Abstract
Detoxification of the maize (Zea mays) antimicrobial compound 2-benzoxazolinone by the fungal endophyte Fusarium verticillioides involves two genetic loci, FDB1 and FDB2, and results in the formation of N-(2-hydroxyphenyl)malonamic acid. Intermediate and branch metabolites were previously suggested to be part of the biotransformation pathway. Evidence is presented here in support of 2-aminophenol as the intermediate metabolite and 2-acetamidophenol as the branch metabolite, which was previously designated as BOA-X. Overall, 2-benzoxazolinone metabolism involves hydrolysis (FDB1) to produce 2-aminophenol, which is then modified (FDB2) by addition of a malonyl group to produce N-(2-hydroxyphenyl)malonamic acid. If the modification is prevented due to genetic mutation (fbd2), then 2-acetamidophenol may accumulate as a result of addition of an acetyl group to 2-aminophenol. This study resolves the overall chemistry of the 2-benzoxazolinone detoxification pathway, and we hypothesize that biotransformation of the related antimicrobial 6-methoxy-2-benzoxazolinone to produce N-(2-hydroxy-4-methoxyphenyl)malonamic acid also occurs via the same enzymatic modifications. Detoxification of these antimicrobials by F. verticillioides apparently is not a major virulence factor but may enhance the ecological fitness of the fungus during colonization of maize stubble and field debris.
The endophytic fungus Fusarium verticillioides (= Fusarium moniliforme) can infect maize kernels following systemic colonization of the plant or the deposition of conidia on silks and growth to the developing kernels (3, 6, 11, 13, 16). Asymptomatic kernel infections are nonembryonic, with the fungus restricted to the maternal pedicel region (2). This spatial proximity strategically positions F. verticillioides for infection of developing seedlings. During the early stages of seedling development, maize accumulates cyclic hydroxamic acids, also known as benzoxazinoids, to very high concentrations (14, 17, 18). Benzoxazinoids are defensive compounds that inhibit growth of bacteria and fungi and also deter insect feeding (1, 4, 5, 11, 14, 17). The compounds produced are 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA) and 2,4-dihydroxy-2H-1,4-benzoxazin-3(4H)-one (DIBOA), which are inherently unstable and spontaneously degrade to the more stable benzoxazolinones 6-methoxy-2-benzoxazolinone (MBOA) and 2-benzoxazolinone (BOA), respectively (Fig. 1) (12, 21). MBOA and BOA are effective in vitro antagonists that inhibit the growth of a wide range of microbes, including a number of Fusarium species (11, 17). Wheat also produces DIMBOA and DIBOA, whereas rye produces only DIBOA (25), while other cultivated grains are not known to produce any benzoxazinoids or benzoxazolinones.
FIG. 1.
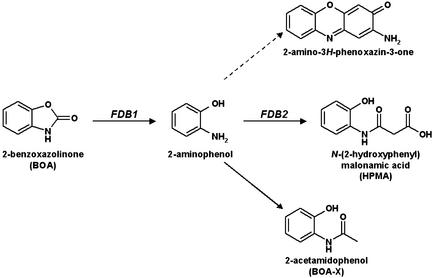
F. verticillioides detoxification of the maize antimicrobial BOA involves two genetic loci, FDB1 and FDB2. BOA is hydrolyzed via FDB1 to produce 2-aminophenol, which is then modified via FDB2 to produce HPMA. An fdb2 mutant may accumulate 2-acetamidophenol as a result of acetylation of 2-aminophenol, which can also spontaneously oxidize to form 2-amino-3H-phenoxazin-3-one.
F. verticillioides can tolerate MBOA and BOA at concentrations that inhibit growth of other fungi (19). Tolerance results from biotransformation of MBOA and BOA into the metabolites N-(2-hydroxy-4-methoxyphenyl)malonamic acid (HMPMA) and N-(2-hydroxyphenyl)malonamic acid (HPMA), respectively, which are nontoxic to the fungus (Fig. 1) (23). A screen of 29 Fusarium species found only 11 species that could detoxify BOA to some extent, with F. verticillioides being the most tolerant followed closely by Fusarium subglutinans, another common maize pathogen (11, 20). The majority of the MBOA- and BOA-tolerant Fusarium species had host associations with the benzoxazinoid producers maize and wheat.
The wheat pathogen Gaeumannomyces graminis var. tritici and the related Gaeumannomyces graminis var. graminis also metabolize MBOA and BOA into the respective nontoxic compounds, HMPMA and HPMA (7). The oat pathogen Gaeumannomyces graminis var. avenae was sensitive to MBOA and BOA and showed no capacity for detoxification. The ability of Gaeumannomyces species to cause root rot symptoms on wheat paralleled their tolerance and detoxification of these antimicrobials, suggesting a role for detoxification in the plant-pathogen interactions (7). In contrast, the tolerance and detoxification exhibited by F. verticillioides did not appear to have a major effect on its ability to infect and cause disease in maize seedlings (10). Some nondetoxifying F. verticillioides strains were as virulent as the detoxifying wild-type strain. Thus, the role of these compounds in plant-pathogen interactions and the impact of tolerance exhibited by the fungi may vary depending on the pathosystem.
The compound 2-amino-3H-phenoxazin-3-one is an additional product of BOA metabolism by G. graminis var. tritici (7). Microbial hydrolysis of BOA is thought to produce 2-aminophenol, which spontaneously oxidizes to produce 2-amino-3H-phenoxazin-3-one (8). Interestingly, 2-amino-3H-phenoxazin-3-one exhibited greater allelopathic phytotoxicity toward barnyard grass (Echinocloa crusgalli), a common agricultural weed, than did BOA (8). Such production of a more effective allelochemical suggests possible ecological consequences of microbial biotransformation.
F. verticillioides requires at least two functional genetic loci, FDB1 and FDB2, for tolerance and detoxification of MBOA and BOA (Fig. 1) (10). Neither an FDB1/fdb2 strain nor an fdb1/FDB2 strain can metabolize BOA to HPMA, but coinoculating such strains on BOA-amended agar medium results in production of HPMA, which suggests that the strains can physiologically complement each other via an intermediate compound. Based on these data, we have previously hypothesized that BOA is hydrolyzed to an intermediate metabolite through an enzyme encoded by FDB1 (10). The intermediate then serves as substrate for an enzyme encoded by FDB2, resulting in formation of HPMA. If fdb2 is nonfunctional, then a small amount of a branch metabolite can accumulate (9, 10).
Production of an intermediate by hydrolysis of BOA is similar to the production of 2-aminophenol as suggested by Gagliardo and Chilton (8). If 2-aminophenol is the intermediate in the F. verticillioides physiological complementation assays, then addition of a malonyl group to 2-aminophenol via FDB2 would produce the nontoxic HPMA (9). A recent analysis of metabolites produced by other BOA-detoxifying fungi is consistent with 2-aminophenol being an intermediate in the pathway (24).
Our objectives in this study were to utilize genetically defined F. verticillioides strains to identify the intermediate and branch metabolites resulting from biotransformation of BOA. Our hypothesis is that 2-aminophenol is the intermediate metabolite and that 2-acetamidophenol is the branch metabolite. Congruent genetic, physiological, and chemical data strongly support the proposed BOA detoxification pathway, which also is hypothesized to be the pathway for MBOA detoxification.
MATERIALS AND METHODS
Fungal strains.
Fungal strains examined in this study are described in the work of Glenn et al. (10). Strain MRC826 (genotype FDB1/FDB2) is wild type for tolerance and detoxification of BOA, while strains AEG3-A3-5 (fdb1/FDB2), AEG3-A3-7 (FDB1/fdb2), and AEG3-A3-8 (fdb1/fdb2) are unable to grow on BOA (7.4 mM) since they each possess mutant alleles at loci necessary for detoxification (10). AEG3-A3-5, AEG3-A3-7, and AEG3-A3-8 are siblings from a tetrad collected from a backcross between MRC826 and AEG1-1-57 (fdb1/fdb2). AEG1-1-57 was derived from a cross between MRC826 and the naturally occurring mutant NRRL25059 (fdb1/fdb2). For long-term storage, strains were frozen at −80°C in 15% glycerol. For routine culturing, the fungi were grown on potato dextrose agar (Difco, Detroit, Mich.) and incubated at 23°C in the dark.
Physiological analyses and metabolite purification.
For feeding experiments, fungal strains (106 conidia) were inoculated into 200-ml potato dextrose broth (Difco) in 500-ml baffled flasks. Experiments were conducted at least twice with two replicates per treatment. Cultures were grown for 3 days in the dark on a rotary shaker (190 rpm) at 23°C and then amended with BOA or 2-aminophenol (both from Aldrich Chemical Co., Milwaukee, Wis.) at a final concentration of 7.4 mM. Both compounds were dissolved in ethanol, and the final ethanol concentration in each culture was 2%. Incubation of each amended culture was continued as described above for an additional 18 h, and then the culture was centrifuged at 12,000 × g for 20 min. The supernatant was acidified to pH 2.6 to 2.8 with 1 M HCl and extracted twice with 200 ml of ethyl acetate, which was combined and dried by rotary evaporation, and the extracted metabolites were dissolved in 6 ml of methanol (19, 23). Metabolic transformation of BOA and 2-aminophenol was assessed by applying extracts (3 μl) to silica gel thin-layer chromatography (TLC) sheets containing UV254 indicator (Whatman Ltd., Maidstone, Kent, England) and developing them in toluene-ethyl acetate-formic acid (50:40:10) as previously described (11). TLC sheets were visualized and photographed under UV254 light by using an Alpha Innotech FluorChem imaging system (San Leandro, Calif.) equipped with epifluorescent lights.
Preparative TLC for specific isolation and semipurification of metabolites was performed by at least one round of applying extracts to the TLC sheet, developing and visualizing them as described above, scraping off the silica gel containing the metabolite of interest, and eluting the compound(s) in the same solvent solution. Also, metabolites were purified through a Sep-Pak silica cartridge (Waters Chromatography Division, Millipore Corporation, Milford, Mass.) or a silica gel column (Pasteur pipette packed with SilicAR CC-7 100/200 mesh; Mallinckrodt Chemical Works, St. Louis, Mo.) by washing the applied extract with chloroform-methanol-formic acid (100:3:0.1) followed by elution with toluene-ethyl acetate-formic acid (50:40:1). HPMA often can be monitored during elution as a yellow-golden pigment on the column (23).
Mass spectrometry and gas chromatography (GC)-mass spectrometry.
Mass spectrometry was performed on isolated compounds by using a Finnigan (San Jose, Calif.) Polaris Q instrument equipped with a direct exposure probe (rhenium loop) to allow in-source vaporization of the compounds prior to any decomposition. Analysis conditions were as follows: ionization energy, 70 eV; mass range, 50 to 350; scan time, 1 s; temperature rise of 10°C s−1 to 675°C; ion source temperature, 200°C. To ensure that samples were not decomposing during purification, GC-mass spectrometry (Polaris Q; Finnigan Corp.) was performed to verify the mass spectra of GC-stable compounds in unpurified extracts or semipurified mixtures. The GC column was a DB 5 mass spectrometer (J&W Scientific, Folsom, Calif.; 30 m by 0.25-mm inside diameter) with a hold for 2 min at 50°C and was programmed at a rate of 4°C min−1 to 275°C. BOA, 2-aminophenol, HPMA, 2-acetamidophenol, and 2-amino-3H-phenoxazin-3-one were confirmed by comparison with the authentic compound and published spectra (7, 8, 23). A standard of HPMA was obtained from M. D. Richardson (Department of Horticulture, University of Arkansas, Fayetteville). BOA, 2-aminophenol, and 2-acetamidophenol were purchased from Aldrich, and a standard of 2-amino-3H-phenoxazin-3-one was prepared by allowing a methanolic solution of 2-aminophenol to oxidize naturally (8).
RESULTS
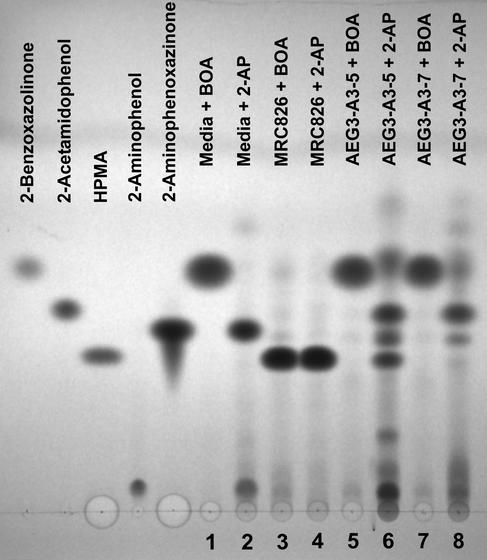
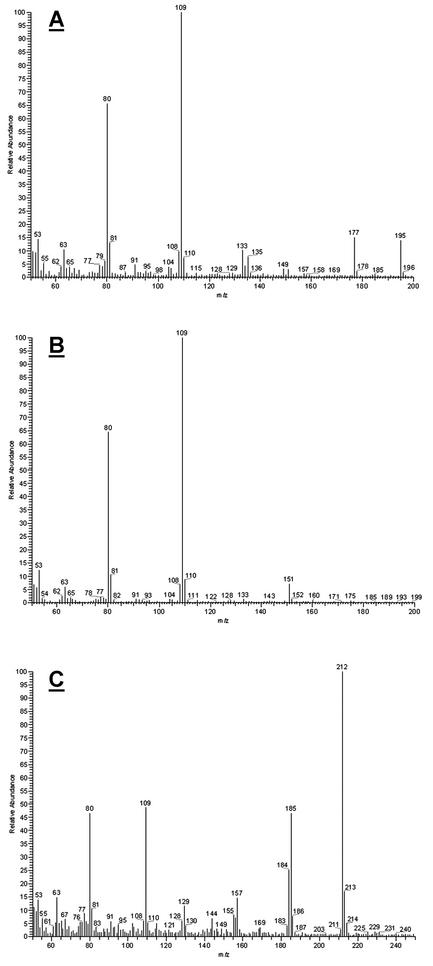
Wild-type strain MRC826 (FDB1/FDB2) transformed BOA into HPMA, while strains AEG3-A3-5 (fdb1/FDB2), AEG3-A3-7 (FDB1/fdb2), and AEG3-A3-8 (fdb1/fdb2) were unable to metabolize BOA (Fig. 2). The double mutant AEG3-A3-8 had the same physiological phenotype in response to BOA as did the fdb1 single mutant AEG3-A3-5 (10). When fed 7.4 mM 2-aminophenol, MRC826 and AEG3-A3-5 produced HPMA, whereas AEG3-A3-7 and AEG3-A3-8 did not (Fig. 2). The HPMA produced from feeding 2-aminophenol was identical to the HPMA produced by MRC826 when fed BOA (Fig. 3A), with a fragmentation pattern that matched previous reports (7, 23). Purification of HPMA and analysis by direct-exposure mass spectrometry were necessary since HPMA decomposed under the GC conditions. The other compounds of interest were GC stable.
FIG. 2.
TLC of solvent extracts made from F. verticillioides liquid cultures supplemented with either BOA or 2-aminophenol (2-AP) at a final concentration of 7.4 mM (18-h incubation). Standards of each compound (10 μg) are indicated. Samples 1 to 8 are the culture extracts (3 μl). Samples 1 and 2 represent uninoculated potato dextrose broth supplemented with BOA and 2-aminophenol, respectively. The strain MRC826 genotype is FDB1/FDB2, while strain AEG3-A3-5 is fdb1/FDB2 and strain AEG3-A3-7 is FDB1/fdb2. The top of the image corresponds with the solvent front.
FIG. 3.
Mass spectrometry m/z data for HPMA (A), 2-acetamidophenol (B), and 2-amino-3H-phenoxazin-3-one (C). Standards and experimental samples for each respective compound were identical.
Transformation of BOA into 2-acetamidophenol by AEG3-A3-7 (FDB1/fdb2) was not detected during the 18-h liquid culture incubation period (Fig. 2) but was detected by TLC when incubation was extended over several days (10) or with incubation at 3.7 mM BOA (data not shown). AEG3-A3-7 readily produced 2-acetamidophenol when it was fed 2-aminophenol (Fig. 2). Comparison of mass spectra confirmed that the metabolites were 2-acetamidophenol (Fig. 3B).
Spontaneous oxidation of 2-aminophenol produced the red-pigmented compound 2-amino-3H-phenoxazin-3-one (Fig. 2 and 3C). Such oxidation was clearly evident in uninoculated culture medium amended with 2-aminophenol (Fig. 2) but also was detected in amended cultures of AEG3-A3-5 and AEG3-A3-7. Production of 2-amino-3H-phenoxazin-3-one was not detected in cultures of MRC826 (Fig. 2), apparently due to efficient transformation of 2-aminophenol into HPMA.
When AEG3-A3-5 and AEG3-A3-7 were incubated with 2-aminophenol, an unidentified metabolite with an Rf very similar to that of 2-amino-3H-phenoxazin-3-one was produced (Fig. 2). This unknown metabolite was not observed as a major product of MRC826 incubated with 2-aminophenol, nor with any of the strains incubated with BOA. It appears to be a product of 2-aminophenol biotransformation easily detected in the mutant strains.
DISCUSSION
FDB1/fdb2 and fdb1/FDB2 strains are sensitive to BOA, since neither can fully metabolize BOA to HPMA when cultured alone. When grown together, however, the two strains can physiologically complement each other and metabolize BOA to HPMA (10), suggesting that an excreted intermediate metabolite is produced by the FDB1/fdb2 strain and utilized as a substrate by the fdb1/FDB2 strain (Fig. 1). Given the chemical structures of BOA and HPMA, the carbonyl group of BOA was hypothesized to be important for biotransformation into HPMA, suggesting that enzymatic hydrolysis and removal of the carbonyl group would produce 2-aminophenol as the intermediate compound (8, 9). If true, 2-aminophenol would serve as substrate for the protein encoded by FDB2 and would be transformed into HPMA.
As predicted, FDB2 strains (MRC826 and AEG3-A3-5) transformed 2-aminophenol into HPMA (Fig. 2). Metabolites produced by other BOA-detoxifying fungi are consistent with the hypothesis that 2-aminophenol is the intermediate in the detoxification pathway (24). Our physiological data and genetically characterized F. verticillioides strains (10), combined with chemical data (Fig. 2 and 3), also support the hypothesis that 2-aminophenol is the intermediate metabolite between BOA and HPMA.
We propose that transformation of BOA to 2-aminophenol involves a hydrolytic enzyme encoded by FDB1. Hydrolysis of the oxazolone ring of BOA would liberate CO2, producing 2-aminophenol (8, 24), which would be modified by addition of a malonyl group to produce HPMA. This modification suggests that FDB2 may encode an N-malonyltransferase (24). Production of the branch metabolite, 2-acetamidophenol, would result from acetylation, presumably by an N-acetyltransferase.
In FDB1/fdb2 mutants (e.g., strain AEG3-A3-7) a branch in the BOA detoxification pathway can result in transformation of 2-aminophenol into 2-acetamidophenol, which was previously designated as BOA-X (Fig. 1 and 2) (10). In typical wild-type situations (e.g., strain MRC826), 2-acetamidophenol is not a common product of BOA biotransformation (Fig. 2), suggesting that the metabolic pathway via FDB2 to HPMA is preferred. The inability of FDB1/fdb2 mutants to produce HPMA may result in cellular toxicity or feedback inhibition, since only a minor amount of BOA is transformed into 2-acetamidophenol. Strain AEG3-A3-7 accumulates 2-acetamidophenol to a limited extent if incubation times are extended or if it is grown on a lower concentration of BOA (10). Since substantial amounts of 2-acetamidophenol are produced when AEG3-A3-7 is grown with 2-aminophenol (Fig. 2), the hydrolysis of BOA via FDB1 appears to be the rate-limiting step in the detoxification pathway.
Transformation of 2-aminophenol to either HPMA or 2-acetamidophenol by FDB2 strains appears dependent upon the allelic state of FDB1. MRC826 (FDB1/FDB2) transformed both BOA and 2-aminophenol into HPMA. However, both HPMA and 2-acetamidophenol were produced at relatively equal levels when AEG3-A3-5 (fdb1/FDB2) was incubated with 2-aminophenol. These data suggest that the BOA-to-HPMA pathway is metabolically preferred in wild-type strains. However, fdb1 mutants may lack metabolic controls for 2-aminophenol transformation, thus allowing alternative pathways for production of HPMA or 2-acetamidophenol.
Gagliardo and Chilton (8) described the formation of 2-amino-3H-phenoxazin-3-one in BOA-amended soil as the result of microbial hydrolysis of BOA, and Friebe et al. (7) identified the same compound as a product of BOA detoxification by the wheat pathogen G. graminis var. tritici. The hydrolysis of BOA by detoxifying fungi produces 2-aminophenol, which spontaneously oxidizes to produce 2-amino-3H-phenoxazin-3-one if it is not modified to form HPMA or 2-acetamidophenol. Production of 2-amino-3H-phenoxazin-3-one may have ecological significance as an allelopathic compound. Rye mulch and cover crops are used for weed suppression, with DIBOA and BOA implicated as the primary phytotoxic allelochemicals, even though 2-amino-3H-phenoxazin-3-one is an order of magnitude more toxic than BOA in barnyard grass radicle elongation assays (8). Thus, biotransformation of BOA may enhance the allelopathic effect of rye.
As with the soil experiments involving BOA, addition of MBOA to nonsterile soil resulted in microbial hydrolysis and production of a substituted aminophenol, which oxidized to form 2-amino-7-methoxy-3H-phenoxazin-3-one (15). This methoxylated aminophenoxazinone is more hydrophobic than 2-amino-3H-phenoxazin-3-one and readily forms red crystals in aqueous solution (15). F. verticillioides metabolizes MBOA into HMPMA (19, 23), and aggregations of red crystals were observed along fungal hyphae in the agar medium (9). These red crystals may have been 2-amino-7-methoxy-3H-phenoxazin-3-one, produced as the result of MBOA hydrolysis to 5-methoxy-2-aminophenol followed by oxidation. Due to its low solubility in water, 2-amino-7-methoxy-3H-phenoxazin-3-one is not readily available for absorption and is not significantly inhibitory to barnyard grass radicle elongation (15). Unlike the interaction with rye, microbial hydrolysis of the maize and wheat antimicrobials DIMBOA and MBOA may not enhance allelopathic weed suppression.
A screen of nearly 60 field isolates of F. verticillioides from various hosts and geographical origins found only one that could not detoxify the maize antimicrobials (11), suggesting that evolutionary maintenance of the biotransformation pathway is strongly favored. The role of BOA and MBOA detoxification in the association between F. verticillioides and maize is still not clear. Detoxification apparently is not a major virulence factor and does not have an obvious effect on the ability of the fungus to infect and colonize maize seedlings (10). Nondetoxifying strains unable to grow on BOA- or MBOA-amended media can still cause seedling disease. However, detoxification may affect the virulence of the wheat pathogen G. graminis var. tritici (7). BOA and MBOA metabolism by fungi such as F. verticillioides might be an ecological fitness factor that provides a competitive advantage when colonizing senescing maize tissues, debris, and field soils that contain levels of these compounds that are suppressive to nondetoxifying fungi and bacteria. The persistence of DIMBOA, DIBOA, MBOA, and BOA in maize stubble and field debris has not been assessed, but 50% of the total content of DIBOA and BOA in rye plant residue disappeared within 12 days (22). Detoxifying fungi may be the primary colonizers during this time and contribute to the disappearance of the antimicrobials.
Our study resolves the overall chemistry of the BOA/MBOA detoxification pathway characterized by Glenn et al. (10). In the earlier study, intermediate and branch metabolites were genetically and physiologically deduced, while in this study we identified these metabolites as 2-aminophenol and 2-acetamidophenol, respectively. Though we examined BOA metabolism, the same detoxification pathway is expected for metabolism of the related benzoxazolinone MBOA (9, 19, 23).
Acknowledgments
We thank Maurice Snook (Department of Entomology, University of Georgia) for technical guidance with metabolite identification and Michael Richardson (Department of Horticulture, University of Arkansas) for helpful discussions and the provision of a standard of HPMA.
REFERENCES
- 1.Argandona, V. H., J. G. Luza, H. M. Niemeyer, and L. J. Corcuera. 1980. Role of hydroxamic acids in the resistance of cereals to aphids. Phytochemistry 19:1665-1668. [Google Scholar]
- 2.Bacon, C. W., R. M. Bennett, D. M. Hinton, and K. A. Voss. 1992. Scanning electron microscopy of Fusarium moniliforme within asymptomatic corn kernels and kernels associated with equine leukoencephalomalacia. Plant Dis. 76:144-148. [Google Scholar]
- 3.Bacon, C. W., and D. M. Hinton. 1996. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 74:1195-1202. [Google Scholar]
- 4.Bravo, H. R., S. V. Copaja, and W. Lazo. 1997. Antimicrobial activity of natural 2-benzoxazolinones and related derivatives. J. Agric. Food Chem. 45:3255-3257. [Google Scholar]
- 5.Corcuera, L. J., M. D. Woodward, J. P. Helgeson, A. Kelman, and C. D. Upper. 1978. 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one, an inhibitor from Zea mays with differential activity against soft rotting Erwinia species. Plant Physiol. 61:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley, D. C. 1962. Systemic infection of corn by Fusarium moniliforme. Phytopathology 52:870-872. [Google Scholar]
- 7.Friebe, A., V. Vilich, L. Hennig, M. Kluge, and D. Sicker. 1998. Detoxification of benzoxazolinone allelochemicals from wheat by Gaeumannomyces graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and Fusarium culmorum. Appl. Environ. Microbiol. 64:2386-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagliardo, R. W., and W. S. Chilton. 1992. Soil transformation of 2(3H)-benzoxazolone of rye into phytotoxic 2-amino-3H-phenoxazin-3-one. J. Chem. Ecol. 18:1683-1691. [DOI] [PubMed] [Google Scholar]
- 9.Glenn, A. E. 2001. Detoxification of corn antimicrobial compounds by the endophytic fungus Fusarium verticillioides and the significance to plant-fungus interactions. Ph.D. thesis. The University of Georgia, Athens.
- 10.Glenn, A. E., S. E. Gold, and C. W. Bacon. 2002. Fdb1 and Fdb2, Fusarium verticillioides loci necessary for detoxification of preformed antimicrobials from corn. Mol. Plant-Microbe Interact. 15:91-101. [DOI] [PubMed] [Google Scholar]
- 11.Glenn, A. E., D. M. Hinton, I. E. Yates, and C. W. Bacon. 2001. Detoxification of corn antimicrobial compounds as the basis for isolating Fusarium verticillioides and some other Fusarium species from corn. Appl. Environ. Microbiol. 67:2973-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto, Y., and K. Shudo. 1996. Chemistry of biologically active benzoxazinoids. Phytochemistry 43:551-559. [DOI] [PubMed] [Google Scholar]
- 13.Kedera, C. J., J. F. Leslie, and L. E. Claflin. 1992. Systemic infection of corn by Fusarium moniliforme. Phytopathology 82:1138. [Google Scholar]
- 14.Klun, J. A., and J. F. Robinson. 1969. Concentration of two 1,4-benzoxazinones in dent corn at various stages of development of the plant and its relation to resistance of the host plant to the European corn borer. J. Econ. Entomol. 62:214-220. [Google Scholar]
- 15.Kumar, P., R. W. Gagliardo, and W. S. Chilton. 1993. Soil transformation of wheat and corn metabolites MBOA and DIM2BOA into aminophenoxazinones. J. Chem. Ecol. 19:2453-2461. [DOI] [PubMed] [Google Scholar]
- 16.Munkvold, G. P., D. C. McGee, and W. M. Carlton. 1997. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 87:209-217. [DOI] [PubMed] [Google Scholar]
- 17.Niemeyer, H. M. 1988. Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defense chemicals in the Gramineae. Phytochemistry 27:3349-3358. [Google Scholar]
- 18.Richardson, M. D., and C. W. Bacon. 1993. Cyclic hydroxamic acid accumulation in corn seedlings exposed to reduced water potentials before, during, and after germination. J. Chem. Ecol. 19:1613-1624. [DOI] [PubMed] [Google Scholar]
- 19.Richardson, M. D., and C. W. Bacon. 1995. Catabolism of 6-methoxy-benzoxazolinone and 2-benzoxazolinone by Fusarium moniliforme. Mycologia 87:510-517. [Google Scholar]
- 20.Vilich, V., B. Lohndorf, R. A. Sikora, and A. Friebe. 1999. Metabolism of benzoxazolinone allelochemicals of Zea mays by Fusarium subglutinans. Mycol. Res. 103:1529-1532. [Google Scholar]
- 21.Woodward, M. D., L. J. Corcuera, J. P. Helgeson, and C. D. Upper. 1978. Decomposition of 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one in aqueous solutions. Plant Physiol. 61:796-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yenish, J. P., A. D. Worsham, and W. S. Chilton. 1995. Disappearance of DIBOA-glucoside, DIBOA, and BOA from rye (Secale cereale L.) cover crop residue. Weed Sci. 43:18-20. [Google Scholar]
- 23.Yue, Q., C. W. Bacon, and M. D. Richardson. 1998. Biotransformation of 2-benzoxazolinone and 6-methoxy-benzoxazolinone by Fusarium moniliforme. Phytochemistry 48:451-454. [Google Scholar]
- 24.Zikmundova, M., K. Drandarov, L. Bigler, M. Hesse, and C. Werner. 2002. Biotransformation of 2-benzoxazolinone and 2-hydroxy-1,4-benzoxazin-3-one by endophytic fungi isolated from Aphelandra tetragona. Appl. Environ. Microbiol. 68:4863-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuniga, G. E., V. H. Argandona, H. M. Niemeyer, and L. J. Corcuera. 1983. Hydroxamic acid content in wild and cultivated Gramineae. Phytochemistry 22:2665-2668. [Google Scholar]