Abstract
Three kinds of lactic acid bacteria were isolated from spoiling cooked meat products stored below 10°C. They were identified as Leuconostoc mesenteroides subsp. mesenteroides, Lactococcus lactis subsp. lactis, and Leuconostoc citreum. All three strains grew well in MRS broth at 10°C. In particular, L. mesenteroides subsp. mesenteroides and L. citreum grew even at 4°C, and their doubling times were 23.6 and 51.5 h, respectively. On the other hand, although the bacteria were initially below the detection limit (<10 CFU/g) in model cooked meat products, the bacterial counts increased to 108 CFU/g at 10°C after 7 to 12 days.
Lactic acid bacteria are widely used in the manufacturing of fermented foods, but occasionally they have a negative impact on beer products (20, 23) and cooked meat products (1, 3, 4, 5, 6, 15, 17, 21), for example. Cooked meat products are hygienically handled and are packaged in a vacuum or modified atmosphere during manufacturing; such products are expected to be able to maintain good sensory quality for 2 to 4 weeks if they are stored below 10°C. However, spoilage sometimes occurs within the shelf-life period, requiring the producer to make recalls (4, 15). Some spoilage bacteria produce such typical changes as souring and the formation of gas, slime, and/or white liquid. Most of the spoilage bacteria have been identified as lactic acid bacteria (4, 6, 15). After products are packaged, populations of these bacteria in the product are not usually detected below 10 CFU/g, even if the product later spoils quickly (2, 3, 17). This inability to detect bacteria leads to increased damage and product recalls. Since the behavior of these bacteria is still poorly understood, this study was designed to identify and elucidate the behavior of spoilage bacteria in cooked meat products, ultimately in order to help predict occurrences of spoilage.
Three spoilage samples of commercially available cooked meat products were obtained. These samples were diluted 10-fold with a sterile 0.85% NaCl solution and treated with a stomacher (Pro-media SH-001; Elmex Ltd., Tokyo, Japan) for 1 min, and serial dilutions were prepared. Bacterial counts were determined by plate counting with MRS agar (Oxoid Ltd., Basingstoke, United Kingdom). The colonies were selected at random from the MRS agar plates and cultured on MRS agar again. MRS agar plates with the pure culture were used for Gram staining, catalase testing, and morphology testing. Each pure culture was further characterized by the measurement of gas production from glucose, 16S rRNA analysis, biochemical tests, and halotolerance analysis. Gas production from glucose in MRS broth (Oxoid Ltd.) at 30°C was estimated. 16S rRNA analysis was performed according to the method of Collins et al. (8). The sequence and search were determined by using a MicroSeq 500 16S ribosomal DNA bacterial sequencing kit (Applied Biosystems Inc., Foster City, Calif.) and the MicroSeq 16S ribosomal DNA sequence database (Applied Biosystems Inc.), respectively. Biochemical tests were performed by using the API 50CH system (bioMerieux s.a., Marcy-l'Etoile, France). Test preparations were incubated at 30°C, and readings were made after 24 and 48 h. Halotolerance was estimated by measuring growth in MRS broth containing 4.0 or 6.5% NaCl at 30°C.
Growth rates at 10 or 4°C were based on cell numbers, determined by plate counting with MRS agar, in MRS broth. Precultures of the isolates were prepared by incubation in MRS broth at 30°C for 24 h. A dilution series to 10−7 was prepared, and the cultures were incubated in MRS broth at 10 or 4°C. The data were collected in a Microsoft Excel spreadsheet, and the growth rates were determined by using exponential curve fitting. Growth curves at 30 to 40°C were observed with optical density at 660 nm. The preculture (1 ml) was added to the MRS broth (100 ml) and incubated at 30, 35, 37, or 40°C.
Sterile cooked meat products were prepared to prevent any influence of bacterial impurities. Commercially available sliced loin hams were vacuum packed and then heated at 70°C for 20 min and subsequently cooled to room temperature once a day. These steps were carried out three times over a period of 3 days, and the hams were held at room temperature between these steps. As no bacterium was detected after incubating the hams in MRS broth at 30°C for 24 h, the hams were regarded as sterile and were used as model cooked meat products. The hams were inoculated with one of the isolates from the spoilage samples. The cells of the isolates were washed with sterile 0.85% NaCl solution twice and diluted to approximately 103 CFU/ml. Random places on the model cooked meat products (50 g each, in plastic bags [PYXON-10; Elmex Ltd.]) were inoculated with cell suspensions (0.1 ml), introduced drop by drop, and the cells were spread by lightly crumpling the bag. The inoculated hams were individually sealed under aseptic aerobic conditions and stored at 10°C. During storage, the packed hams were subsequently removed one by one for microbiological analysis.
The three spoilage samples showed different types of spoilage. In one vacuum-packaged sliced loin ham (product A), slime and gas were formed, and in another package of the same type (product B), the meat was soured. On the other hand, white liquid appeared in a modified-atmosphere-packaged wiener sausage (product C). The bacterial numbers were over 108 CFU/g, even if bacteria were not detected (<10 CFU/g) in the initial product analysis. Several spoilage organisms were isolated from the plate with the highest dilution and classified into three groups based on their characteristics. All of them were gram positive, catalase negative, and coccoid. The other characteristics are shown in Table 1. Strain MCRI 1 (culture collection of Marudai Central Research Institute) isolated from product A was consistent with Leuconostoc mesenteroides subsp. mesenteroides or subsp. dextranicum according to a comparison with its 16S rRNA sequence. According to acid production from l-arabinose, 6.5% NaCl tolerance, and the other characteristics, strain MCRI 1 was identified as L. mesenteroides subsp. mesenteroides (9, 11). Strain MCRI 3, isolated from product B, was closest to Lactococcus lactis subsp. lactis or subsp. hordniae according to 16S rRNA analysis. It produced acid from ribose, galactose, maltose, and lactose and grew in 4.0% NaCl. Based on these characteristics, strain MCRI 3 was identified as Lactococcus lactis subsp. lactis (22). Strain MCRI 4, isolated from product C, was identified as Leuconostoc citreum by 16S rRNA analysis and other characteristics (9).
TABLE 1.
Characteristics of isolates from spoiling cooked meat products
| Characteristic | Test result witha:
|
||
|---|---|---|---|
| Leuconostoc mesenteroides subsp. mesenteroides MCRI 1 | Lactococcus lactis subsp. lactis MCRI 3 | Leuconostoc citreum MCRI 4 | |
| Gas production from glucose | + | − | + |
| Acid production fromb: | |||
| l-Arabinose | + | + | + |
| Ribose | + | + | − |
| Galactose | + | + | − |
| d-Fructose | + | + | + |
| d-Mannose | + | + | + |
| Sorbitol | − | − | − |
| Amygdalin | + | + | + |
| Arbutin | + | + | + |
| Esculin | + | + | + |
| Maltose | + | + | + |
| Lactose | + | + | − |
| Melibiose | + | − | − |
| Sucrose | + | + | + |
| Trehalose | + | + | + |
| d-Raffinose | + | − | − |
| Starch | − | + | − |
| d-Turanose | + | − | + |
| Growth in: | |||
| 4.0% NaCl | + | + | + |
| 6.5% NaCl | + | + | + |
+, positive; −, negative. Based on their 16S rRNA sequences, the closest relative of Leuconostoc mesenteroides subsp. mesenteroides MCRI 1 was Leuconostoc mesenteroides subsp. mesenteroides or subsp. dextranicum (100% identity; 535 of 535 bases), that of Lactococcus lactis subsp. lactis MCRI 3 was Lactococcus lactis subsp. lactis or subsp. hordniae (99.81% identity; 534 of 535 bases), and that of Leuconostoc citreum MCRI 4 was Leuconostoc citreum (99.81% identity; 534 of 535 bases).
Tests were performed by using the API 50CH system; readings were taken at 48 h.
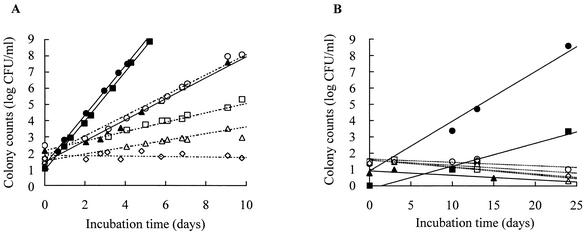
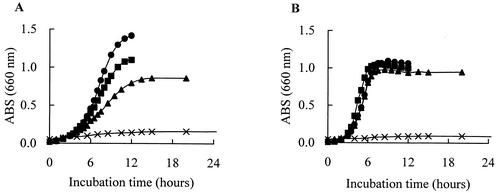
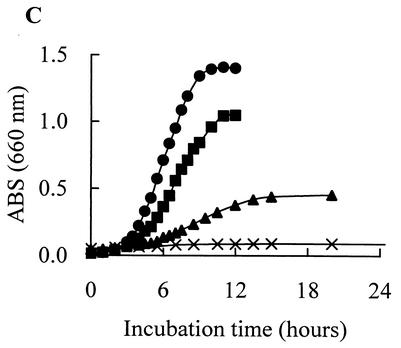
All of these organisms grew at 10°C in MRS broth and tended to grow more rapidly than the other lactic acid bacteria: Pediococcus acidilactici JCM 5885, Lactobacillus plantarum JCM 1149, Enterococcus faecalis IFO 12968, and Lactobacillus fermentum IFO 3956 (Fig. 1A). L. mesenteroides subsp. mesenteroides MCRI 1 and L. citreum MCRI 4 grew even at 4°C (Fig. 1B). L. mesenteroides subsp. mesenteroides MCRI 1 showed the most rapid growth; its doubling times were 4.7 h at 10°C and 23.6 h at 4°C. The doubling times of L. citreum MCRI 4 were 4.8 h at 10°C and 51.5 h at 4°C, and that of Lactococcus lactis subsp. lactis MCRI 3 was 11.1 h at 10°C. The optimum growth temperature of L. mesenteroides subsp. mesenteroides MCRI 1 and of L. citreum MCRI 4 was 30°C, and the maximum for each was 37°C (Fig. 2A and C). In the growth of Lactococcus lactis subsp. lactis MCRI 3, it was expected that the optimum and the maximum temperatures would be 30 to 35 and 37 to 40°C, respectively (Fig. 2B).
FIG. 1.
Growth rates of Leuconostoc mesenteroides subsp. mesenteroides MCRI 1 (•), Lactococcus lactis subsp. lactis MCRI 3 (▴), Leuconostoc citreum MCRI 4 (▪), P. acidilactici JCM 5885 (○), Lactobacillus plantarum JCM 1149 (□), Enterococcus faecalis IFO 12968 (▵), and Lactobacillus fermentum IFO 3956 (◊) in MRS broth at 10°C (A) and 4°C (B).
FIG. 2.
Growth curves of Leuconostoc mesenteroides subsp. mesenteroides MCRI 1 (A), Lactococcus lactis subsp. lactis MCRI3 (B), and Leuconostoc citreum MCRI4 (C) in MRS broth at 30°C (•), 35°C (▪), 37°C (▴), and 40°C (X). ABS, absorbance.
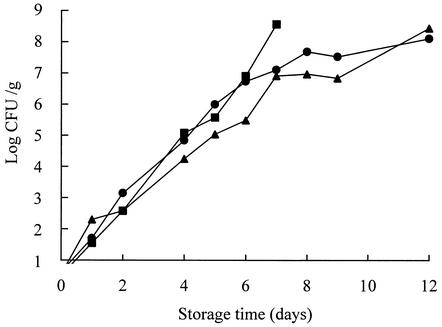
In the model cooked meat products, the isolates grew rapidly at 10°C and finally caused spoilage similar to that found in their isolation sources. Although the bacterial counts were initially <10 CFU/g, this increased to 108 CFU/g after 7 to 12 days (Fig. 3) of shelf life.
FIG. 3.
Growth of Leuconostoc mesenteroides subsp. mesenteroides MCRI 1 (•), Lactococcus lactis subsp. lactis MCRI 3 (▴), and Leuconostoc citreum MCRI 4 (▪) in the model cooked meat products at 10°C.
In this study, three bacteria were isolated and identified. L. mesenteroides subsp. mesenteroides and L. citreum had already been isolated from various types of cooked meat products and identified as a frequent source of spoilage (4, 15, 21). Lactococcus lactis subsp. lactis is known to be an important organism for milk fermentation in dairy products. On the other hand, it has also been isolated from bean sprouts (7), fermented fish products (18), and fermented sauerkraut (12). In addition, Barakat et al. (1) and Rodriguez et al. (19) isolated Lactococcus lactis from meat products. In this study, Lactococcus lactis subsp. lactis was isolated from a spoiling loin ham, so it seems to have a more widespread habitat than that recognized up to the present time and to be one of the spoilage organisms in cooked meat products. Furthermore, other lactic acid bacteria, for example, Carnobacterium spp., Enterococcus spp., Lactobacillus spp., Pediococcus spp., and Weissella spp., have also been identified as spoilage organisms in cooked meat products (2, 5, 6, 15, 21). Various genera and species of lactic acid bacteria, in particular, are regarded as such.
L. mesenteroides subsp. mesenteroides MCRI 1 and L. citreum MCRI 4 are regarded as psychrotrophs, because they grow at temperatures between 0 and 7°C (13). Also, Lactococcus lactis subsp. lactis MCRI 3 seems to be close to psychrotrophs, with relatively rapid growth at 10°C. Most psychrotrophs important in foods belong to Pseudomonas spp., Vibrio spp., Brochothrix spp., and other genera (13), but usually they are not related to the spoilage of cooked meat products. Psychrotrophs are probably destroyed during heating at temperatures of approximately 70°C (3, 5, 6, 15, 17, 21). After sterilization by cooking, products may be recontaminated with a few microorganisms, including psychrotrophs, by processes such as slicing and packaging, even if such processes are hygienic (3, 15). In cooked meat products, nitrite inhibits most bacterial growth by suppressing the electron transport system in the respiratory chain (16), but the growth of lactic acid bacteria is not inhibited (6, 14) because they do not possess an electron transport system. Furthermore, vacuum packaging, modified-atmosphere packaging, and other types of packaging may support the inhibitory effect. In results consistent with that phenomenon, Blickstad and Molin (5) found that the predominant organisms on fresh smoked pork loin products and frankfurter sausages were Bacillus spp., coryneform bacteria, Flavobacterium spp., and Pseudomonas spp., but at the end of the storage time, the microflora consisted mainly of Lactobacillus spp.
In conclusion, the results of this study suggest that psychrotrophic lactic acid bacterial contamination, even at bacterial counts below 10 CFU/g, can cause rapid spoilage of cooked meat products in the refrigerator. Although the presence of nitrite inhibits most psychrotrophs from contaminating products after they are cooked, this is not the case with psychrotrophic lactic acid bacteria, whose growth continues and eventually causes spoilage. Usually, a routine bacterial analysis of a product cannot detect a small quantity of microorganisms, because such analysis is quantitative, taking the form of plate counting with a detection limit of 10 CFU/g (2, 3, 10, 17). With such methods, initial product analysis will not enable producers to predict spoilage. Producers should therefore adopt a qualitative and selective analysis for lactic acid bacteria.
REFERENCES
- 1.Barakat, R. K., M. W. Griffiths, and L. J. Harris. 2000. Isolation and characterization of Carnobacterium, Lactococcus, and Enterococcus spp. from cooked, modified atmosphere packaged, refrigerated, poultry meat. Int. J. Food Microbiol. 62:83-94. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkroth, K. J., and H. J. Korkeala. 1996. Evaluation of Lactobacillus sake contamination in vacuum-packaged sliced cooked meat products by ribotyping. J. Food Prot. 59:398-401. [DOI] [PubMed] [Google Scholar]
- 3.Björkroth, K. J., and H. J. Korkeala. 1997. Use of rRNA gene restriction patterns to evaluate lactic acid bacterium contamination of vacuum-packaged sliced cooked whole-meat product in a meat processing plant. Appl. Environ. Microbiol. 63:448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björkroth, K. J., P. Vandamme, and H. J. Korkeala. 1998. Identification and characterization of Leuconostoc carnosum, associated with production and spoilage of vacuum-packaged, sliced, cooked ham. Appl. Environ. Microbiol. 64:3313-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blickstad, E., and G. Molin. 1983. The microbial flora of smoked pork loin and frankfurter sausage stored in different gas atmospheres at 4°C. J. Appl. Bacteriol. 54:45-56. [DOI] [PubMed] [Google Scholar]
- 6.Borch, E., M. L. Kant-Muermans, and Y. Blixt. 1996. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 33:103-120. [DOI] [PubMed] [Google Scholar]
- 7.Cai, Y., L. K. Ng, and J. M. Farber. 1997. Isolation and characterization of nisin-producing Lactococcus lactis subsp. lactis from bean-sprouts. J. Appl. Microbiol. 83:499-507. [DOI] [PubMed] [Google Scholar]
- 8.Collins, M. D., U. Rodrigues, C. Ash, M. Aguirre, J. A. E. Farrow, A. Martinez Murcia, B. A. Phillips, A. M. Williams, and S. Wallbanks. 1991. Phylogenetic analysis of the genus Lactobacillus and related lactic acid bacteria as determined by reverse transcriptase sequencing of 16S rRNA. FEMS Microbiol. Lett. 77:5-12. [Google Scholar]
- 9.Farrow, J. A. E., R. R. Facklam, and M. D. Collins. 1989. Nucleic acid homologies of some vancomycin-resistant leuconostocs and description of Leuconostoc citreum sp. nov. and Leuconostoc pseudomesenteroides sp. nov. Int. J. Syst. Bacteriol. 39:279-283. [Google Scholar]
- 10.Franz, C. M., and A. von Holy. 1996. Thermotolerance of meat spoilage lactic acid bacteria and their inactivation in vacuum-packaged vienna sausages. Int. J. Food Microbiol. 29:59-73. [DOI] [PubMed] [Google Scholar]
- 11.Garvie, E. I. 1986. Genus Leuconostoc van Tieghem 1878, 198AL emend mut. char. Hucker and Pederson 1930, 66AL, p. 1071-1075. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md.
- 12.Harris, L. J., H. P. Fleming, and T. R. Klaenhammer. 1992. Characterization of two nisin-producing Lactococcus lactis subsp. lactis strains isolated from a commercial sauerkraut fermentation. Appl. Environ. Microbiol. 58:1477-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jay, J. M. 2000. Modern food microbiology, 6th ed., p. 323-339. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 14.Korkeala, H., T. Alanko, and T. Tiusanen. 1992. Effect of sodium nitrite and sodium chloride on growth of lactic acid bacteria. Acta Vet. Scand. 33:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korkeala, H. J., and K. J. Bjorkroth. 1997. Microbiological spoilage and contamination of vacuum-packaged cooked sausages. J. Food Prot. 60:724-731. [DOI] [PubMed] [Google Scholar]
- 16.Morita, H., H. Yoshikawa, Y. Kato, S. Hisamatsu, R. Sakata, H. Fukuoka, and T. Yoshimura. 2002. Antibacterial mechanism of nitric oxide (NO) derived from sodium nitrite for verotoxigenic Escherichia coli. Magn. Reson. Med. 13:96-100. [Google Scholar]
- 17.Nerbrink, E., and E. Borch. 1993. Evaluation of bacterial contamination at separate processing stages in emulsion sausage production. Int. J. Food Microbiol. 20:37-44. [DOI] [PubMed] [Google Scholar]
- 18.Paludan-Muller, C., H. H. Huss, and L. Gram. 1999. Characterization of lactic acid bacteria isolated from a Thai low-salt fermented fish product and the role of garlic as substrate for fermentation. Int. J. Food Microbiol. 46:219-229. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez, J. M., L. M. Cintas, P. Casaus, N. Horn, H. M. Dodd, P. E. Hernandez, and M. J. Gasson. 1995. Isolation of nisin-producing Lactococcus lactis strains from dry fermented sausages. J. Appl. Bacteriol. 78:109-115. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto, K., A. Margolles, H. W. van Veen, and W. N. Konings. 2001. Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J. Bacteriol. 183:5371-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samelis, J., and K. G. Georgiadou. 2000. The microbial association of Greek taverna sausage stored at 4 and 10 degrees C in air, vacuum or 100% carbon dioxide, and its spoilage potential. J. Appl. Microbiol. 88:58-68. [DOI] [PubMed] [Google Scholar]
- 22.Schleifer, K. H., J. Kraus, C. Dvorak, R. Kilpper-Balz, M. D. Collins, and W. Fischer. 1985. Transfer of Streptococcus lactis and related streptococci to the genus Lactococcus gen. nov. Syst. Appl. Microbiol. 6:183-195. [Google Scholar]
- 23.Simpson, W. J., and J. L. Fernandez. 1992. Selection of beer spoilage lactic acid bacteria and induction of their ability to grow in beer. Lett. Appl. Microbiol. 14:13-16. [Google Scholar]