Abstract
Adhesion is a crucial first step in Listeria monocytogenes pathogenesis. In this study, we examined how the adhesion properties of serotypes correlate with their invasion efficiencies in a cell culture model (Caco-2) and in a mouse model. Adhesion characteristics of all 13 serotypes of L. monocytogenes (25 strains) were analyzed, which yielded three distinct groups (P < 0.05) with high-, medium-, and low-level-adhesion profiles. The efficiency of these strains in invading the Caco-2 cell line was analyzed, which produced two groups; however, the overall correlation (R2) was only 0.1236. In the mouse bioassay, all selected strains, irrespective of their adhesion profiles, translocated to the liver and the spleen with almost equal frequencies that did not show any clear relationship with adhesion profiles. However, the serotypes with increased adhesion showed a slightly increased translocation to the brain (R2 = 0.3371). Collectively, these results indicate that an in vitro adhesion profile might not be an accurate assessment of a strain's ability to invade a cultured cell line or organs or tissues in a mouse model.
Listeria monocytogenes is a facultative intracellular gram-positive bacterium that is responsible for food-borne illnesses and may lead to encephalitis, meningitis, or stillbirths and infection of the central nervous system in newborn or immunocompromised patients (1, 31). Despite the widespread distribution of L. monocytogenes, the incidence of listeriosis is not high. Furthermore, not all L. monocytogenes serotypes are capable of causing food-borne illnesses (2, 12). In fact, serotypes 4b, 1/2a, 1/2b, and 3b were responsible for more than 90% of listeriosis outbreaks that have had occurred in the past 2 decades (12, 16). The factors other than serotype that may also affect infectivity are the infective dose, survival of the bacteria in the stomach, and the host immunity (31). Epidemiological evidence shows that the gastrointestinal tract is the primary route of infection and that penetration of the intestinal epithelial cell barrier is the first step in the infection process (17). Moreover, the ability of L. monocytogenes to invade epithelial cells correlates with bacterial virulence (5, 19, 27).
The virulence of L. monocytogenes stems from its capacity to adhere, invade, and multiply within professional and nonprofessional phagocytes (31). Several surface proteins are involved in these processes. Ami (20), fibronectin binding protein (10), and Listeria adhesion protein (LAP) (23) are responsible for adhesion; internalin A and internalin B are required for intracellular invasion; Act A is involved in the cell-to-cell spread; and listeriolysin O and phospholipases are responsible for the disruption of the phagosomal membrane (31).
Listeriolysin O is an important virulence factor in L. monocytogenes pathogenicity (2, 3, 22), and quantitative analysis of this factor may not accurately determine the pathogenicity potential of a strain. Therefore, in vitro assays with intestinal cell lines (5, 19, 24, 27, 30) and in vivo animal models are important in confirming the pathogenicity characteristics of isolates (1-3, 18, 21, 29, 30). The pathogenicity of L. monocytogenes in mice following intragastric, intraperitoneal, or intravenous injections has been evaluated, and the majority of those studies have determined the translocation of the bacteria only to the liver or the spleen, except for some instances of translocation to the Peyer's patches following intragastric inoculation (1-3, 18, 21). However, only a limited number of studies have focused on the translocation of L. monocytogenes to the brain.
L. monocytogenes adhesion to and invasion of intestinal epithelial cells and the subsequent translocation to distant organs are critical in establishing a systemic infection in a host (31). Therefore, it is necessary to determine whether there exists any correlation between adhesion, invasion, and translocation of a strain for the purpose of assessing its pathogenic potential. In this study, we tested the adhesion properties of different serotypes using an enterocyte-like Caco-2 cell line and examined the invasion properties of the same cell line to determine the relationship between adhesion and invasion among serotypes. Translocation of selected serotypes with varied profiles of adhesion to the liver, spleen, and brain were examined following oral administration of the strains to mice.
Twenty-five L. monocytogenes strains belonging to 13 serotypes were used in this study (Table 1). Cultures were stored in brain heart infusion (BHI) slants at room temperature, and fresh cultures were obtained after growth for 18 to 20 h at 37°C in BHI. All isolates were tested for hemolysin production with the CAMP test on sheep blood agar plates (26) and were found to be hemolytic.
TABLE 1.
L. monocytogenes serotypes used in this study
| Strain | Serotype | Sourcea |
|---|---|---|
| F4263 | 1/2a | Human CSF and blood (CDC) |
| ATCC 35152 | 1/2a | Guinea pig (ATCC) |
| V7 | 1/2a | Raw milk (U.S. FDA) |
| EGD | 1/2a | S. Kathariou (NCSU) |
| ATCC 19111 | 1/2a | Poultry (ATCC) |
| F4260 | 1/2b | Human CSF and blood (CDC) |
| F4233 | 1/2b | Human CSF and blood (CDC) |
| ATCC 19112 | 1/2c | Human CSF (ATCC) |
| ATCC 7644 | 1/2c | Human CSF (ATCC) |
| ATCC 19113 | 3a | Human (ATCC) |
| SLCC 2540 | 3b | Human CSF |
| SLCC 2479 | 3c | Unknown |
| ATCC 19114 | 4a | Bovine brain (ATCC) |
| Murray B | 4ab | Human (CDC) |
| ATCC 19115 | 4b | Human CSF (ATCC) |
| F4244 | 4b | Human CSF (CDC) |
| F4393 | 4b | Cheese (CDC) |
| F5069 | 4b | Raw milk (CDC) |
| Scott A | 4b | Human blood (CDC) |
| CAP | 4b | Human CSF (ADH) |
| F2379 | 4b | Cheese (CDC) |
| ATCC 19116 | 4c | Poultry (ATCC) |
| ATCC 19117 | 4d | Sheep (ATCC) |
| ATCC 19118 | 4e | Poultry (ATCC) |
| SLCC 2482 | 7 | Human feces (SLCC) |
Abbreviations: ADH, Arkansas Department of Health; ATCC, American Type Culture Collection; CAP, College of American Pathologists; CSF, cerebrospinal fluid; CDC, Centers for Disease Control and Prevention; NCSU, North Carolina State University; SLCC, Special Listeria Culture Collection; U.S. FDA, U.S. Food and Drug Administration.
Adhesion analysis.
The adhesion assays were performed using 3H-labeled Listeria species according to the method of Hagman et al. (11), with modifications (14, 15). Bacteria were grown overnight at 37°C in BHI containing 20 μCi of [methyl-3H]thymidine (79 Ci/mmol; Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) per ml. One hundred microliters of 3H-labeled bacteria (∼5 × 108/ml) was added to the Caco-2 cell monolayers in 24-well tissue culture plates to obtain a multiplicity of infection (MOI) of 100:1, and the bacteria were incubated for 30 min at 37°C (28). The plates were washed five times with 20 mM phosphate-buffered saline, pH 7.0 (PBS), and treated with 0.5 ml of 0.5% sodium dodecyl sulfate per well, the well contents were mixed thoroughly with 10 ml of a scintillation cocktail (CytoScint; ICN Biomedicals, Inc., Costa Mesa, Calif.), and the radioactivity was enumerated using a liquid scintillation counter (Beckman Coulter, Fullerton, Calif.). All adhesion assays were performed at least three times in triplicate.
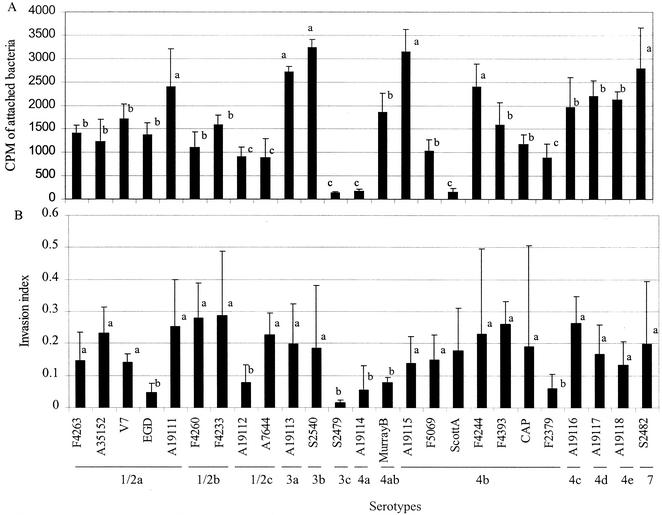
All L. monocytogenes serotypes were able to attach to Caco-2 cells; however, the attachment varied greatly, with counts from 140 to 3,242 cpm (Fig. 1A), while the negative control (L. innocua NCTC 10528) had a count of 111 cpm. The background counts for Caco-2 cell monolayers without labeled bacteria were generally around 40 cpm/well. By using a statistical analysis software (SAS, Cary, N.C.) and Tukey's test, adhesion profiles could be divided into three groups, which were significantly different (P < 0.05) from one another. The serotypes that exhibited the highest levels of adhesion (2,396 to 3,242 cpm) were serotypes 3b (SLCC 2540, ATCC 19113), 4b (ATCC 19115, F4244), 7 (SLCC 2482), and 1/2a (ATCC 19111). The serotypes with medium levels of adhesion (1,121 to 2,197 cpm) were serotypes 4d (ATCC 19117), 4e (ATCC 19118), 4c (ATCC 19116), 4ab (MurrayB), 1/2a (V7, F4263, EGD, ATCC 35152), 1/2b (F4233, F4260), and 4b (F4393, CAP, F5069). The lowest levels of adhesion (140 to 908 cpm) were observed for serotypes 1/2c (ATCC 19112, ATCC 7644), 4b (F2379, Scott A), 4a (ATCC 19114), and 3c (SLCC 2479). In this study, a uniform MOI was used for most of the adhesion analyses. In some cases, however, we used MOIs of 300:1, 1,000:1, or even 5,000:1, and no variation in adhesion within a serotype was observed (data not shown). Direct microscopic examination of the adhesion of nonlabeled L. monocytogenes strains to Caco-2 cells supported the adhesion profiles obtained from the radiolabeled bacteria (data not shown). Meyer et al. (19) also reported a high variation in adhesion among different serotypes of L. monocytogenes when the serotypes were tested with a primary cell line from guinea pig.
FIG. 1.
Adhesion (A) and invasion (B) analyses of 25 strains (13 serotypes) of L. monocytogenes with the secondary intestinal cell line Caco-2 (colon). (A) Adhesion profiles of serotypes (adhesion values are expressed as counts per minute/well ± standard deviations) are grouped as high (a), medium (b), and low (c), and the results of the groups are significantly different (P < 0.05) from each other. (B) Invasion index ± standard deviation results of the same serotypes with the Caco-2 cell line. Serotypes were grouped as having high (a) and low (b) invasion efficiencies, and the results for the groups are significantly different (P < 0.05) from each other. Invasion efficiency is the ratio of invasion values to the total counts of invasion and adhesion (see the text for details).
Invasion assay.
The invasion properties of serotypes were analyzed by a gentamicin-based invasion assay with Caco-2 cell monolayers in 24-well plates according to the method of Gaillard et al. (9), with modifications. Briefly, Caco-2 monolayers were inoculated with 100 μl of the L. monocytogenes suspension (∼5 × 108 CFU/ml) and incubated for 1.5 h at 37°C in 7% CO2. The monolayers were washed with PBS, followed by the addition of 100 μl of gentamicin (1 mg/ml) and an additional 1.5-h incubation. However, to determine total counts of bacteria associated with the cell (counts of adhered bacteria plus counts of intracellular bacteria), duplicate wells with Caco-2 cells were analyzed without gentamicin treatment. The cell monolayers with or without gentamicin treatments were then washed with PBS and lysed with 1% Triton X-100 (Sigma). Appropriate dilutions were plated on BHI plates, and the CFU were enumerated. The invasion efficiency (invasion index) for each serotype was calculated by dividing the number of CFU that invaded the cells (with gentamicin) by the total number of CFU obtained without gentamicin treatment (both the invasion and the adhesion counts).
Statistical analysis, using Tukey's test at a P of <0.05, revealed that the serotypes could be divided into two major groups based on their invasion profiles (Fig. 1B). Group 1 contained 19 strains with invasion efficiencies of 0.14 to 0.314. This group comprised six of seven strains of serotype 4b, four of five strains of serotype 1/2a, two of two strains of serotype 1/2b, one of two strains of serotype 1/2c, and one strain each of serotypes 3a, 3b, 4c, 4d, 4e, and 7. Group 2 contained six strains whose invasion efficiency values ranged from 0.018 to 0.086, and the serotypes were one of seven strains of serotype 4b, one of five strains of serotype 1/2a, one of two strains of serotype 1/2c, and one strain each of serotypes 3c, 4ab, and 4a (Fig. 1B). The differences in invasion efficiencies between the serotypes of the two groups were significant (P < 0.05), while the differences in results for members within the same group were not significant (P < 0.05).
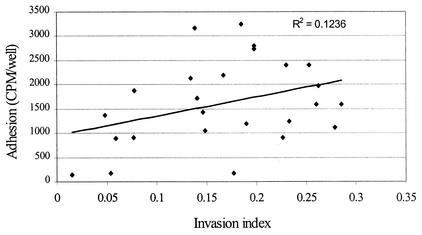
When the adhesion results were compared with the invasion efficiencies of all 13 serotypes (25 strains), using the Microsoft Excel software, the data showed a poor correlation (R2 = 0.1236) (Fig. 2). When the adhesion results and invasion efficiencies were compared for different serotypes within serovar 1, 3, or 4, we observed varied correlations. Results for serovars 1 (1/2a, 1/2b, 1/2c) and 4 (4a, 4b, 4c, 4d, 4e, 4ab) showed the lowest correlation (R2), 0.0506 and 0.0951, respectively, while results for serovar 3 (3a, 3b, 3c) showed the highest correlation, 0.9539.
FIG. 2.
Correlation plot of the adhesion and invasion index profiles of 25 L. monocytogenes strains with the secondary intestinal cell line Caco-2.
Using the RPMI 60 and HT-29 cell lines, Meyer et al. (19) reported that all L. monocytogenes strains had similar invasion efficiencies. They also found that nonhemolytic L. monocytogenes strains exhibited higher efficiencies of invasion than many hemolytic L. monocytogenes strains. In our study using the Caco-2 cell line, we found significant variation in efficiencies of invasion among L. monocytogenes strains, ranging from 1.8 to 31.4% of the initial inoculum. Similarly, Van Langendonck et al. (30) observed that all clinical L. monocytogenes isolates entered Caco-2 cells even though the invasion efficiency of each strain varied from 0.6 to 23% of the initial inoculum. The nonhemolytic or weakly hemolytic strains showed entry into the cells, but the invasion efficiencies were minimal (0.2 to 0.6% of the initial inoculum). In a recent study, Olier et al. (22) reported that two human fecal isolates with very high hemolytic activities displayed varied abilities to invade Caco-2 cells and varied levels of virulence in a chicken embryo test.
Meyer et al. (19) and Bunduki et al. (5) reported that there is no correlation between invasion and adhesion among different L. monocytogenes serotypes. However, they used only 10 serotypes in their study. Barbour et al. (2) observed a wide variation in infectivity of seven L. monocytogenes serotypes following an intragastric inoculation of mice. In our study, we used all 13 serotypes (25 strains) of L. monocytogenes and observed a low correlation (R2) of 0.1236 (Fig. 2). As stated earlier, only the results for serotypes 3a, 3b, and 3c (belonging to serovar 3) had a very high correlation of 0.9539, which indicated that the strains with a high level of adhesion also had a high efficiency of invasion into Caco-2 cells, while the results for the remaining serotypes had a very poor correlation between levels of adhesion and efficiencies of invasion. Observed discrepancies in adhesion and invasion among certain serotypes could be attributed to the variation in expression of different adhesion factors such as fibronectin binding proteins (10), LAP (14, 15, 28), and Ami (20) and invasion factors such as internalins (8, 25) in different serotypes (13, 22). Del Corral et al. (7) analyzed the adhesion and invasion properties of numerous food and clinical isolates with the HEp-2 cell line and found that the abilities of L. monocytogenes strains to adhere vary greatly and that the degree of invasion and adhesion overlaps those of nonpathogenic strains.
Analysis of the translocation of L. monocytogenes serotypes to the liver, spleen, and brain in a mouse bioassay.
Six serotypes representing high (3b, SLCC 2540; 4b, ATCC 19115), medium (1/2a, EGD), and low (1/2c, ATCC 19112; 3c, SLCC 2479; 4a, ATCC 19114) adhesion profiles, along with an L. innocua strain, were chosen to determine their ability to translocate to the liver, the spleen, and the brain following oral inoculation. Male BALB/c mice (8 to 12 weeks old) were purchased from Hilltop Lab Animals (Scottdale, Pa.) and housed in the animal facility at Purdue University Veterinary School. The mice were divided into eight groups (three to six mice per group); one group was left as a negative control without any inoculation, while the other seven groups were subjected to oral gavage with an animal feeding needle (Popper, Inc., New Hyde Park, N.Y.) with viable L. monocytogenes or L. innocua strains at a concentration of 5 × 108 CFU/mouse. Mice were sacrificed 72 h after inoculation, and livers, spleens, and brains were aseptically removed and blended with PBS (9 ml of PBS for the liver and spleen samples and 4 ml for the brain samples). Diluted samples were plated onto BHI agar plates by a pour plate method and incubated for 24 to 36 h.
Of the six serotypes tested, four (3b, 4b, 3c, and 4a) translocated to the spleen, the liver, and the brain in 72 h, while two others (1/2a and 1/2c) were isolated from the spleen and the liver but not from the brain (Table 2). In the liver, serotype 3c showed the highest cell counts (6.99 log10 CFU/ml), followed by serotypes 1/2a (4.83 log10 CFU/ml), 3b (4.81 log10 CFU/ml), 4b (4.55 log10 CFU/ml), 4a (4.3 log10 CFU/ml), and 1/2c (4.19 log10 CFU/ml). The counts for serotype 3c in the liver were significantly different (P < 0.05) from those for the other serotypes. An almost similar trend was observed for cell counts in the spleen (3c > 3b > 1/2a > 4b > 4a > 1/2c). Generally, counts for each serotype from the spleen were lower than those from the liver. Only four strains translocated to the brain, with serotype 4b showing the highest count, namely, 4.35 log10 CFU/ml, which is significantly different from those for serotypes 3b (3.55 log10 CFU/ml), 3c (2.69 log10 CFU/ml), and 4a (1.83 log10 CFU/ml). L. innocua, which was used as a negative control, showed some counts in the liver (2.5 log10 CFU/ml) and the spleen (0.3 log10 CFU/ml) that were significantly different (P < 0.05) from those of L. monocytogenes, and L. innocua also did not translocate to the brain. Lammerding et al. (16) also reported similar translocation patterns for L. innocua to livers and spleens.
TABLE 2.
Comparison of translocations of different serotypes from high-, medium-, and low-level-adhesion groups to different organs in a mouse bioassay after 72 h of oral administrationa
| Serotype | Strain | Adhesion (avg ± SD)b | Invasion index (avg ± SD)b | Avg log10 CFU/ml ± SD
|
||
|---|---|---|---|---|---|---|
| Liver | Spleen | Brain | ||||
| 3b | SLCC 2540 | 3,242 ± 169 A | 0.185 ± 0.19 A | 4.81 ± 3.62 A | 4.54 ± 4.45 A | 3.55 ± 3.53 A |
| 4b | ATCC 19115 | 3,165 ± 454 A | 0.146 ± 0.08 A | 4.55 ± 4.37 A | 3.91 ± 3.58 A | 4.35 ± 3.62 B |
| 1/2a | EGD | 1,375 ± 253 B | 0.047 ± 0.026 B | 4.83 ± 4.72 A | 3.97 ± 4.17 A | <5 C |
| 1/2c | ATCC 19112 | 899 ± 399 C | 0.226 ± 0.068 A | 4.19 ± 4.22 A | 3.25 ± 2.76 A | <5 C |
| 4a | ATCC 19114 | 172 ± 38 C | 0.054 ± 0.076 B | 4.30 ± 4.30 A | 3.90 ± 3.48 A | 1.83 ± 1.71 C |
| 3c | SLCC 2479 | 140 ± 26 C | 0.015 ± 0.007 B | 6.99 ± 5.83 B | 6.25 ± 6.28 B | 2.69 ± 2.46 C |
Three to six mice were used for each group.
Values are taken from Fig. 1. Adhesion values are counts per minute of 3H-labeled bacteria. Invasion index values are ratios of intracellular counts to the total counts (intracellular and adhering bacteria). Values marked with different capital letters (A, B, C) in the same column are significantly different at a P of < 0.05.
The in vitro adhesion values of selected serotypes (3b, 4b, 1/2a, 1/2c, 3c, 4a) were compared with the counts of bacteria from the liver, spleen, and brain, and correlation values (R2) were determined to be 0.1085, 0.0553, and 0.3371, respectively. The most striking discrepancy was observed for serotype 3c (ATCC 19113), which had the lowest adhesion and invasion counts in the Caco-2 cell assay but showed the highest counts in the liver and spleen (Table 2). This result clearly indicates the lack of a positive correlation between adhesion to and invasion of organs or tissues in the mouse model (Table 2). All together, these observations further indicate that adhesion is essential for L. monocytogenes strains but that the degree of adhesion may not accurately reflect the virulence potential, invasiveness, or multiplication and survival properties of a strain in organs or tissues in an animal model.
A variation in translocation to the brain was observed for some strains in that serotypes 1/2a (EGD) and 1/2c (ATCC 19112) were not detected at all in the brain (Table 2). The reason why some bacteria did not reach the brain despite reaching the liver and spleen may be that they might not have survived the immune system while they were in the bloodstream. Apparently those strains were not virulent enough to circumvent the mouse immune system; therefore, they were cleared from the blood before reaching the brain (31). Berche (4) indicated that if the bacteremic phase does not last long enough to seed the brain, there will be no invasion of the brain. The 4b serotype, which is frequently implicated in human listeriosis (2, 6), was isolated from the brain, which suggests this strain to be highly invasive.
We have observed some variations in the translocations of different serotypes administered orally to healthy mice after 72 h. L. monocytogenes serotypes were better at translocating to the liver than to the spleen or the brain. These results differ from results obtained by Barbour et al. (2), who reported that L. monocytogenes serotypes grew better in the spleen than in the liver following an intravenous injection. This result indicates that the route of administration dictates the distribution of L. monocytogenes among the target tissues. They also reported that most virulent strains grew more in the liver than in the spleen. The reasons behind this variation are that the numbers of macrophages differ between the spleen and the liver and that the mechanisms of growth also differ between serotypes (2). However, Barbour et al. did not analyze the translocation of L. monocytogenes to the brain tissues. Okamoto et al. (21) indicated that variation could also arise from the host cellular immunity and variation in the intestinal bacterial flora. In this study, it is noteworthy that strain 4b counts were not the highest in the liver or spleen but were the highest in the brain. This could be an indicator of its virulence, as not all L. monocytogenes strains were able to cross the blood-brain barrier.
In conclusion, we observed different adhesion and invasion profiles among different serotypes of L. monocytogenes with which the serotypes could be divided into groups of high, medium, and low levels of adhesion or invasion. Furthermore, we found a low correlation between adhesion and invasion. In the mouse bioassay, serotypes from the high-level-adhesion group had slightly higher counts in the brain than those of medium- and low-level-adhesion groups, whereas no significant differences were observed in the translocations to livers and spleens.
Acknowledgments
We sincerely thank Jennifer Wampler for statistical analysis and Brad Reuhs for critical reading of the manuscript.
Part of this project was supported through a cooperative agreement with the Agricultural Research Service of the U.S. Department of Agriculture (1935-42000-035) and the Center for Food Safety and Engineering at Purdue University.
REFERENCES
- 1.Altimira, J., N. Prats, S. Lopez, M. Domingo, V. Briones, L. Dominguez, and A. Marco. 1999. Repeated oral dosing with Listeria monocytogenes in mice as a model of central nervous system listeriosis in man. J. Comp. Pathol. 121:117-125. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 1996. Comparison of the infectivity of isolates of Listeria monocytogenes following intragastric and intravenous inoculation in mice. Microb. Pathog. 20:247-253. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 2001. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect. Immun. 69:4657-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berche, P. 1995. Bacteremia is required for invasion of murine central nervous system by Listeria monocytogenes. Microb. Pathog. 18:323-336. [DOI] [PubMed] [Google Scholar]
- 5.Bunduki, M. C., C. M. Beliveau, and C. W. Donnelly. 1993. Examination of attachment and phagocytic uptake of Listeria species by mammalian intestinal cells. Food Microbiol. 10:507-516. [Google Scholar]
- 6.Czuprynski, C. J., N. G. Faith, and H. Steinberg. 2002. Ability of the Listeria monocytogenes strain Scott A to cause systemic infection in mice infected by the intragastric route. Appl. Environ. Microbiol. 68:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Corral, F., R. L. Buchanan, M. M. Bencivengo, and P. H. Cooke. 1990. Quantitative comparison of selected virulence associated characteristics in food and clinical isolates of Listeria. J. Food Prot. 53:1003-1009. [DOI] [PubMed] [Google Scholar]
- 8.Dramsi, S., C. Kocks, C. Forestier, and P. Cossart. 1993. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and the pleiotropic activator prfA. Mol. Microbiol. 9:931-941. [DOI] [PubMed] [Google Scholar]
- 9.Gaillard, J.-L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilot, P., P. André, and J. Content. 1999. Listeria monocytogenes possesses adhesins for fibronectin. Infect. Immun. 67:6698-6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagman, M. M., J. B. Dale, and D. L. Stevens. 1999. Comparison of adherence to and penetration of a human laryngeal epithelial cell line by group A streptococci of various M protein types. FEMS Immunol. Med. Microbiol. 23:195-204. [DOI] [PubMed] [Google Scholar]
- 12.Hof, H., T. Nichterlein, and M. Kretschmar. 1994. When are Listeria in foods a health risk? Trends Food Sci. Technol. 5:185-190. [Google Scholar]
- 13.Jacquet, C., E. Gouin, D. Jeannel, P. Cossart, and J. Rocourt. 2002. Expression of ActA, Ami, InlB, and listeriolysin O in Listeria monocytogenes of human and food origin. Appl. Environ. Microbiol. 68:616-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaradat, Z. W., and A. K. Bhunia. 2002. Glucose and nutrient concentrations affect the expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl. Environ. Microbiol. 68:4876-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaradat, Z. W., J. L. Wampler, and A. K. Bhunia. 2003. A Listeria adhesion protein-deficient Listeria monocytogenes strain shows reduced adhesion to intestinal cell lines. Med. Microbiol. Immunol. 192:85-91. [DOI] [PubMed] [Google Scholar]
- 16.Lammerding, A. M., K. A. Glass, A. Gendron-Fitzpatrick, and M. P. Doyle. 1992. Determination of virulence of different strains of Listeria monocytogenes and Listeria innocua by oral inoculation of pregnant mice. Appl. Environ. Microbiol. 58:3991-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 18.Marco, A. J., J. Altimira, N. Prats, S. Lopez, L. Dominguez, M. Domingo, and V. Briones. 1997. Penetration of Listeria monocytogenes in mice infected by the oral route. Microb. Pathog. 23:255-263. [DOI] [PubMed] [Google Scholar]
- 19.Meyer, D. H., M. Bunduki, C. M. Beliveau, and C. W. Donnelly. 1992. Differences in invasion and adherence of Listeria monocytogenes with mammalian gut cells. Food Microbiol. 9:115-126. [Google Scholar]
- 20.Milohanic, E., R. Jonquieres, P. Cossart, P. Berche, and J.-L. Gaillard. 2001. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto, M., A. Nakane, and T. Minagawa. 1994. Host resistance to an intragastric infection with Listeria monocytogenes in mice depends on cellular immunity and intestinal bacterial flora. Infect. Immun. 62:3080-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olier, M., F. Pierre, J.-P. Lemaitre, C. Divies, A. Rousset, and J. Guzzo. 2002. Assessment of the pathogenic potential of two Listeria monocytogenes human faecal carriage isolates. Microbiology 148:1855-1862. [DOI] [PubMed] [Google Scholar]
- 23.Pandiripally, V. K., D. G. Westbrook, G. R. Sunki, and A. K. Bhunia. 1999. Surface protein p104 is involved in adhesion of Listeria monocytogenes to human intestinal cell line, Caco-2. J. Med. Microbiol. 48:117-124. [DOI] [PubMed] [Google Scholar]
- 24.Pine, L., S. Kathariou, F. Quinn, V. George, J. D. Wenger, and R. E. Weaver. 1991. Cytopathogenic effects in enterocytelike Caco-2 cells differentiate virulent from avirulent Listeria strains. J. Clin. Microbiol. 29:990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renzoni, A., A. Klarsfeld, S. Dramsi, and P. Cossart. 1997. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect. Immun. 65:1515-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripio, M. T., C. Geoffroy, G. Dominguez, J. E. Alouf, and J. A. Vazquez-Boland. 1995. The sulphydryl-activated cytolysin and a sphingomyelinase C are the major membrane-damaging factors involved in cooperative (CAMP-like) hemolysis of Listeria spp. Res. Microbiol. 146:303-313. [DOI] [PubMed] [Google Scholar]
- 27.Roche, S. M., P. Velge, E. Bottreau, C. Durier, N. Marquet-van der Mee, and P. Pardon. 2001. Assessment of the virulence of Listeria monocytogenes: agreement between a plaque-forming assay with HT-29 cells and infection of immunocompetent mice. Int. J. Food Microbiol. 68:33-44. [DOI] [PubMed] [Google Scholar]
- 28.Santiago, N. I., A. Zipf, and A. K. Bhunia. 1999. Influence of temperature and growth phase on expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl. Environ. Microbiol. 65:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stelma, G. N., A. L. Reyes, J. T. Peeler, D. W. Francis, J. M. Hunt, P. L. Spaulding, C. H. Johnson, and J. Lovett. 1987. Pathogenicity test for Listeria monocytogenes using immunocompromised mice. J. Clin. Microbiol. 25:2085-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Langendonck, N., E. Bottreau, S. Bailly, M. Tabouret, J. Marly, P. Pardon and P. Velge. 1998. Tissue culture assays using Caco-2 cell line differentiate virulent from non-virulent Listeria monocytogenes strains. J. Appl. Microbiol. 85:337-346. [DOI] [PubMed] [Google Scholar]
- 31.Vásquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Domínguez-Bernal, W. Goebel, B. González-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]