Abstract
While several studies on the ecology of Vibrio vulnificus in Gulf Coast environments have been reported, there is little information on the distribution of this pathogen in East Coast waters. Thus, we conducted a multiyear study on the ecology of V. vulnificus in estuarine waters of the eastern United States, employing extensive multiple regression analyses to reveal the major environmental factors controlling the presence of this pathogen, and of Vibrio spp., in these environments. Monthly field samplings were conducted between July 2000 and April 2002 at six different estuarine sites along the eastern coast of North Carolina. At each site, water samples were taken and nine physicochemical parameters were measured. V. vulnificus isolates, along with estuarine bacteria, Vibrio spp., Escherichia coli organisms, and total coliforms, were enumerated in samples from each site by using selective media. During the last 6 months of the study, sediment samples were also analyzed for the presence of vibrios, including V. vulnificus. Isolates were confirmed as V. vulnificus by using hemolysin gene PCR or colony hybridization. V. vulnificus was isolated only when water temperatures were between 15 and 27°C, and its presence correlated with water temperature and dissolved oxygen and vibrio levels. Levels of V. vulnificus in sediments were low, and no evidence for an overwintering in this environment was found. Multiple regression analysis indicated that vibrio levels were controlled primarily by temperature, turbidity, and levels of dissolved oxygen, estuarine bacteria, and coliforms. Water temperature accounted for most of the variability in the concentrations of both V. vulnificus (47%) and Vibrio spp. (48%).
Vibrio vulnificus, a gram-negative, halophilic bacterium found in estuarine environments, causes primary septicemia and wound infections in humans (28). Primary septicemia usually occurs through ingestion of raw shellfish, especially oysters, by persons who are predisposed to infection by increased serum iron levels or who are immunocompromised. Wound infections, however, have been shown to occur in otherwise healthy individuals who come in contact with this bacterium via contamination of a previously inflicted wound or one incurred in an estuarine environment.
One of the main virulence factors associated with infection with V. vulnificus is an antiphagocytic, polysaccharide capsule (35), and encapsulated cells are highly virulent, with a 50% lethal dose of less than 10 CFU (38, 49). The V. vulnificus endotoxin is also important in the virulence of this pathogen (21), and the hypotension produced by this factor is eliminated when nitric oxide synthase is inhibited (6). Interestingly, we found that estrogen induces a protective response against V. vulnificus-induced toxic shock (22). This likely accounts for the fact that 82% of infections occur in males (28). The pathogenesis of V. vulnificus infection has been reviewed in several recent papers (18, 28, 39).
Several studies have reported on the presence of V. vulnificus, as well as other estuarine vibrios, along the Gulf Coast (5, 15, 17, 40), the West Coast (14), and the East Coast (29, 30) of the United States, as well as in coastal waters of Denmark (8), Hong Kong (2), Japan, and countries in Africa and South America (25). However, except for two studies in the northeast portion of the United States (9, 32), the only reported long-term studies on the ecology of V. vulnificus were conducted along the Gulf Coast. In 1997, V. vulnificus infections became reportable in North Carolina. Therefore, we conducted a multiyear study on the ecology of V. vulnificus along the eastern coast of North Carolina. This area is unique because of the small islands that make up the Outer Banks, which block the flow of water to the rivers and sounds in the eastern part of the state and to the Atlantic Ocean. The lack of flushing which results could lead to serious environmental damage from bacterial overload in instances such as hog waste spills. For this reason, relationships between culturable levels of estuarine bacteria, total coliforms, Escherichia coli, Vibrio spp., and V. vulnificus were investigated at six estuarine sites. In addition, nine chemical and physical parameters were measured at each site. Statistically significant relationships existing between these environmental parameters and the isolation of vibrios and, more specifically, V. vulnificus were determined.
MATERIALS AND METHODS
Sample collection and parameter measurements.
During the 22-month period between July 2000 and April 2002, 1,000-ml water grab samples were collected monthly for 18 months (no samples were collected from July to October 2001) from six sites along the Neuse, Pungo, and Pamlico Rivers of North Carolina (Fig. 1). During the last six months of the study, sediment grab samples were also collected. Water and sediment samples were transported on ice from each site to the laboratory (a maximum of 4 h) for bacteriological analysis. Environmental parameters measured at each site included water temperature, salinity, turbidity, and pH and levels of ferrous iron, phosphate, ammonia nitrogen, dissolved organic carbon (DOC), and dissolved oxygen. Water temperature was measured with a waterproof thermometer (Fisher Scientific, Pittsburgh, Pa.). Salinity was measured with a handheld refractometer (Schuco, Chiyoda-ku, Japan). Turbidity and pH and levels of ferrous iron, phosphate, ammonia nitrogen, and dissolved oxygen were measured by using reagents and a DR/850 portable colorimeter according to the instructions of the manufacturer (Hach Co., Love, Colo.). In order to measure DOC levels, water was filtered through a 47-mm cellulose nitrate membrane with a 0.2-μm pore size (Nalgene, Rochester, N.Y.) and kept at −20°C until measured with a Total Organic Carbon Analyzer (TOC-5000A) with an ASI-5000A autosampler (Shimadzu Instruments, Inc., Kyoto, Japan). Background levels of DOC present in these membranes were determined and subtracted from the DOC values of each water sample analyzed.
FIG. 1.
Locations of the six estuarine sites in eastern North Carolina.
Bacterial enumeration.
On each sampling trip, water taken from each of the six sites was plated directly onto half-strength modified salt water-yeast extract agar (31) for enumeration of CFU of estuarine bacteria, thiosulfate-citrate-bile salts-sucrose (TCBS) agar (Difco, Sparks, Md.) for enumeration of CFU of Vibrio spp., and colistin-polymyxin B-cellobiose (CPC) agar (20) for detection of V. vulnificus. Plates were incubated at 22, 37, and 40°C, respectively. In addition, 10 g of sediment was diluted with 90 ml of one-half-strength artificial seawater (48) and plated directly onto half-strength modified salt water-yeast extract, TCBS, and CPC. When water temperatures dropped below ca. 13°C, 25 to 50 ml of sample water was filtered through a 0.2-μm-pore-size cellulose nitrate membrane filter (Nalgene), which was plated onto CPC agar to increase the detection limit of V. vulnificus. Similarly, between November 2001 and April 2002, 10 to 50 ml of water was filtered and plated onto TCBS to increase the detection of Vibrio spp. Water was also routinely filtered through 0.2-μm-pore-size filters, which were incubated in alkaline peptone water (45) at 37°C for 12 to 18 h prior to being plated on CPC as an enrichment for V. vulnificus. This procedure was used for detection, rather than for enumeration, of this pathogen. For total coliforms and E. coli, 1 to 10 ml of water was filtered through a gridded 0.45-μm-pore-size mixed cellulose ester filter (Millipore, Bedford, Mass.) and incubated with M-ColiBlue24 broth (Hach Co.) for 18 to 24 h at 34.5°C.
Identification methods.
Following purification, cells from sucrose-negative colonies on TCBS and cellobiose-positive colonies on CPC were confirmed as V. vulnificus by hemolysin gene PCR, by colony hybridization, or by both methods. For hemolysin gene PCR, cells were grown overnight at 22°C in heart infusion broth (Difco, Detroit, Mich.) and cell lysates were prepared as follows: 200 μl of the broth culture was centrifuged, resuspended in 200 μl of filtered, autoclaved, deionized water, and boiled for 5 min. PCR was conducted using the cycling parameters described by Coleman and Oliver (3) in a Genius thermal cycler (Techne, Princeton, N.J.). Briefly, cell lysates (5 μl) were added to a master mix consisting of 17.75 μl of diethyl pyrocarbonate-treated water, 5 mM (each) deoxynucleoside triphosphates (8 μl; Promega, Madison, Wis.), 20 mM (each) primers (0.09 μl; Bio-Synthesis, Lewisville, Tex.), 5 U (0.25 μl) of Taq polymerase (Promega), 25 mM (3.2 μl) MgCl2 (Promega), and 10× (4 μl) Mg-free buffer (Promega) for a final reaction volume of 40 μl. The hemolysin gene was amplified by using 24-bp oligonucleotides that are specific for a 340-bp fragment located within this 1,416-bp gene unique to V. vulnificus (23). The primers utilized were Vv1 (5′-CGC CGC TCA CTG GGG CAG TGG CTG-3′) and Vv2 (5′-CCA GCC GTT AAC CGA ACC ACC CGC-3′). For all experiments, a negative control containing all PCR reagents and sterile heart infusion broth was employed. The reaction mixture was overlaid with 20 μl of sterile mineral oil. For visualization, gel electrophoresis was performed using a 2% agarose gel (NuSieve 3:1; BioWhittaker Molecular Applications, Rockland, Maine) with PCR products that were stained with ethidium bromide (1.25 μg/ml).
Some V. vulnificus colonies were confirmed by using a colony hybridization probe (13) employing an alkaline phosphatase-conjugated oligonucleotide (DNA Technology A/S, Aarhus, Denmark) with the sequence 5′ XCG GCT GTC ACG GCA GTT GGA ACC A 3′, which detects the V. vulnificus hemolysin gene.
Statistical analysis.
A one-way analysis of variance (37) was conducted, analyzing the effect of site on each environmental parameter. The sequential Bonferroni adjustment (33) was then used to determine significance levels, assuring that the experimentwise error did not exceed 5%. When it was demonstrated that salinity (F = 7.51; P ≤ 0.01) and turbidity (F = 4.09; P ≤ 0.05) measurements differed based on sampling site, an unplanned comparison using the Tukey statistic (43) was then conducted to ascertain which sites were significantly different with regard to salinity and turbidity values.
Environmental parameter data were plotted and log transformed as necessary to achieve normality. Linear regressions were conducted, followed by examination of the standardized residuals to determine if linear relationships existed. A standardized multiple regression analysis (37) was then completed.
In order to compare our data to the correlations reported in previously published research, Spearman's coefficient of rank correlation (37), followed by the sequential Bonferroni adjustment, was used to detect correlations between the presence of vibrios and V. vulnificus and each of the environmental parameters that were measured.
RESULTS AND DISCUSSION
Distribution of Vibrio spp.
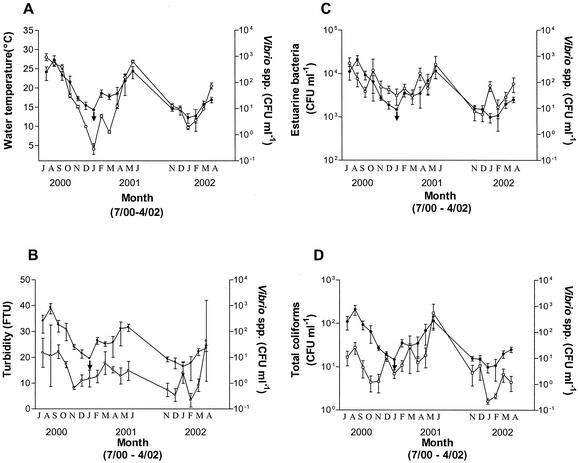
Analysis of our environmental data with Spearman's correlation coefficient (rs) revealed that the isolation of Vibrio spp. was positively correlated with water temperature (rs = 0.65449; P < 0.01), turbidity (rs = 0.29081; P < 0.05), the presence of estuarine bacteria (rs = 0.41151; P < 0.01), and levels of total coliforms (rs = 0.34935; P < 0.01) and negatively correlated with dissolved oxygen levels (rs = −0.30737; P < 0.05). As also reported by Barbieri et al. (1), we found water temperature to be the parameter most highly correlated with the isolation of Vibrio spp. at our sample sites (Fig. 2A). Figure 2B and C show correlations between the isolation of vibrios and estuarine turbidity and bacteria, respectively. These indicate that levels of Vibrio spp. and both levels of estuarine bacteria and turbidity vary together in a positive manner. Earlier studies by Oliver et al. (29, 30) also reported a positive correlation between Vibrio spp., estuarine bacteria, and turbidity.
FIG. 2.
Relationship between numbers of CFU of Vibrio spp. and four of the environmental parameters measured at the six estuarine sites. Averages of numbers of CFU and of each parameter were taken for each month from July 2000 to April 2002 (no data were obtained for July through October 2001). (A) Vibrio spp. (○) and water temperature (•). (B) Vibrio spp. (○) and turbidity (•) in formazan turbidity units (FTU). (C) Vibrio spp. (○) and estuarine bacteria (•). (D) Vibrio spp. (○) and total coliforms (•). Names of months are abbreviated by their first letters.
Koh et al. (16) reported that correlations between vibrios and indicator bacteria, such as total and fecal coliforms, enterococci, and E. coli, were either negative or not present at two test sites in Apalachicola Bay, Florida. In contrast, and in agreement with the results of an earlier study from our laboratory (29), we found the frequencies of isolation of Vibrio spp. and total coliforms to vary together (Fig. 2D); statistical analysis also indicated a positive correlation between these two groups. This is a substantial finding for the rivers along the eastern coast of North Carolina, where waste runoff from hog farms is a principal concern for the preservation of the natural environment. According to our data, monitoring total coliform counts could be useful in indicating the level of Vibrio spp.
Whereas most environmental studies have performed correlation analyses to determine significant relationships between physicochemical and bacteriological parameters, we employed a standardized multiple regression analysis to determine which parameters accounted for the variability in the frequency of vibrio isolation. As expected, water temperature (coefficient of multiple determination [R2] = 0.4819; standardized partial regression coefficient [b′] = 0.64146) accounted for most (48%) of the variability in the retrieval of this genus from the environment (Table 1). As reported by Koh et al. (16), Vibrio spp. were difficult to isolate during the cold-weather months, with few vibrios isolated when water temperatures were ca. <10°C. Only during the last 6 months of the study, when filtration was used to increase the limit of detection, were Vibrio spp. isolated at this temperature from nearly all sites.
TABLE 1.
Water temperature, estuarine bacterial numbers, levels of phosphorous and ammonia nitrogen, salinity, turbidity, and pH account for 48, 7, 3, 2, 2, 2, and 2% of the change in the level of Vibrio spp., respectively (see R2 below)
| Environmental parameter | R2 | b′ | P value |
|---|---|---|---|
| Water temperature | 0.4819 | 0.64146 | <0.01 |
| Number of estuarine bacteria | 0.0772 | 0.31181 | <0.01 |
| Phosphorous level | 0.0303 | 0.16405 | <0.01 |
| Ammonia nitrogen level | 0.0248 | −0.21605 | <0.05 |
| Salinity | 0.0207 | 0.21461 | <0.05 |
| Turbidity | 0.0203 | 0.18568 | <0.05 |
| pH | 0.0184 | −0.13912 | <0.05 |
To a lesser extent, the quantity of estuarine bacteria (R2 = 0.0772; b′ = 0.31181), phosphorous levels (R2 = 0.0303; b′ = 0.16405), ammonia nitrogen levels (R2 = 0.0248; b′ = −0.21605), salinity (R2 = 0.0207; b′ = 0.21461), turbidity (R2 = 0.0203; b′ = 0.18568), and pH (R2 = 0.0184; b′ = −0.13912) were responsible for a small but statistically significant amount (2 to 8%) of the variability in Vibrio spp. isolation (Table 1). Together with water temperature, these parameters accounted for 67% of the variability associated with the isolation of vibrios from the environment. Thus, it appears that no single parameter primarily affects the isolation of vibrio levels in these estuarine environments.
Distribution of V. vulnificus.
Quantities of estuarine bacteria ranged from 2.3 × 102 to 6 × 104 CFU ml−1 among the six sampling sites, with Vibrio spp. averaging 2% of these levels. While V. vulnificus represented an average of 7.7% of the Vibrio spp. cultured from all sites, the average monthly levels of this species ranged from <0.01 to 23 CFU ml−1, with the highest concentrations detected during the warm-weather months. These results are similar to those of other environmental studies in which V. vulnificus was isolated from estuarine waters (19, 32, 42). Total coliform and E. coli levels ranged from 1.3 to 4.6 × 102 CFU ml−1 and <0.1 to 28 CFU ml−1, respectively.
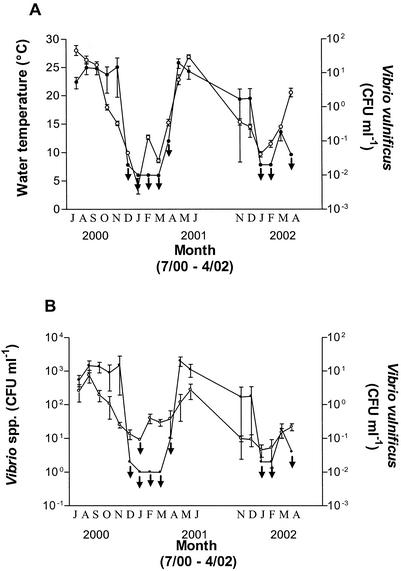
Previous studies along the Gulf of Mexico and the eastern and western coasts of the United States have shown strong relationships between water temperature, salinity, and the isolation of V. vulnificus (5, 14, 15, 25, 29, 30, 32, 40, 46). Generally, estuarine waters with salinities between 6 and 16 ppt have been found most likely to sustain populations of this bacterium, whereas low water temperatures (<10°C) have a negative effect on its isolation. In the present study, temperatures ranged from 0.9 to 30.7°C. Consistent with findings of previous studies, V. vulnificus isolation was most prevalent at the six sample sites when average water temperatures ranged from 15 to 27°C with average salinity levels between 8 and 14 ppt. While V. vulnificus isolation from environments with temperatures as low as 11°C has been reported (5), V. vulnificus was not isolated in the present study between December 2000 and March 2001 or between January and February of 2002, when water temperatures were <14°C. The extremely close relationship between the isolation of V. vulnificus and water temperature is shown in Fig. 3A.
FIG. 3.
Relationship between average numbers of CFU of V. vulnificus (○) and average water temperatures (•) (A) and average numbers of CFU of Vibrio spp. (•) (B). Average bacterial counts and temperatures were determined for each month from July 2000 to April 2002 (no data were obtained for July through October 2001). Arrows indicate culturability below the limit of detection. Names of months are abbreviated by their first letters.
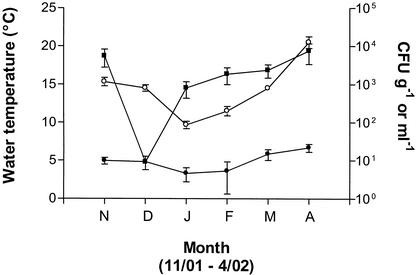
There have been several suggestions offered to explain the seasonality observed with the isolation of V. vulnificus. One possible reason that this bacterium is difficult to culture during cold-weather months is that V. vulnificus enters a viable but nonculturable state (26, 27). Oliver et al. (31) demonstrated this phenomenon in situ in an estuarine environment through the use of membrane diffusion chambers. In that study, when water temperatures averaged <15°C, V. vulnificus cells no longer grew on routine media but were still alive, as indicated by direct viable counts. In the same study, exit from the viable but nonculturable state (“resuscitation”) during warm-weather months (water temperatures of 22 to 24°C) was also demonstrated. The decrease in frequency of V. vulnificus isolation from estuarine waters in winter months has also been suggested to result from an overwintering of the cells in sediment, fish, or oysters (5, 41, 46). In the present study, while the occurrence of Vibrio spp. was more frequent in sediment than in water (Fig. 4), only a single V. vulnificus isolate was obtained from sediment during the 6 months that sediments were studied. This particular strain was obtained in November when the water temperature was 15°C, but V. vulnificus was also isolated from the water at this site during November. It is not clear why sediments did not contain more V. vulnificus cells. Most of the sediments taken from these sites were either very grainy or made up of more clay than sand, which may not have been suitable for the persistence of this species.
FIG. 4.
Occurrence of Vibrio spp. (▪) and estuarine bacteria (•) in sediments. Also shown are average water temperatures (○) during the sampling period of November 2001 through April 2002. Vibrio spp. and estuarine bacteria counts (in CFU g of sediment−1) are averages of those from the six estuarine sites. Names of months are abbreviated by their first letters.
Results demonstrated a positive correlation between the occurrence of V. vulnificus and water temperature (rs = 0.81845; P < 0.01), and multiple regression analysis indicated that 47% of the variability in V. vulnificus isolation depended upon water temperature (R2 = 0.4693; b′ = 0.68502). This is very similar to data reported by Motes et al. (24), who found that water temperature accounted for 60% of the change in the frequency of V. vulnificus isolation from oysters.
Contrary to the findings of most environmental studies of this bacterium (9, 19, 24, 30, 31, 32, 40), salinity was not correlated with V. vulnificus isolation frequency in our study, nor did it have an apparent effect on the variability of V. vulnificus isolation from estuarine waters. However, salinities measured at our study sites were always between 5 and 20 ppt, values within the optimal growth range for V. vulnificus (12, 15, 32). Thus, it is likely that no significant relationship was found between salinity and the detection of V. vulnificus because salinity levels at our study sites were not limiting to the growth of this bacterium.
While other studies have examined dissolved oxygen and its relationship to the isolation of Vibrio spp. (1, 34), this parameter has not been reported to correlate with the presence of V. vulnificus in estuarine waters. A negative correlation was demonstrated between dissolved oxygen level and the isolation of V. vulnificus (rs = −0.48619; P < 0.01) in the present study. This is understandable since water temperature and dissolved oxygen level are negatively correlated (i.e., when the temperature of the water increases, the level of dissolved oxygen decreases).
A previous study by Høi et al. (8) observed no relationship between the occurrence of Vibrio spp. and V. vulnificus. Further, a report prepared for the European Commission (7) suggested that a correlation between Vibrio spp. and V. vulnificus should not be expected as the presence of vibrios is not necessarily indicative of the presence of pathogenic Vibrio spp. However, a correlation between the occurrence of Vibrio spp. (rs =0.65761; P < 0.01) and V. vulnificus was detected in the present study (Fig. 3B). While water temperature and salinity in the Danish estuarine environments were similar to those of our estuarine sites, the involvement of other parameters not accounted for in either study (see below) might explain these conflicting results.
Unlike the studies of Tamplin et al. (40), who reported correlations between the density of V. vulnificus and the presence of fecal coliforms, and Høi et al. (8), who reported a correlation with total coliforms, the present study revealed no such relationships. This finding agrees with those of several earlier studies (29, 30, 32) that also reported no correlation between V. vulnificus and the presence of fecal coliforms.
While other studies have also reported significant relationships between the isolation of V. vulnificus and both turbidity and pH (29, 30, 40), the statistical analyses in the present study determined no such results. Jones and Summer-Brason (9) reported that turbidity was the major factor affecting the concentrations of V. vulnificus isolated from northeastern U.S. estuarine waters, with few or no isolates of V. vulnificus obtained when total suspended solids were especially high. Although no statistically significant relationship was detected in our study, this could explain why V. vulnificus isolates made up a low percentage of vibrios at those sites with the highest turbidity values. It is likely that pH was not a significant variable in the present study because the values at the six sample sites were always within a range (6.4 to 8.7) that readily supports the growth of V. vulnificus (44).
In summary, no significant relationships were found between the isolation of V. vulnificus and pH, turbidity, salinity, or levels of ferrous iron, ammonia nitrogen, phosphorous, DOC, estuarine bacteria, total coliforms, or E. coli. Only water temperature accounted for any variability (47%) in the frequency of V. vulnificus isolation. This suggests that another unknown variable(s) is responsible for 53% of the variability in the abundance of V. vulnificus. Other researchers (4, 11, 36, 47) have suggested that the presence of host organisms, such as copepods or crabs, may have an effect on certain Vibrio spp. because of the nutritional value of the chitinous exoskeletons of these animals. Kaneko and Colwell (10) reported that Vibrio spp. make up a large percentage of the bacteria associated with plankton in warm-weather months and decrease substantially in the winter months when water temperatures drop. Alternatively, certain protozoa that feed upon, or bacteriophages that infect, Vibrio spp. may have a deleterious effect on the presence of vibrios, including V. vulnificus.
A variety of pathogenic vibrios, including V. vulnificus, are routinely isolated from estuarine waters worldwide. Several of these environments are used not only for recreational purposes, such as swimming and water sports, but also for occupational purposes, such as fishing and crabbing. If relationships between one or more environmental parameters and the incidence of V. vulnificus can be elucidated, it may be possible to restrict recreational and occupational activities during those periods when the levels of this pathogen are increased. By doing so, it may be possible to reduce the incidence of the potentially fatal infections which are caused by this bacterium.
REFERENCES
- 1.Barbieri, E., L. Falzano, C. Fiorentini, A. Pianetti, W. Baffone, A. Fabbri, P. Matarrese, A. Casiere, M. Katouli, I. Kühn, R. Möllby, F. Bruscolini, and G. Donelli. 1999. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl. Environ. Microbiol. 65:2748-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, K.-Y., M. L. Woo, K. W. Lo, and G. L. French. 1986. Occurrence and distribution of halophilic vibrios in subtropical coastal waters of Hong Kong. Appl. Environ. Microbiol. 52:1407-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman, S. S., and J. D. Oliver. 1996. Optimization of conditions for the polymerase chain reaction amplification of DNA from culturable and nonculturable cells of Vibrio vulnificus. FEMS Microbiol. Ecol. 19:127-132. [Google Scholar]
- 4.Davis, J. W., and R. W. Sizemore. 1982. Incidence of Vibrio species associated with blue crabs (Callinectes sapidus) collected from Galveston Bay, Texas. Appl. Environ. Microbiol. 43:1092-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DePaola, A., G. M. Capers, and D. Alexander. 1994. Densities of Vibrio vulnificus in the intestines of fish from the U.S. Gulf Coast. Appl. Environ. Microbiol. 60:984-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmore, S. P., J. A. Watts, L. M. Simpson, and J. D. Oliver. 1992. Reversal of hypotension induced by Vibrio vulnificus lipopolysaccharide in the rat by inhibition of nitric oxide synthase. Microb. Pathog. 13:391-397. [DOI] [PubMed] [Google Scholar]
- 7.Gerner-Smidt, P. 2001. Opinion of the scientific committee on veterinary measures relating to public health on Vibrio vulnificus and Vibrio parahaemolyticus (in raw and undercooked seafood). European Commission Health and Consumer Protection Directorate-General, Brussels, Belgium.
- 8.Høi, L., J. L. Larsen, I. Dalsgaard, and A. Dalsgaard. 1998. Occurrence of Vibrio vulnificus biotypes in Danish marine environments. Appl. Environ. Microbiol. 64:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, S. H., and B. Summer-Brason. 1998. Detection of pathogenic Vibrio sp. in a northern New England estuary, USA. J. Shellfish Res. 17:1665-1669. [Google Scholar]
- 10.Kaneko, T., and R. R. Colwell. 1973. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J. Bacteriol. 113:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko, T., and R. R. Colwell. 1975. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl. Microbiol. 29:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaspar, C. W., and M. L. Tamplin. 1993. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl. Environ. Microbiol. 59:2425-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaysner, C. A., and A. DePaola, Jr. 2001. Vibrio, p. 405-420. In F. P. Downes and K. Ito (ed.), Compendium of methods for the microbiological examination of foods. American Public Health Association, Washington, D.C.
- 14.Kaysner, C. A., C. Abeyta, Jr., M. M. Wekell, A. DePaola, Jr., R. F. Scott, and J. M. Leitch. 1987. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States west coast. Appl. Environ. Microbiol. 53:1349-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly, M. T. 1982. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl. Environ. Microbiol. 44:820-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh, E. G. L., J. H. Huyn, and P. A. LaRock. 1994. Pertinence of indicator organisms and sampling variables to Vibrio concentrations. Appl. Environ. Microbiol. 60:3897-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine, W. C., P. M. Griffin, and the Gulf Coast Working Group. 1993. Vibrio infections on the Gulf Coast: results of first year of regional surveillance. J. Infect. Dis. 167:479-483. [DOI] [PubMed] [Google Scholar]
- 18.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 19.Lipp, E. K., C. Rodriguez-Palacios, and J. B. Rose. 2001. Occurrence and distribution of the human pathogen Vibrio vulnificus in a subtropical Gulf of Mexico estuary. Hydrobiologia 460:165-173. [Google Scholar]
- 20.Massad, G., and J. D. Oliver. 1987. New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl. Environ. Microbiol. 53:2262-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McPherson, V. M., J. A. Watts, L. M. Simpson, and J. D. Oliver. 1991. Physiological effects of the lipopolysaccharide of Vibrio vulnificus on mice and rats. Microbios 67:141-149. [PubMed] [Google Scholar]
- 22.Merkel, S. M., S. Alexander, E. Zufall, J. D. Oliver, and Y. M. Huet-Hudson. 2001. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect. Immun. 69:6119-6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris, J. G., Jr., A. C. Wright, D. M. Roberts, P. K. Wood, L. M. Simpson, and J. D. Oliver. 1986. Identification of environmental Vibrio vulnificus isolates with a DNA probe for the cytotoxin-hemolysin gene. Appl. Environ. Microbiol. 53:193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motes, M. L., A. DePaola, D. W. Cook, J. E. Veazey, J. C. Hunsucker, W. E. Garthright, R. J. Blodgett, and S. J. Chirtel. 1998. Influence of water temperature and salinity on Vibrio vulnificus in northern Gulf and Atlantic coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 64:1459-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver, J. D. 1989. Vibrio vulnificus, p. 569-600. In M. P. Doyle (ed.), Foodborne bacterial pathogens. Marcel Dekker, Inc., New York, N.Y.
- 26.Oliver, J. D. 1993. Formation of viable but nonculturable cells, p. 239-272. In S. Kjelleburg (ed.), Starvation in bacteria. Plenum Press, New York, N.Y.
- 27.Oliver, J. D. 2000. The public health significance of viable but nonculturable bacteria, p. 277-300. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 28.Oliver, J. D., and J. B. Kaper. 2001. Vibrio species, p. 228-264. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers. ASM Press, Washington, D.C.
- 29.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1982. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl. Environ. Microbiol. 44:1404-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1983. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl. Environ. Microbiol. 45:985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver, J. D., F. Hite, D. McDougald, N. L. Andon, and L. M. Simpson. 1995. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl. Environ. Microbiol. 61:2624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Neill, K. R., S. H. Jones, and D. J. Grimes. 1992. Seasonal occurrence of Vibrio vulnificus in the Great Bay Estuary of New Hampshire and Maine. Appl. Environ. Microbiol. 58:3257-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice, W. R. 1989. Analyzing tables of statistical tests. Evolution 43:223-225. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, N. C., R. J. Siebeling, J. B. Kaper, and H. B. Bradford, Jr. 1982. Vibrios in the Louisiana Gulf Coast environment. Microb. Ecol. 8:299-312. [DOI] [PubMed] [Google Scholar]
- 35.Simpson, L. M., V. K. White, S. F. Zane, and J. D. Oliver. 1987. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 55:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sochard, M. R., D. F. Wilson, B. Austin, and R. R. Colwell. 1979. Bacteria associated with the surface and the gut of marine copepods. Appl. Environ. Microbiol. 37:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokal, R. R., and F. J. Rohlf. 1995. Biometry: the principles and practice of statistics in biological research, 3rd ed. W. H. Freeman and Company, New York, N.Y.
- 38.Starks, A. M., T. R. Schoeb, M. L. Tamplin, S. Parveen, T. J. Doyle, P. E. Bomeisl, G. M. Escudero, and P. A. Gulig. 2000. Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 68:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 40.Tamplin, M., G. E. Rodrick, N. J. Blake, and T. Cuba. 1982. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl. Environ. Microbiol. 44:1466-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamplin, M. L. 1994. The seasonal occurrence of Vibrio vulnificus in shellfish, seawater, and sediment of United States coastal waters and the influence of environmental factors on survival and virulence. Final report to Saltonstal-Kennedy Grant Program. Project NA27.FD0117-01. U.S. Department of Commerce, Seattle, Wash.
- 42.Tilton, R. C., and R. W. Ryan. 1987. Clinical and ecological characteristics of Vibrio vulnificus in the northeastern United States. Diagn. Microbiol. Infect. Dis. 6:109-117. [DOI] [PubMed] [Google Scholar]
- 43.Tukey, J. W. 1977. Exploratory data analysis. Addison-Wesley, Reading, Mass.
- 44.U.S. Food and Drug Administration. 1998. Fish and fishery products hazards and controls guide. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Washington, D.C.
- 45.U.S. Food and Drug Administration. 2001. FDA bacteriological analytical manual. [Online.] http://www.cfsan.fda.gov/∼ebam/m10.html.
- 46.Vanoy, R. W., L. Tamplin, and J. R. Schartz. 1992. Ecology of Vibrio vulnificus in Galveston Bay oysters, suspended particulate matter, sediment and seawater: detection by monoclonal antibody—immunoassay—most probable number procedures. J. Ind. Microbiol. 9:219-223. [Google Scholar]
- 47.Williams, L. A., and P. A. LaRock. 1985. Temporal occurrence of Vibrio species and Aeromonas hydrophila in estuarine sediments. Appl. Environ. Microbiol. 50:1490-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf, P. W., and J. D. Oliver. 1992. Temperature effects on the viable but non-culturable state of Vibrio vulnificus. FEMS Microbiol. Ecol. 101:33-39. [Google Scholar]
- 49.Wright, A. C., L. M. Simpson, and J. D. Oliver. 1981. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]