kunji, K. J. Leenhouts, M. A. Hellendorn, A. J. Haandrikman, B. Poolman, W. N. Konings, G. Venema, and J. Kok. 1996. Multiple-peptidase mutants of Lactococcus lactis are severely impaired in their ability to grow in milk. J. Bacteriol. 178:2794-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Sullivan, D. J., and T. R. Klaenhammer. 1993. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl. Environ. Microbiol. 59:2730-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rijnen, L., S. Bonneau, and M. Yvon. 1999. Genetic characterization of the major lactococcal aromatic aminotransferase and its involvement in conversion of amino acids to aroma compounds. Appl. Environ. Microbiol. 65:4873-4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rijnen, L., P. Courtin, J.-C. Gripon, and M. Yvon. 2000. Expression of a heterologous glutamate dehydrogenase gene in Lactococcus lactis highly improves the conversion of amino acids to aroma compounds. Appl. Environ. Microbiol. 66:1354-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Smid, E. J., and W. N. Konings. 1990. Relationship between utilization of proline and proline-containing peptides and growth of Lactococcus lactis. J. Bacteriol. 172:5286-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner, K., and H. Malke. 2001. relA-Independent amino acid starvation response network of Stretococcus pyogenes. J. Bacteriol. 183:7354-7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas, T. D., and O. E. Mills. 1981. Proteolytic enzymes of starter bacteria. Neth. Milk Dairy J. 35:255-273. [Google Scholar]
- 32.Thomas, T. D., and K. W. Turner. 1977. Preparation of skim milk to allow harvesting of starter cells from milk cultures. N. Z. J. Dairy Sci. Technol. 12:15-21. [Google Scholar]
- 33.Von Wright, A., S. Tynkkynen, and M. Souminen. 1987. Cloning of a Streptococcus lactis subsp. lactis chromosomal fragment associated with the ability to grow in milk. Appl. Environ. Microbiol. 53:1584-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, H., D. J. O'Sullivan, K. A. Baldwin, and L. L. McKay. 2000. Cloning, sequencing, and expression of the pyruvate carboxylase gene in Lactococcus lactis subsp. lactis C2. Appl. Environ. Microbiol. 66:1223-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, H., W. Yu, T. Coolbear, D. O'Sullivan, and L. L. McKay. 1998. A deficiency in aspartate biosynthesis in Lactococcus lactis subsp. lactis C2 causes slow milk coagulation. Appl. Environ. Microbiol. 64:1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yvon, M., E. Chambellon, A. Sorokine, and F. Roudot-Algaron. 2000. Characterization and role of the branched-chain aminotransferase (BcaT) isolated from Lactococcus lactis subsp. cremoris NCDO 763. Appl. Environ. Microbiol. 66:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yvon, M., and L. Rijnen. 2001. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 11:185-201. [Google Scholar]
- 38.Yvon, M., S. Thirouin, L. Rijnen, D. Fromentier, and J. C. Gripon. 1997. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl. Environ. Microbiol. 63:414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]