Abstract
Dental unit water system (DUWS) tubing harbors complex multispecies biofilms that are responsible for high microbial levels at the distal outlet. The aim of this study was to use an established biofilm laboratory model to simulate biofouling of DUWS to evaluate practical, cost-effective, and evidence-based methods of microbial decontamination. Reproducible biofilms were developed in the model over 14 days; decontamination was assessed using total viable counts (TVC) and microscopic-image analysis techniques to view the inner surface of tubing. Flushing did not reduce the biofilm coverage or TVC. Combizyme and ozone did not completely eliminate the viable bacteria (70 and 65% reduction in biofilm TVC, respectively), nor did they remove the biofilm (45 and 57% reduction in biofilm coverage, respectively). Chlorhexidine and Bio2000 (active agent: ethanol and chlorhexidine), Tegodor and Gigasept Rapid (aldehyde based), and Grotanol (hydroxide based) completely eliminated the TVC but did not completely remove biofilm (31, 53 33, 34, and 64.9% reduction of biofilm coverage, respectively). Other products including Grotanol Flussig (phenol based), Betadine (povidone-iodine based), Alpron (chlorite based), and the hydroxide-containing products Sporklenz, Sterilex Ultra, Dialox, Sterilox, Sanosil, Oxigenal, and Grotanat Bohrerbad resulted in a 100% reduction in the biofilm TVC and a >95% reduction in biofilm coverage. The study demonstrated that while many disinfectants achieve a sufficient reduction in TVC they may not necessarily remove unwanted biofilm from the tubing surfaces as tested in this laboratory-controlled biofilm model.
Dental unit water systems (DUWS) are used to irrigate the oral cavity during dental treatment. Water delivered from these devices is not sterile and has been shown to contain high numbers of bacteria (6, 12, 22, 30, 38). Biofilms accumulating on the inner surface of the tubing are responsible for high levels of contamination of DUWS (30, 37). Currently, dentists across the world have no evidence-based guidelines to control bacterial numbers in DUWS. A number of surveys have demonstrated that the majority of DUWS are supplied by tap water (40). European Union (EU) guidelines recommend that tap water should be delivered at <100 CFU · ml−1 at 22°C and <20 CFU · ml−1 at 37°C (2); however, once the water enters the DUWS the number of bacteria can begin to increase, with numbers as high as 1.6 × 105 CFU · ml−1 having been recovered in the outflow (12). Such high numbers can result from numerous factors including ambient temperatures, stagnation, and the presence of biofilms (22). In the United States, the American Dental Association (ADA) and the Centers for Disease Control and Prevention (CDC) have suggested a standard for DUWS water of no more than 200 CFU · ml−1 (1). The EU has yet to set an equivalent standard.
Pathogens such as Legionella pneumophila, Mycobacterium spp., Pseudomonas aeruginosa, and Candida spp. have been recovered from DUWS so it is evident that these medical devices have the potential to harbor opportunistic or frank pathogens (4, 5, 25). Exposure of dental personnel to such pathogens has been implied, since dentists practicing in dental schools have a significantly higher antibody titer to L. pneumophila than other equivalent employment sectors (8, 21). P. aeruginosa has been responsible for the illness of two immunocompromised patients due to a cross infection incident while at a dental surgery (17). A wide range of products are now being developed for use in DUWS; some examples include chlorine dioxide, oral antiseptics, sodium hypochlorite, and hydrogen peroxide (13, 19, 27, 29, 42). These products are being evaluated using a variety of approaches (34).
There is a clear requirement for a reliable, relevant laboratory model system to simulate microbial contamination of DUWS systems, thereby permitting the objective evaluation of antimicrobial and antibiofilm products to control such contamination.
The aims of this study were, therefore, (i) to establish the ability of the laboratory model to generate reproducible, multiple biofilms on relevant surfaces, and then (ii) to use this model to evaluate and compare the efficacy of a variety of products based on different classes of active compound, which could then be proposed for use within DUWS in general dental practice (GDP).
MATERIALS AND METHODS
Laboratory model.
The laboratory model was based on a continuous-culture chemostat design and has been described previously (34). Materials used for the tubing included medical-grade silicone tubing (Portex, Ltd., Kent, United Kingdom) and small-bore polyurethane DUWS tubing (Woodlane Dental equipment, Bristol, United Kingdom) attached using polypropylene connectors (Jencons PLS, Leighton Buzzard, United Kingdom). This model consists of a glass chemostat vessel with a titanium top plate, with filter sterilized tap water as the microbial growth medium. The working volume of 1 liter was maintained automatically at 20 ± 0.1°C with a constant dilution rate (D) = 0.05 h−1 (mean generation time of 13.9 h).
Chemostat operation and generation of biofilm.
The chemostat was inoculated with 1.0 ml of a mixed inoculum pooled by filtering water from 10 DUWS through 0.2-μm-pore-size analytical test filter funnels (Techware, Poole, United Kingdom) and then recovering the waterborne microorganisms in sterile phosphate-buffered saline (PBS) before storing over liquid nitrogen. The chemostat was operated in batch mode for 48 h before continuous culture was commenced. After 7 days growth at D = 0.05 h−1 (equivalent to 12 mean generation times) to establish a steady state, the microbial effluent was passed through multiple parallel lines of DUWS tubing at a flow rate of 12.5 ml · h−1 for up to a further 14 days to establish biofilm. Biofilm analysis was initially carried out on a frequent basis to assess biofilm development over the 14 day period, by both total viable count (TVC) and (microscopic) percentage coverage (methods described below).
Efficacy of flushing.
Biofilms were formed as above over a two-week period. According to the British Dental Association (BDA)-recommended guidelines, water (sterile) was passed through the DUWS lines for 2 min (flow rate of 80 ml · min−1, which is typical of a GDP DUWS on full flow) (3). Samples of tubing were then analyzed for biofilm TVC and coverage.
Use of disinfectants.
In order to test the efficacy of disinfectants, biofilms were generated for two weeks and analysis undertaken to assess the TVC and percentage coverage. Each of the disinfectants (Table 1) were prepared as per manufacturers instructions and tested against sterile water as a negative control. Disinfectants where required were prepared using sterile water (WFI, Miza Pharmaceuticals, Wolverhampton, United Kingdom). Disinfectants were placed in a bottle and pumped through the DUWS tubing from a t-junction connected to the distal outlet until visual detection and then pumped for a further two minutes. Repeats were carried out in fresh parallel lines to remove the problem of residual activity. Following disinfection, each line was renewed with fresh tubing. The contact time for the products was 16 h (overnight), although ozone was applied for 10 min. For Alpron, the first and second stages were held for a contact time of 20 min, and the third stage was then left for a contact time of 16 h (overnight).
TABLE 1.
List of active ingredients and concentrations of agents used in the disinfectant trial
| Trade name or ingredient | Active agent(s) | Concn used | Manufacturer |
|---|---|---|---|
| Alpron BRS solution | Sodium hypochlorite, citric acid | 1-2%, 70% | Alpro Dental Products GmbH, St. Georgen, Germany |
| Alpron Mint | Sodium-p-toluolsulfonechloramide, EDTA | <0.2%, 1-5% | |
| Bilpron | Hydroxy benzoin acid ester polyhexamethylene- biguanide EDTA phenylalanine | Undiluted | Alpro Dental Products GmbH |
| Bio2000 | Ethanol | 12% | Micrylium, Toronto, Canada |
| Chlorhexidine | 0.12% (undiluted) | ||
| Chlorhexidine | Chlorhexidine | 0.2% | Sigma, Poole, United Kingdom |
| Combizyme | Proteinases and carbohydrases | 1.25% | Biocatalyst, Pontypridd, United Kingdom |
| Dentasept | Hydrogen peroxide | 1% | Muller Dental, Cologne, Germany |
| Dioxiclear | Chlorine dioxide | As per instructions | Frontier Pharmaceutical, Inc., Melville, N.Y. |
| Dialox | Hydrogen peroxide, peracetic acid, acetic acid | Undiluted | Schülke and Mayr UK Ltd, Rotherham, United Kingdom |
| Grotanat Bohrerbad | Calcium hydroxide, propanol, ethylhexanol | Undiluted | Schülke and Mayr, Norderstedt, Germany |
| Grotanol Flussig | Chloro-methylphenol Chlor-benzylphenol, biphenol | 2% | Schülke and Mayr |
| Gigasept Rapid | Glutaraldehyde, formaldehyde, didecyldimethyl- ammoniumchloride | 4% | Schülke and Mayr |
| Grotanol | Triazine-triethanol, sodium hydroxide | 3% | Schülke and Mayr |
| Oxigenal | Hydrogen peroxide | 0.4% | Kavo, Maersham, United Kingdom |
| Ozone | Ozone | 200 mg/h (liquid and gas phase = 10 min) | Onnic Ltd, Waterlooville, United Kingdom |
| Parmetol | Butylhydroperoxide | 1% | Schülke and Mayr |
| Betadine | Povidone iodine solution | 10% | Seton Scholl Healthcare Group plc, Knutsford, United Kingdom |
| Sanosil | Hydrogen peroxide and silver | 5% | Sanosil Ltd, Feldmeilen, Switzerland |
| Sodium hypochlorite | Chlorite | 0.5% | P & R Laboratory Supplies, St. Helen's, United Kingdom |
| Sporklenz | Hydrogen peroxide, peracetic acid, acetic acid | Undiluted | Steris, Camberley, Surrey, United Kingdom |
| Sterilox | Superoxidized water | 2.5% | Sterilox Technologies, Abingdon, United Kingdom |
| Sterilex Ultra | Alkaline peroxide | 5% | Prestige, Bradford, United Kingdom |
| Tegodor | Benzalkoniumchloride, formaldehyde, glutaraldehyde | 1% | TH Goldschmidt Ltd, Ruislip, Middlesex, United Kingdom |
Determination of water TVC.
Water samples were removed from the chemostat via a sample port and total viable counts (TVC) were carried out on decimal dilutions in sterile PBS containing sodium thiosulfate (3.5 g/liter) plated onto R2A agar (24) (37°C for 7 days). These counts were used as the definitive measure of total microbial contamination of the water passing through the DUWS model.
Analysis of biofilm accumulation.
Biofouling was assessed by image analysis of biofilm coverage and TVC of defined areas of tubing as previously described (34). Briefly, sections (50 mm) of the DUWS tubing were sectioned longitudinally into two equal portions. One half was rinsed twice in nonflowing sterile diluent (PBS containing sodium thiosulfate, 3.5 g/liter) to remove nonadhered cells. The surface biofilm was removed by scraping with a sterile dental probe into 1 ml of sterile diluent and samples were vortexed for 15 s. Any potentially residual disinfectant was removed by the tubing rinse step and by inclusion of the sodium thiosulfate in the diluent. TVC was then determined as described as above. The extent of DUWS tubing biofouling was assessed on the other half of the same piece of tubing using image analysis. Lengths of tubing were aseptically sectioned into thin strips and stained for 1 min with 50 μl of prefiltered (pore size, 0.2 μm; Sartorious, Epsom, United Kingdom) propidium iodide (Sigma, Poole, United Kingdom; 1 mg · ml−1 stock in sterile distilled water) before being gently rinsed twice in nonflowing sterile distilled water to remove planktonic and loosely adhering cells. The stained biofilm on the tubing surface was examined using a Nikon Labophot 2 microscope with episcopic fluorescence and a ×50 water immersion lens, as described previously (36). Ten representative images of each tubing sample were captured as computer (*.jpg) images, and the images were analyzed for percentage coverage using AxioVision 2.0.5 software (Imaging Associates, Thame, United Kingdom).
Statistical analysis.
Log10 CFU per unit volume (milliliter) (for water-planktonic samples) or per unit area (centimeter2) for biofilm were compared between four independent chemostat runs by one-way analysis of variance (ANOVA), to determine the degree of reproducibility of the model system. Similarly, treatment effects were also investigated by ANOVA, and significant differences between individual treatments were investigated using the Scheffé test. Significance of differences was assumed at P < 0.05 (5%).
RESULTS
Biofilm development.
Effluent from the continuous culture vessel containing a geometric mean of 2.0 × 105 CFU · ml−1 (range 8.5 × 104 to 6.8 × 105 CFU · ml−1) was passed through the DUWS tubing. This level was comparable to that observed in some GDP DUWS (33). Gram-negative and oxidase-negative bacteria were the predominant groups detected. Bacterial numbers recovered from the biofilm were initially low, at <1.0 × 103 CFU · cm−2 over the first 7 days, but then increased and stabilized at a geometric mean of 3.4 × 104 CFU · cm−2 (range, 1.3 × 104 to 1.1 × 105 CFU · cm−2) by day 14. Biofilm coverage was initially low (geometric mean, 0.5%; range, 0.3 to 1.9%) for the first week; during the period 8 to 14 days, the surface coverage stabilized at a geometric mean value of 8.9% (range, 3.3 to 26%). Since the variability of 14 day biofilms was lower than that of the younger biofilms, these were used for comparison of products in all subsequent studies.
Reproducibility within and between different runs.
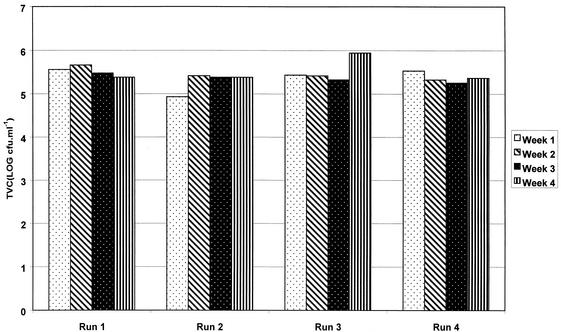
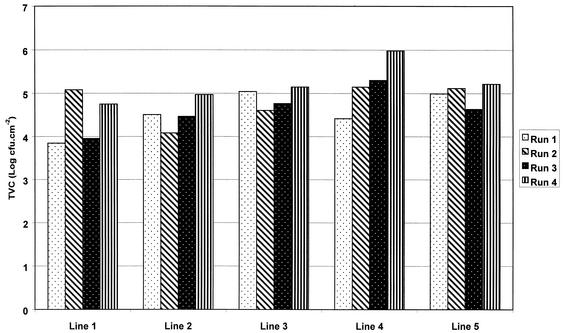
Comparison of within-run versus between-run variations in planktonic counts showed no significant differences (by ANOVA, F = 1.44 and P = 0.27) (Fig. 1). Similarly, biofilm counts were reproducible in repeat runs, compared to within an individual run (by ANOVA, F = 1.23 and P = 0.24) (Fig. 2).
FIG. 1.
Reproducibility of planktonic counts from chemostat water samples from DUWS model within and between four independent experiments.
FIG. 2.
Reproducibility of biofilm counts after 14 days of generation within and between four independent experiments in the DUWS model.
Effect of flushing and disinfectants on biofilm viability and coverage.
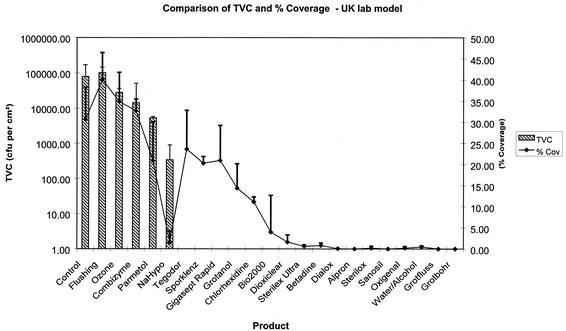
Biofilms generated over 14 days had a geometric mean of 3.4 × 103 CFU · cm−2. Flushing as recommended by the BDA, resulted in only a small reduction in the biofilm TVC (9.1%) and percentage coverage (0.5%). A number of disinfectants such as Combizyme and ozone did not completely eliminate viable bacteria (70 and 65% reduction, respectively), nor did they remove the biofilm (45 and 57% reduction in coverage, respectively). Chlorhexidine and Bio2000 (active agents, ethanol and chlorhexidine) both completely eliminated viable bacteria but were not able to remove the biofilm from the surface (31 and 53% reduction in coverage, respectively). Likewise, Tegodor and Gigasept Rapid (aldehyde based) and Grotanol (hydroxide based) achieved a 100% reduction in the biofilm viability but did not remove the biofilm adhered to the tubing surface (only a 33, 34, and 65% reduction in coverage, respectively). Other products including Grotanol Flussig (phenol based), Betadine (povidone-iodine based), Alpron (chlorite based), and the hydroxide-containing products Sporklenz, Sterilex Ultra, Dialox, Sterilox, Sanosil, Oxigenal, and Grotanat Bohrerbad resulted in a 100% reduction in the biofilm TVC and a >95% reduction of the biofilm coverage (Table 2 and Fig. 3).
TABLE 2.
Percentage reduction of viable counts and biofilm coverage after exposure to disinfectants and flushing
| Treatment | % Reduction of:
|
|
|---|---|---|
| Viable count | Biofilm coverage | |
| Flushing | 9.1 | 0.5 |
| Ozone | 65 | 57.8 |
| Combizyme | 70 | 45 |
| Tegodor | 100 | 33 |
| Sporklenz | 100 | 92.6 |
| Sodium hypochlorite | 100 | 94.4 |
| Chlorhexidine | 100 | 31.77 |
| Dialox | 100 | 99.77 |
| Betadine | 100 | 97.3 |
| Parmetol | 94.8 | 31.3 |
| Gigasept | 100 | 34 |
| Grotanol | 100 | 64.9 |
| Dioxiclear | 100 | 94.7 |
| Alpron | 100 | 100 |
| Sterilox | 100 | 99.3 |
| Sanosil | 100 | 100 |
| Oxigenal | 100 | 99.2 |
| Bio2000 | 100 | 53.2 |
| Sterilex Ultra | 100 | 97.3 |
FIG. 3.
Effect of 16-h contact time of disinfectants on TVC and percentage surface coverage of 14-day-old biofilms. NaHypo, sodium hypochlorite. Error bars indicate standard deviations.
DISCUSSION
Bacterial fouling of dental water systems has been recognized as a problem for almost 40 years (6). Although the majority of studies have been carried out in dental hospitals (4, 8), a more recent survey in the United Kingdom demonstrated that DUWS in “High Street” GDPs are also contaminated by relatively high numbers of bacteria (mean, 2.9 × 103 CFU · ml−1; range, 7 CFU · ml−1 to 6.4 × 104 CFU · ml−1) (33). Occasionally these DUWS systems also contained opportunistic pathogens and oral bacteria. The latter observations suggested failure of antiretraction valves and demonstrated the potential for patient to patient cross infection. With the ever-increasing presentation of human immunodeficiency virus-positive patients and hepatitis B virus carriers, care and precautions must be undertaken to minimize the risk for cross infection resulting from the use of DUWS, particularly to immunocompromised patients (23).
In the study on GDP-DUWS, there was a direct correlation between the numbers of bacteria in biofilm and planktonic samples (33). Since tap water going in to the DUWS should have a microbial load of ca. ≤100 CFU · ml−1, the high levels of bacteria are assumed to be due to build up of biofilm, followed by shedding from the biofilm lining the tubing into the water phase (33). As the water from the DUWS is used to irrigate the oral cavity during dental treatment, it has been recommended that the levels of microbial contamination should be reduced (for example, the ADA have proposed a threshold value of <200 CFU · ml−1) (16). Therefore, the biofilm that accumulates on the tubing must be a major target for any control strategy.
Statistical analyses confirmed that there were no significant differences in either the planktonic or biofilm microbial loads, either between or within experimental runs. Thus, the model could be used with confidence not only to compare the efficacy of products and control strategies within an experiment but also between replicate experiments.
Current BDA guidelines suggest that dentists should allow any hand piece that delivers water (low or high speed) to discharge (flush) for at least 2 min at the beginning of the day to reduce microbial contamination (3). Although it has been reported that this approach could reduce contamination by around 30% (41), our data agree with previous in-practice studies, which demonstrated that 2 min of flushing did not significantly reduce microbial counts (11). Longer periods of flushing may be more effective (26), but this may not always be practical in a clinical setting. In addition, our data suggest that the biofilm which was not removed by flushing would rapidly reseed the water and could act as a trap to capture planktonic bacteria into an existing biofilm.
There are considered to be four categories of products that are available to address microbial contamination in DUWS: independent water systems, sterile water delivery systems, filtration, and chemical treatment protocols (15). A previous study demonstrated that there were no significant differences between different DUWS systems, regardless of whether these systems were mains or bottle or header tank fed or whether the water supplied to them was hard, soft deionized, or distilled (33).
An increasing number of disinfectant products are available for microbial decontamination, many of which are being promoted for use in DUWS (12, 19, 27, 29, 31, 42). In our study a number of criteria needed to be satisfied in order for a product to be considered for use in DUWS in GDP. These included (i) killing of bacteria in the water phase, (ii) killing of biofilm bacteria (since the biofilms are largely responsible for the high microbial load in the water phase), and (iii) removal of biofilm from the surfaces (the reason for this is that a “killed” biofilm could still act as a source of endotoxin, as well as allowing rapid recolonization of new, viable biofilm that could occlude the tubing). There may be important implications if the biofilms are not removed from the dental unit water line. Residual biofilm may facilitate the colonization of waterborne bacteria to existing cells (coaggregation), and subsequently contaminate fresh incoming water (7), as well as provide a haven for human pathogens (35).
Combizyme (proteinases and carbohydrases) and ozone did not completely reduce the biofilm TVC, nor reduce the percentage biofilm coverage. Chlorhexidine and Bio2000 (active agents ethanol and chlorhexidine) achieved a complete kill of the TVC but did not completely remove the biofilm. The aldehyde-containing products Tegodor and Gigasept Rapid also eliminated the biofilm TVC (i.e., no viable cells were detected) but were unable to completely remove biofilm from the surface. Aldehydes are widely used as fixatives for bacterial cells and so this may explain this observation. However, it should be noted that the use of aldehyde-containing products may require occupational exposure monitoring for dental personnel.
The other products, including Dialox, Sporklenz, Sterilex Ultra, Betadine (20), Alpron, Sterilox, Sanosil, Oxigenal, Grotanol Flussig, and Grotanat Bohrerbad, resulted in a complete elimination of viable bacteria in biofilm and an almost total removal of biofilm coverage.
Dialox and Sanosil (both hydrogen peroxide) are currently used in the reuse of dialysis machines (32), while Sporklenz (blend of hydrogen peroxide, peracetic acid and acetic acid) is a liquid commercial product formulated for the disinfection of hard surfaces (14). Sterilox, a liquid biocide of super-oxidized water, containing a mixture of oxidizing substances produced by electrolysis of a dilute saline solution, was demonstrated to provide an efficient reduction in the biofilm TVC and coverage (28, 43; J. B. Selkon, Letter, J. Hosp. Infect. 48:154-155, 2001). Similarly, Grotanat Flussig (instrument cleaner) and Grotanat Bohrerbad (drill bath cleaner) achieved efficient biofilm kill and removal. Although providing efficient biofilm kill and removal, neither Grotanat Flussig nor Grotanat Bohrerbad is currently approved or marketed for use in DUWS. These products would require further evaluation, for example, in terms of materials compatibility and copper solvation (cuprosolvency) before they could be recommended for routine use in DUWS (9).
Particular parameters may have to be addressed with respect to the compatibility of DUWS construction materials with any proposed agent. For example, the brass components that are used as connectors in DUWS may be incompatible with some disinfectants. The pH of the water can be critical to the cuprosolvency of brass fittings (9).
A number of the other products, including Alpron (a three-part component cleaner containing sodium hypochlorite, citric acid, and sodium-p-toluolsulfonechloramide) (31), Sterilex Ultra (alkaline peroxide), and Oxigenal (hydrogen peroxide), are all currently approved for use in DUWS and resulted in a complete kill with biofilm removal. A number of these products will be evaluated in a later study in due course in GDPs.
In the long term, the redesign of dental units may be necessary to reduce biofilms and microbial contamination. However, in the short term, effective disinfectants are required that will control biofilm formation.
The model system described here demonstrated that biofilm regrowth following disinfection would occur within 7 to 14 days (results not shown). This suggests that weekly treatment programs may not be sufficient to reduce microbial counts to levels that comply with EU drinking water standards or ADA DUWS guidelines and that daily or continuous treatment may be more suitable. It is therefore important to determine the kinetics of microbial killing. In this way, appropriate contact times, frequency, and mode of application can be developed for clinical use. The model system described in this work can provide robust data of this type to inform public health policy with respect to DUWS contamination. When using products constantly in the DUWS there may be an inherent risk of microbial resistance occurring (10, 18). Hence, a monitoring program to determine that disinfectants are maintaining reduced microbial numbers in DUWS should play a role in the GDP health care policy (39).
Acknowledgments
This investigation was supported by (i) the Primary Dental Care R&D Programme and National Research Register research grant PDC97-213 from the NHS Executive, North West, Warrington, United Kingdom, and (ii) the European Commission, specific RTD program Quality of Life and Management of Living Resources 4.1 Environment and Health (QLK4-2000-00097).
We acknowledge the assistance of the disinfectant manufacturers where appropriate for the supply of disinfectants.
REFERENCES
- 1.Anonymous. 1996. ADA statement on dental unit waterlines. J. Am. Dent. Assoc. 127:185-186. [Google Scholar]
- 2.Anonymous. 1998. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Official J. Eur. Commun. L330:32-54. [Google Scholar]
- 3.Anonymous. 2000. Infection control in dentistry, Advice sheet A12, p. 6. British Dental Association, London, United Kingdom.
- 4.Atlas, R. M., J. F. Williams, and M. K. Huntington. 1995. Legionella contamination of dental-unit waters. Appl. Environ. Microbiol. 61:1208-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbeau, J., C. Gauthier, and P. Payment. 1998. Biofilms, infectious agents, and dental unit waterlines: a review. Can. J. Microbiol. 44:1019-1028. [DOI] [PubMed] [Google Scholar]
- 6.Blake, G. C. 1963. Incidence and control of bacteria infection in dental spray reservoirs. Br. Dent. J. 115:413-416. [DOI] [PubMed] [Google Scholar]
- 7.Buswell, C. M., Y. M. Herlihy, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1997. Co-aggregation amongst aquatic biofilm bacteria. J. Appl. Bacteriol. 83:477-484. [Google Scholar]
- 8.Challacombe, S. J., and L. L. Fernandes. 1995. Detecting Legionella pneumophila in water systems: a comparison of various dental units. J. Am. Dent. Assoc. 126:603-608. [DOI] [PubMed] [Google Scholar]
- 9.Critchley, M. M., N. J. Cromar, N. McClure, and H. J. Fallowfield. 2001. Biofilms and microbially influenced cuprosolvency in domestic copper plumbing systems. J. Appl. Microbiol. 91:646-651. [DOI] [PubMed] [Google Scholar]
- 10.Foley, I., and P. Gilbert. 1996. Antibiotic resistance of biofilms. Biofouling 10:331-346. [DOI] [PubMed] [Google Scholar]
- 11.Gross, A., M. J. Devine, and D. E. Cutright. 1976. Microbial contamination of dental units and ultrasonic scalers. J. Periodont. 47:670-673. [DOI] [PubMed] [Google Scholar]
- 12.Karpay, R. I., T. J. Plamondon, and S. E. Mills. 1999. Comparison of methods to enumerate bacteria in dental unit water lines. Curr. Microbiol. 38:132-134. [DOI] [PubMed] [Google Scholar]
- 13.Karpay, R. I., T. J. Plamondon, S. E. Mills, and S. B. Dove. 1999. Combining periodic and continuous sodium hypochlorite treatment to control biofilms in dental unit water systems. J. Am. Dent. Assoc. 130:957-965. [DOI] [PubMed] [Google Scholar]
- 14.Knowles, J., and S. Roller. 2001. Efficacy of chitosan, carvacrol, and a hydrogen peroxide-based biocide against foodborne microorganisms in suspension and adhered to stainless steel. J. Food. Prot. 64:1542-1548. [DOI] [PubMed] [Google Scholar]
- 15.Lee, T. K., E. J. Waked, L. E. Wolinsky, R. S. Mito, and R. E. Danielson. 2001. Controlling biofilm and microbial contamination in dental unit waterlines. J. Calif. Dent. Assoc. 29:679-684. [PubMed] [Google Scholar]
- 16.Linger, J. B., J. A. Molinari, W. C. Forbes, C. F. Farthing, and W. J. Winget. 2001. Evaluation of a hydrogen peroxide disinfectant for dental unit waterlines. J. Am. Dent. Assoc. 132:1287-1291. [DOI] [PubMed] [Google Scholar]
- 17.Martin, M. V. 1987. The significance of the bacterial contamination of dental unit water systems. Br. Dent. J. 163:152-154. [DOI] [PubMed] [Google Scholar]
- 18.McBain, A. J., and P. Gilbert. 2001. Biocide tolerance and the harbingers of doom. Int. Biodet. Biodeg. J. 47:55-61. [Google Scholar]
- 19.Meiller, T. F., J. I. Kelley, A. A. Baqui, and L. G. DePaola. 2000. Disinfection of dental unit waterlines with an oral antiseptic. J. Clin. Dent. 11:11-15. [PubMed] [Google Scholar]
- 20.Mills, S. E., P. W. Lauderdale, and R. B. Mayhew. 1986. Reduction of microbial contamination in dental units with povidone-iodine 10%. J. Am. Dent. Assoc. 113:280-284. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheim, B. A., A. M. Sefton, O. N. Gill, J. E. Tyler, M. C. O'Mahony, J. M. Richards, P. J. Dennis, and T. G. Harrison. 1987. Widespread Legionella pneumophila contamination of dental stations in a dental school without apparent human infection. Epidemiol. Infect. 99:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pankhurst, C. L., N. W. Johnson, and R. G. Woods. 1998. Microbial contamination of dental unit waterlines: the scientific argument. Int. Dent. J. 48:359-368. [DOI] [PubMed] [Google Scholar]
- 23.Porter, S. R. 2002. Prions and dentistry. J. R. Soc. Med. 95:178-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reasoner, D. J., and E. E. Geldreich. 1985. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 49:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinthaler, F., and F. Mascher. 1986. Demonstration of Legionella pneumophila in dental units. Zentbl. Bakteriol. Mikrobiol. Hyg. B 183:86-88. [PubMed] [Google Scholar]
- 26.Scheid, R. C., S. Rosen, and F. M. Beck. 1990. Reduction of CFUs in high-speed handpiece water lines over time. Clin. Prevent. Dent. 12:9-12. [PubMed] [Google Scholar]
- 27.Shepherd, P. A., M. A. Shojaei, P. D. Eleazer, A. Van Stewart, and R. H. Staat. 2001. Clearance of biofilms from dental unit waterlines through the use of hydroperoxide ion-phase transfer catalysts. Quintessence Int. 32:755-761. [PubMed] [Google Scholar]
- 28.Shetty, N., S. Srinivasan, J. Holton, and G. L. Ridgway. 1999. Evaluation of microbicidal activity of a new disinfectant: Sterilox 2500 against Clostridium difficile spores, Helicobacter pylori, vancomycin resistant Enterococcus species, Candida albicans and several Mycobacterium species. J. Hosp. Infect. 41:101-105. [DOI] [PubMed] [Google Scholar]
- 29.Smith, A. J., J. Bagg, and J. Hood. 2001. Use of chlorine dioxide to disinfect dental unit waterlines. J. Hosp. Infect. 49:285-288. [DOI] [PubMed] [Google Scholar]
- 30.Smith, A. J., J. Hood, J. Bagg, and F. T. Burke. 1999. Water, water everywhere but not a drop to drink? Br. Dent. J. 186:12-14. [DOI] [PubMed] [Google Scholar]
- 31.Smith, A. J., S. McHugh, I. Aitkin, and J. Hood. 2002. Evaluation of the efficacy of alpron disinfectant in dental unit water lines. Br. Dent. J. 193:593-596. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka, N., T. Fujisawa, T. Daimon, K. Fujiwara, M. Yamamoto, and T. Abe. 2000. The use of electrolyzed solutions for the cleaning and disinfecting of dialyzers. Artificial Organs 24:921-928. [DOI] [PubMed] [Google Scholar]
- 33.Walker, J. T., D. J. Bradshaw, A. M. Bennett, M. R. Fulford, M. V. Martin, and P. D. Marsh. 2000. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl. Environ. Microbiol. 66:3363-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker, J. T., D. J. Bradshaw, M. R. Fulford, M. V. Martin, and P. D. Marsh. 2001. Controlling mixed species biofilm contamination in dental unit water systems (DUWS) using a laboratory simulation model: a choice of products, p. 333-340. In P. Gilbert, D. Allison, M. Brading, J. Verran, and J. Walker (ed.), Biofilm community interactions: chance or necessity. BioLine, Cardiff, United Kingdom.
- 35.Walker, J. T., C. W. Mackerness, J. Rogers, and C. W. Keevil. 1995. Biofilm: a haven for waterborne pathogens, p. 196-204. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms. Cambridge University Press, London, United Kingdom.
- 36.Walker, J. T., A. D. G. Roberts, V. J. Lucas, M. M. Roper, and R. Brown. 1999. Quantitative assessment of biocide control of biofilms and Legionella using total viable counts, fluorescent microscopy and image analysis. Methods Enzymol. 310:629-637. [DOI] [PubMed] [Google Scholar]
- 37.Williams, H. N., M. L. Baer, and J. I. Kelley. 1995. Contribution of biofilm bacteria to the contamination of the dental unit water supply. J. Am. Dent. Assoc. 126:1255-1260. [DOI] [PubMed] [Google Scholar]
- 38.Williams, H. N., A. Johnson, J. I. Kelley, M. L. Baer, T. S. King, B. Mitchell, and J. F. Hasler. 1995. Bacterial contamination of the water supply in newly installed dental units. Quintessence Int. 26:331-337. [PubMed] [Google Scholar]
- 39.Williams, H. N., J. Kelley, D. Folineo, G. C. Williams, C. L. Hawley, and J. Sibiski. 1994. Assessing microbial contamination in clean water dental units and compliance with disinfection protocol. J. Am. Dent. Assoc. 125:1205-1211. [DOI] [PubMed] [Google Scholar]
- 40.Williams, J. F., N. Andrews, and J. I. Santiago. 1996. Microbial contamination of dental unit waterlines: current preventive measures and emerging options. Compend. Cont. Educ. Dentist. 17:691-694. [PubMed] [Google Scholar]
- 41.Williams, J. F., A. M. Johnston, B. Johnson, M. K. Huntington, and C. D. Mackenzie. 1993. Microbial contamination of dental unit waterlines: prevalence, intensity and microbiological characteristics. J. Am. Dent. Assoc. 124:59-65. [DOI] [PubMed] [Google Scholar]
- 42.Wirthlin, M. R., and G. J. Marshall. 2001. Evaluation of ultrasonic scaling unit waterline contamination after use of chlorine dioxide mouthrinse lavage. J. Periodont. 72:401-410. [DOI] [PubMed] [Google Scholar]
- 43.Zinkevich, V., I. B. Beech, R. Tapper, and I. Bogdarina. 2000. The effect of super-oxidized water on Escherichia coli. J. Hosp. Infect. 46:153-156. [DOI] [PubMed] [Google Scholar]