Abstract
Toxin complex a (Tca), a high molecular weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens, has been found to be orally toxic to both the Colorado potato beetle, Leptinotarsa decemlineata, and the sweet potato whitefly, Bemisia tabaci biotype B. The 48 hour LC50 for Tca against neonate L. decemlineata was found to be 2.7 ppm, and the growth of 2nd instar L. decemlineata exposed to Tca for 72 hours was almost entirely inhibited at concentrations above 0.5 ppm. B. tabaci was highly susceptible to Tca as well; newly emerged nymphs that were artificially fed Tca developed poorly, or not at all. Tca concentrations between 0.1 and 0.2 ppm reduced the number of nymphs reaching the second instar by 50%. In addition, a preparation of Tca missing two prominent subunits, TcaAii and TcaAiii, was found to be at least as toxic to L. decemlineata and B. tabaci as Tca itself, indicating that the activity of Tca is not dependant on the presence of these subunits at the time of ingestion.
| Abbreviations | |
|---|---|
| Tca, Tcb, Tcc, Tcd |
toxin complex a through d Toxin complex a (Tca), a high molecular weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens, has been found to be orally toxic to both the Colorado potato beetle, Leptinotarsa decemlineata, and the sweet potato whitefly, Bemisia tabaci biotype B. The 48 hour LC50 for Tca against neonate L. decemlineata was found to be 2.7 ppm, and the growth of 2nd instar L. decemlineata exposed to Tca for 72 hours was almost entirely inhibited at concentrations above 0.5 ppm. B. tabaci was highly susceptible to Tca as well; newly emerged nymphs that were artificially fed Tca developed poorly, or not at all. Tca concentrations between 0.1 and 0.2 ppm reduced the number of nymphs reaching the second instar by 50%. In addition, a preparation of Tca missing two prominent subunits, TcaAii and TcaAiii, was found to be at least as toxic to L. decemlineata and B. tabaci as Tca itself, indicating that the activity of Tca is not dependant on the presence of these subunits at the time of ingestion. |
Keywords: entomopathogenic bacteria, Photorhabdus luminescens, Tca, toxin complex
Introduction
The widespread deployment of transgenic crops expressing δ-endotoxins of Bacillus thuringiensis has evoked widespread concern over the potential development of pest resistance to the toxins. One means of averting or delaying the development of such resistance is the deployment of toxins with alternate modes of action. Bowen and Ensign (1998) demonstrated that P. luminescens produced high molecular weight toxin complexes that were orally toxic to insects. Bowen et al . (1998) subsequently isolated several of these toxins, and elucidated the genes that encode them. Since that time, a great deal of progress has been made in describing the arrangement of toxin genes in P. luminescens (Waterfield et al., 2002; Duchaud et al., 2003), and in determining the minimal genetic requirements for the expression of active toxins (Waterfield et al., 2001). In addition, other insect-associated bacteria have been shown to produce, or possess genes that encode, homologues of these toxin complexes. Similar toxin loci have been described from the taxonomically related Xenorhabdus nematophilus (Morgan et al., 2001, as well as Serratia entomophila (Hurst et al., 2000) and Yersinia pestis (Parkhill et al., 2001).
An important consideration in the development of any insecticide is the activity spectrum. Although a great deal is now known about the toxin genes and their arrangement, comparatively little information is available on the specific activities of these toxin complexes for different insect species. The oral activity of three isolated toxin complexes from P. luminescens W14 have been described in relative detail. Bowen et al . (1998) assayed Toxin complex a (Tca) against neonate Manduca sexta, and found that the 7- day LC50 was 875 ng per cm2 of diet, with a substantial suppression of growth at much lower concentrations; at 40 ng per cm2, larvae gained weight at only 14% of their normal rate. Guo et al. (1999) determined that the LC50s of Tcd and Tcb against southern corn rootworm were 5 and 87 ng per cm2 of diet. Against Ostrinia nubilalis and M. sexta, Tcd was found to have LC50s of 93 and 63 ng per cm2 of diet, while LC50s for Tcb were >5000 and >2500 ng per cm2 for these insects, respectively (Ensign, 1998).
The tca locus consists of four open reading frames: tcaA, tcaB, and tcaC, which are transcribed in the same direction, and a terminal tcaZ, which is in the opposite orientation. Products of the tcaA, tcaB, and tcaC genes are ultimately represented in the toxin complex. The TcaA and TcaB proteins are proteolytically cleaved into the smaller TcaAi, TcaAii, TcaAiii, TcaBi, and TcaBii subunits (Figure 1). Mature Tca consists primarily of TcaAii, TcaAiii, TcaBi, TcaBii, and TcaC, with variable quantities of partially cleaved subunits such as TcaAii+TcaAiii (Bowen et al., 1998; Blackburn et al., 1998). In contrast, the mature Tcb or Tcd proteins are the products of single genes. Like the TcaA-TcaB proteins, TcbA and TcdA are proteolytically cleaved in the fully processed toxin complexes (Bowen et al., 1998; Guo et al., 1999). Tca, Tcb, and Tcd share significant similarities. Tcb and Tcd are 51.6% identical and they, in turn, share similarities with TcaA and TcaB. The N-terminal half of either TcbA or TcdA shares some similarities with TcaA, while the C-terminal half of TcbA/dA shares striking similarities to TcaB. Thus, if considered sequentially, TcaA and TcaB constitute a homologue of Tcb/d (Waterfield et al., 2001).
Figure 1.

Schematic representation of the tca and tcd genetic loci with their respective products superimposed upon them. Similarly shaded areas indicate regions of similar amino acid sequences. Proteolytic cleavage sites are indicated by arrowheads. Peptides which have been determined to occur in the mature Tca and Tcd complexes are labeled in red italics (Bowen et al., 1998; Guo et al., 1999).
Thus far, the toxicity of Tca from P. luminescens W14 has only been documented against M. sexta . In order to determine the toxicity of Tca against other insects, we isolated Tca from P. luminescens W14 and tested it against both the Colorado potato beetle (Leptinotarsa decemlineata) and the sweet potato whitefly (Bemisia tabaci biotype B). In addition, we have isolated Tca that was lacking two proteolytically cleaved subunits, TcaAii and TcaAiii, and tested the remaining complex against CPB and SPWF.
Materials and Methods
Bacterial culture
P. luminescens W14 (ATCC 55397) was maintained on plates containing 2% Proteose Peptone #3 (PP3) and 1.6% agar (Difco Laboratories, www.voigtglobal.com/DIFCO.htm). For toxin production, the bacteria were cultured in 1 liter flasks containing 200 ml of 2% PP3 and 0.5% Tween 60; the cultures were 3 liters in total. The flasks were maintained at 29° C for 72 h on an orbital shaker at 200 rpm. After 72 h, the cultures were centrifuged for 30 min at 6000 × g. The supernatant was decanted and retained for purification of toxins.
Preparation of a crude toxin fraction
The preparation of a crude toxin fraction was similar to that described by Bowen and Ensign (1998). Briefly, 150 ml of DEAE Sephacel equilibrated with 50 mM K2HPO4 was batch-mixed with 3 liters of culture supernatant, mixed on a drum roller for 3 h, then allowed to settle. Excess supernatant was decanted and discarded. The DEAE Sephacel slurry was poured into a 5 cm × 30 cm column, and washed with 50 mM K2HPO4 until the 280 nm absorbance had stabilized. Toxins were eluted with 300 mM KCl. The eluted material was concentrated in a stirred ultrafiltration cell with a 100 kDa regenerated cellulose membrane to about 20 ml. This concentrated fraction was then applied to a 2.6 cm × 90 cm Sephacryl S400 column in 10 ml aliquots and eluted with 100 mM potassium phosphate buffer (pH 6.9) at 0.3 ml per min. Toxin-containing fractions from the S400 column were identified based on SDS-PAGE analysis, and pooled.
Isolation of toxin complexes
Separations were performed on a Hewlett-Packard 1100 HPLC (Hewlett-Packard GmbH, www.hp.com) equipped with an external injector and 10 ml SuperLoop® (Amersham Pharmacia, www.apbiotech.com). The crude toxin fraction was concentrated by ultrafiltration using a 100 kDa cutoff regenerated cellulose membrane, then diafiltered with 10 mM piperazine-HCl (pH 5.5). Aliquots of the resulting material were loaded on a 1 cm × 7 cm column packed with Source 15Q anion exchanger (Amersham Pharmacia) that was equilibrated with the piperazine-HCl buffer. Proteins were eluted with a 50 min gradient of 0-500 mM KCl in the piperazine-HCl buffer. Fractions containing Tca, based on SDS-PAGE analysis, were pooled and re-fractionated on the Source 15Q column equilibrated with 15 mM triethanolamine-HCl buffer (pH 7.5). Aliquots were diluted 5-fold with the triethanolamine-HCl buffer prior to loading to allow proteins to bind to the exchanger. The bound proteins were then eluted with a 50 min gradient of 0-500 mM KCl in the triethanolamine-HCl buffer. The identity of Tca was verified by SDS-PAGE analysis. The concentration of purified Tca was determined using the Coomassie Plus® protein assay (Pierce, www.piercenet.com) using the manufacturer's protocol with bovine serum albumin serving as the standard. SDS-PAGE analysis of fractions and purified toxins were performed on 10% gels, using the buffer system of Laemmli (1970). The gels were stained with Phast-Blue® (Amersham Pharmacia) according to the manufacturer's instructions.
Bioassay
For CPB assays, serial dilutions of Tca were applied to lyophilized pellets of an artificial diet for CPB (Gelman et al., 2001). The diet pellets were formed by pouring the diet into plastic forms fashioned from pipette tip racks, freezing, and then lyophilizing the diet while still in the forms. The lyophilized pellets weighed 92.5 mg ± 5.5 mg, and each would absorb 200 µ l of water or a Tca dilution for bioassay. Serial dilutions of Tca were prepared in water immediately before applying to the diet. CPB larvae were placed individually on the rehydrated diet pellets in 24-well tissue culture plates. LC50 experiments were conducted for 48 h with neonate larvae, and were replicated on three separate occasions with 12 larvae per treatment. Growth inhibition studies were 72 h in duration using newly ecdysed second instar larvae, and were repeated twice with 12 individuals per treatment. Mortality data from the LC50 experiments was analyzed by Probit analysis (Finney, 1971). Both mortality and growth inhibition data were subjected to regression analysis to a logistic 4-parameter model using SigmaPlot software (SPSS, www.spssscience.com/). Tca missing subunits Aii and Aiii, referred to as Tca(-Aii, Aiii), and TcaAiii were assayed on neonate CPB at concentrations corresponding to the LC10, LC50, and LC90 for Tca; mortality was scored at 48 h. The assays were performed twice, with 12 larvae at each concentration of each complex, and 24 control larvae in each assay.
Sweet potato whitefly assays were performed on nymphs and adults. Whiteflies were reared on a variety of plants in climate-controlled insect chambers or incubators with a 16 h light: 8 h dark photoperiod at 26 ± 2 °C and about 60% relative humidity (Gelman et al, 2002). The technique for artificial feeding of nymphs was as described by Jancovich et al. (1997). The aqueous artificial diet consisted of 5% yeast extract and 30% sucrose. Dilutions of Tca were made in artificial diet and filter sterilized. Surface sterilized 6-day old B. tabaci eggs were placed on the feeding apparatus membranes and allowed to hatch. For the assay of Tca, each dilution was assayed 3-15 times, with 269 to 1719 first instar nymphs and the control diet was assayed 20 times with a total of 2713 first instar nymphs. For comparisons of Tca with Tca(-Aii, Aiii), each dilution was replicated 6 times with a minimum of 608 first instar nymphs tested at each concentration. Insects were maintained for 40 days; at the end of this period the number of whiteflies that reached a given instar at each concentration of toxin and in controls was determined. The effect of Tca(-Aii, Aiii) concentration on adult whitefly longevity was also determined. For adult tests, 1-2 day old adult whiteflies were aspirated from plant leaves into small vials, anaesthetized by brief exposure to CO2 and transferred to small petri dishes. The lid of each petri dish contained an opening sized to fit an inverted sterile 20 ml glass vial. The top of the vial was covered by a stretched piece of Parafilm® held in place by a screw cap into which a 20 mm hole had been drilled. The vials were filled with 1.5 ml of sterile aqueous diet containing 15% sucrose, 10% free amine, and various concentrations of Tca(-Aii, Aiii) prior to sealing with Parafilm, and inversion into the opening in the petri dish cover. Each day the number of surviving adults was determined and the mean day of survival was calculated for each treatment. For each dilution of Tca(-Aii, Aiii) and for the control, there were 12-20 replicates of (30-90) B. tabaci adults.
Histology
Midguts of second instar L. decemlineata fed 1.7 ppm Tca for 24 hours were dissected from the larvae and placed in Bouin's fixative for 24 hours. Following fixation, midguts were washed in 30% ethanol, then dehydrated in an ethanol series (including 1% LiCl in 70% ethanol to remove excess picric acid), transferred to xylene, and embedded overnight in Paraplast-Xtra® (Oxford Labware, www.biobank.co.kr/maker/ooo/oxford-labware.shtml). Embedded tissue was sectioned on a rotary microtome at 5 µm. Sections were relaxed on water at 40° C, mounted on albumin-coated slides, dried, and placed horizontally in a drying oven at 40° C overnight.
Mounted sections were deparaffinized in 3 changes of xylene, transferred through three changes of absolute ethanol and rehydrated through a series of aqueous ethanol solutions. Sections were stained with Carazzi's hematoxylin (Carazzi, 1911) followed by Casson's trichrome (Kiernan, 1990) and examined with a Nikon Eclipse 600 compound microscope (www.nikon.com).
Results
Isolation of toxin complexes
The primary components of the crude toxin fraction prepared as described by Bowen and Ensign (1998) were Tca and Tcb. Sequential fractionation of the crude preparation by anion-exchange chromatography using piperazine-HCl (pH 5.5) and triethanolamine-HCl (pH 7.5) buffer systems yielded highly purified Tca and Tcb. The Source 15Q anion-exchanger used in these separations was found to be much less prone to fouling than that used in previous studies, and when employed with the piperazine-HCl buffer system, adequate resolution of TcA and TcB was achieved (Figure 2A). The final purification of Tca and Tcb was accomplished by discarding fractions between the Tca and Tcb peaks, and re-fractionating each peak using the triethanolamine-HCl buffer system.
Figure 2.
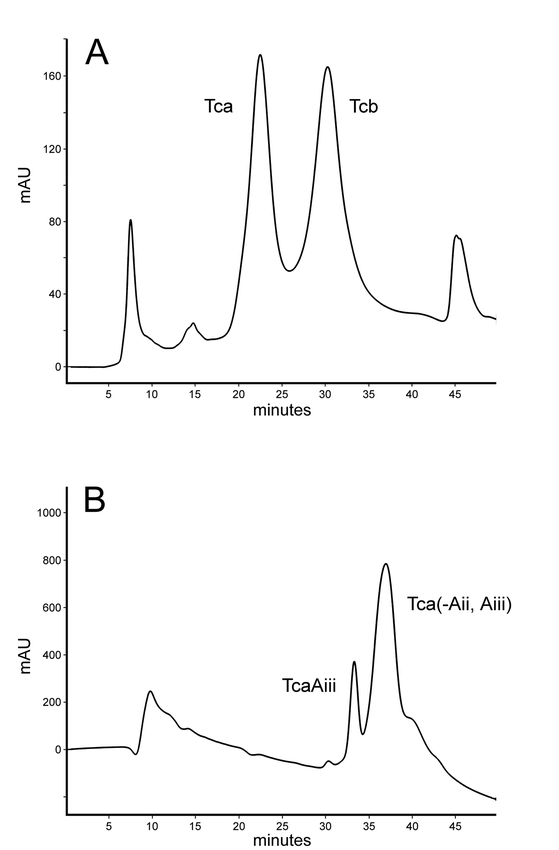
(A) Fractionation of the crude toxin preparation by anion exchange chromatography on a 1 × 7 cm Source 15Q column at pH 5.5. Tca and Tcb represent the major complexes in the crude fraction. Subsequent purifications were performed with the same column at pH 7.5. In one preparation, after prolonged storage at pH 5.5, TcaAiii was separated from the parent Tca (B).
The purity of the Tca produced in this manner was assessed by SDS-PAGE; the purified Tca displayed a banding pattern (Figure 3A) that was virtually identical to that described in earlier reports (Bowen et al., 1998; Blackburn et al., 1998). In these earlier studies, Tca purified by similar methods was shown to migrate as a single band on native agarose gels, and dissociate into the pattern shown in Figure 3A The absence of bands higher in molecular weight than TcaC (the highest band evident) indicates that Tcd/Tcb type complexes were not present in the sample.
Figure 3.
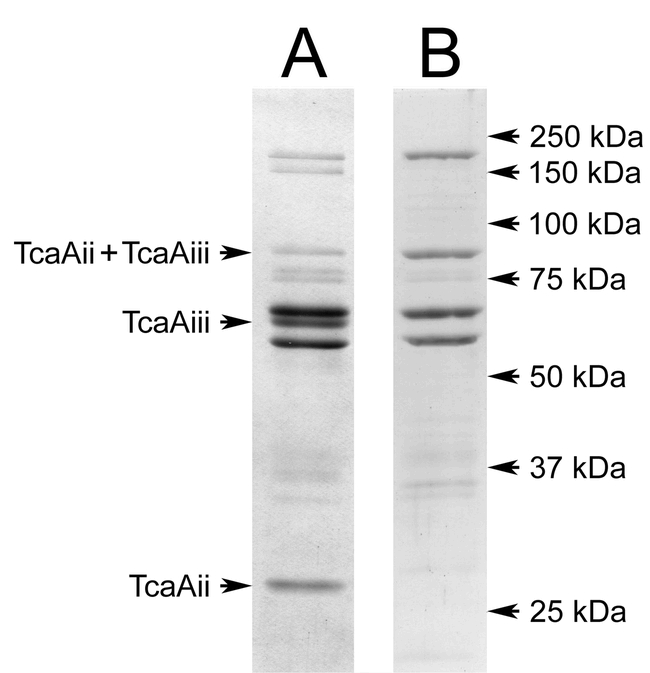
SDS-PAGE analysis of purified Tca. A typical preparation of Tca is shown in lane A, while the Tca(-Aii, Aiii) purified in Figure 2B is shown in lane B.
In one instance, after prolonged storage (ca. 1.5 months) in the piperazine-HCl buffer, subsequent fractionation of Tca in the triethanolamine-HCl buffer system resulted in the loss of TcaAii and TcaAiii subunits; TcaAiii was observed to elute earlier than the remaining complex as a separate peak (Figure 2B), while TcaAii was not recovered. We suspect that this may be due to prolonged exposure to the relatively low pH of the piperazine-HCl buffer. Although lacking cleaved TcaAii and TcaAiii, the Tca (-Aii, Aiii) appeared to possess somewhat more of the un-cleaved TcaAii+TcaAiii than the “typical” Tca preparation used for bioassay in this study (Figure 3B). In addition to the absence of the cleaved TcaAii and TcaAiii subunits, this preparation lacked the band at 150 kDa that is apparent in Figure 3A; this band is often evident in Tca preparations and appears to be a proteolytic fragment of TcaC, based on western-blot analysis using anti-TcaC serum (D. Bowen, personal communication).
The effect of Tca on L. decemlineata larvae
Mortality of neonate L. decemlineata larvae exposed to varying concentrations of Tca for 48 h is shown in Figure 4A. Probit analysis of the mortality data indicates an LC50 of 2.7 ppm Tca, with upper and lower 95% confidence limits of 3.6 ppm and 2.0 ppm, respectively. However, profound growth inhibition was evident at much lower concentrations. With 72 h of exposure, the growth of second instar L. decemlineata was almost entirely inhibited by concentrations of Tca greater than about 0.5 ppm, with an apparent EC50 in the range of 0.1 to 0.2 ppm (Figure 4B). At the highest concentration of Tca tested in the growth inhibition assay (3.4 ppm) 45% of the larvae had died by the end of the experiment. Second instar larvae fed 1.7 ppm Tca for 24 h exhibited severe damage to the midgut epithelium (Figure 5). Tca(-Aii, Aiii) was found to be at least as toxic to neonates as Tca, while TcaAiii was found to be inactive at the concentrations tested (Figure 6).
Figure 4.
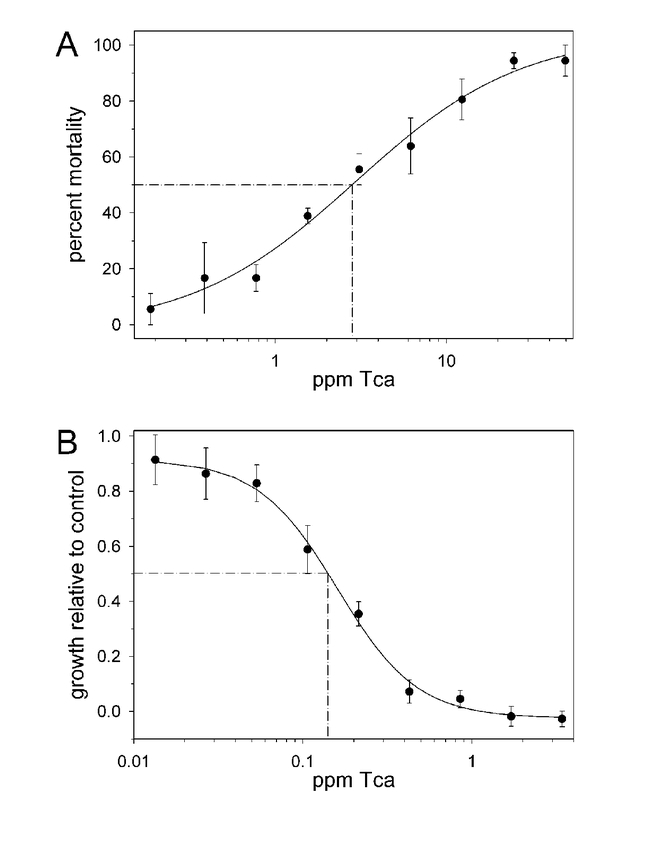
(A) Mortality of neonate Leptinotarsa decemlineata larvae exposed to Tca for 48 h. Each point represents the mean mortality (± SEM) in three separate assays of 12 larvae. (B) Growth of second instar L. decemlineata larvae exposed to Tca for 72 h. Each point represents the mean weight gain (± SEM) of larvae fed Tca relative to control larvae. For each point, 12 larvae were assayed on two separate occasions. At the highest concentrations, mortality was observed (ca. 50% at the highest dose).
Figure 5.
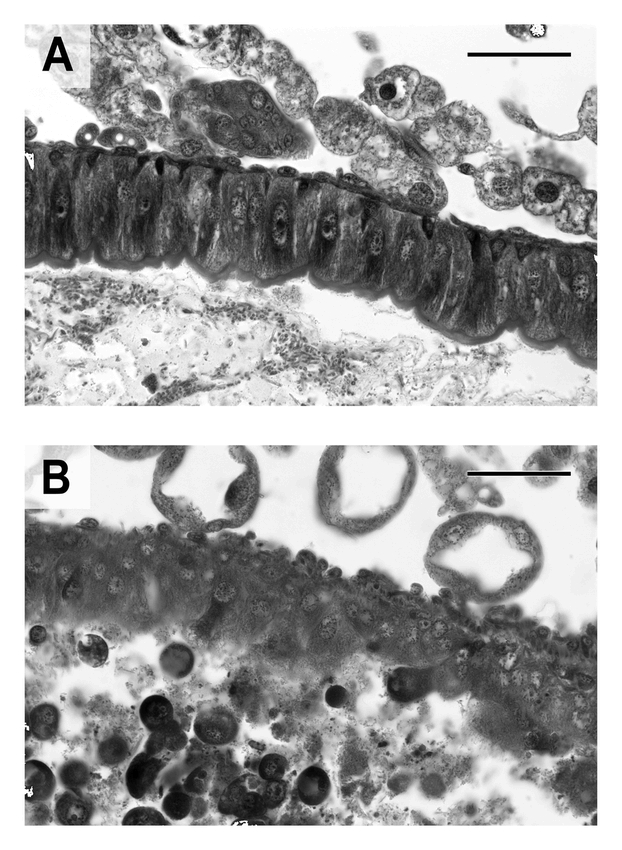
(A) Midgut epithelium of a second instar Leptinotarsa decemlineata larvae fed artificial diet alone. (B) Midgut epithelium of a second instar L. decemlineata larvae fed artificial diet containing 1.7 ppm Tca for 24 h. The gut epithelium is severely disrupted, and the gut lumen is packed with cellular debris (scale bars = 50 µm)
Figure 6.
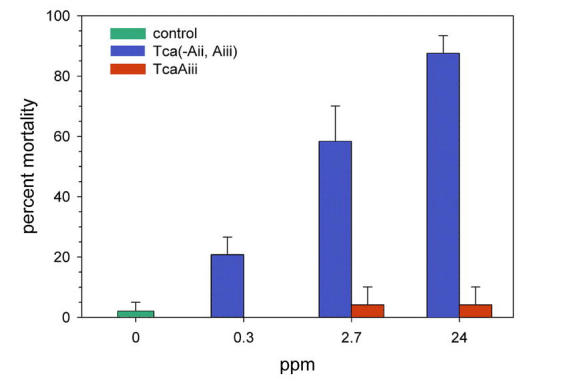
Mortality of neonate Leptinotarsa decemlineata larvae exposed to Tca (-Aii, Aiii) and TcaAiii for 48 h. Each point represents the mean (± SD) of two assays with 12 larvae each (controls had 24 larvae per assay).
The effect of Tca of B. tabaci
Immature B. tabaci were found to be highly susceptible to Tca, as shown in Figure 7. The EC50 for preventing first instar nymphs from progressing to the second instar was in the range of 0.1 to 0.3 ppm. In our experiments, no nymphs feeding on 2.16 ppm Tca reached the second instar (985 nymphs were tested at this dose). In contrast, 79% of nymphs fed the control diet reached the second instar. In similar experiments, Tca(-Aii and Aiii) appeared to be slightly more active against SPWF nymphs than Tca itself (Figure 8A). Tca(-Aii, Aiii) was also found to reduce the longevity of adult B. tabaci . Mean day survival was reduced from 6.7 days for controls to 2.6 days at 4 ppm Tca(-Aii, Aiii) (Figure 8B).
Figure 7.
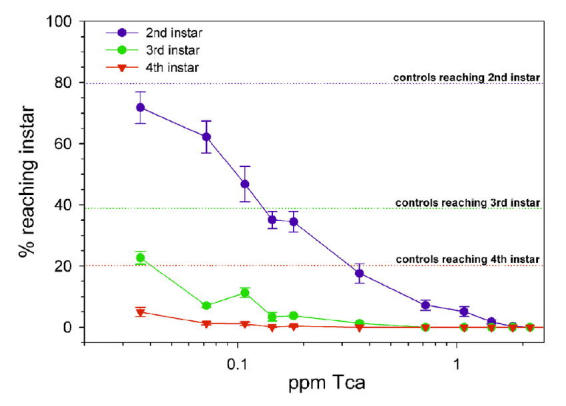
The percentage (± SEM) of first instar Bemesia tabaci nymphs successfully developing to the second, third, and fourth instars at different concentrations of Tca. Reference lines indicate the percentage of controls reaching the indicated instar.
Figure 8.
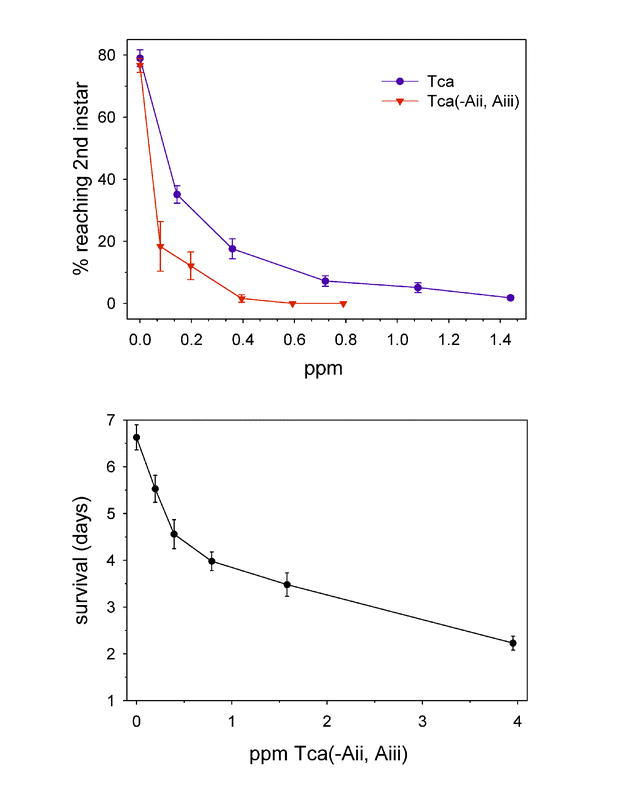
(A) Comparison of Tca and Tca(-Aii, Aiii) toxicity to first instar Bemisia tabaci . The data represent the mean percentage of first instar nymphs developing to the second instar (± SEM) at different concentrations of each complex. (B) The mean length of survival (± SEM), in days, of adult sweet potato whiteflies at varying concentrations of Tca(-Aii, Aiii).
Discussion
We have found that purified Tca from P. luminescens strain W14 is orally toxic to both L. decemlineata and B. tabaci. Although we chose a 48 h LC50 for L. decemlineata to demonstrate that Tca is capable of rapid kill, it is clear that Tca is lethal at much lower concentrations. Based on our growth inhibition results it seems reasonable to conclude that Tca would ultimately prove fatal to young L. decemlineata larvae at concentrations below 1 part per million; very little growth occurred at concentrations above 0.4-0.5 ppm. Similar results were found for the B. tabaci ; when fed to first instar nymphs, concentrations of Tca in the 0.1 to 0.3 ppm range resulted in a 50% reduction of nymphs progressing to the second instar. Although the artificial diet/feeding technique for B. tabaci has not yet been perfected, the impressive toxicity of Tca to both immature and adult whiteflies is clear. The effect of Tca on the midgut epithelium of L. decemlineata larvae was similar to that described for larvae of M. sexta (Blackburn et al., 1998). Ingestion of Tca led to extensive apical swelling and blebbing of the epithelium, and the gut lumen became packed with cytoplasmic vesicles. Superficially, these effect of Tca on the midgut are similar to other gut-active toxins such as the δ-endotoxins and Vip3A from B. thuringiensis (Sutter and Raun, 1967; Kinsinger and McGaughey, 1979; Endo and Nisiitsutsuji-Uwo, 1980; Yu et al., 1997), as well as cholesterol oxidase (Purcell et al., 1993).
In this report, we document the serendipitous isolation of a Tca complex free of cleaved Aii and Aiii subunits. Despite the absence of these subunits, the toxicity of the remaining complex to both L. decemlineata and B. tabaci is little changed from intact Tca; if anything, our results suggest that the Tca (-Aii, Aiii) is somewhat more toxic on a weight basis. It seems clear from these results that the toxicity of Tca is not dependant on the existence of cleaved Aii and Aiii subunits at the time of ingestion. Because the Tca(-Aii, Aiii) still possessed un-cleaved Aii+Aiii, we cannot rule out the possibility that the un-cleaved peptide is sufficient for toxicity, or that active subunits could be generated in the gut.
Combining these results with previous studies, Tca has been found to be active against lepidopteran, coleopteran, and homopteran insect species. The breadth of activity of Tca contrasts starkly with the generally narrow selectivity of B. thuringiensis δ-endotoxins; the expression of toxin complexes in transgenic plants may allow development of crops that are broadly resistant to insect pests, allowing further reduction of insecticide applications now needed to control secondary pests. Of equal or greater importance is the potential for delaying the development of resistance to B. thuringiensis by deploying an alternative mode of action.
Acknowledgments
The authors wish to thank Dr. David Bowen (Department of Entomology, University of Wisconsin, Madison, WI) for helpful discussions and kindly providing P. luminescens strain W14, and Dr. Patrick Dowd (USDA-ARS, National Center for Agricultural Utilization Research, Peoria, IL) for suggesting the use of lyophilized diet for toxin bioassays.
Disclaimer:
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the USDA and does not imply its approval to the exclusion of other products that may also be suitable.
References
- Blackburn M, Golubeva E, Bowen D, ffrench-Constant RH. A novel insecticidal toxin from Photorhabdus luminescens : histopathological effects of toxin complex A (Tca) on the midgut of Manduca sexta. Applied and Environmental Microbiology. 1998;64:3036–3041. doi: 10.1128/aem.64.8.3036-3041.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen DJ, Ensign JC. Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Applied and Environmental Microbiology. 1998;64:3029–3035. doi: 10.1128/aem.64.8.3029-3035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen D, Rocheleau TA, Blackburn M, Andreev O, Golubeva E, Bhartia R, ffrench-Constant RH. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science. 1998;280:2129–2132. doi: 10.1126/science.280.5372.2129. [DOI] [PubMed] [Google Scholar]
- Carazzi D. Eine neue Haematoxylinloesung. Zeitschrift fuer Wissenschaftliche Mikroskopie und fuer Mikroscopische Technik. 1911;28:273. [Google Scholar]
- Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, Taourit S, Bocs S, Boursaux-Eude C, Chandler M, Charles JF, Dassa E, Derose R, Derzelle S, Freyssinet G, Gaudriault S, Medigue C, Lanois A, Powell K, Siguier P, Vincent R, Wingate V, Zouine M, Glaser P, Boemare N, Danchin A, Kunst F. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nature Biotechnology. 2003;21:1307–13. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nishiitsutsuji-Uwo J. Mode of action of Bacillus thuringiensis δ-endotoxin: histopathological changes in the silkworm midgut. Journal of Invertebrate Pathology. 1980;36:90–103. [Google Scholar]
- Ensign JC. 1998 Insecticidal protein toxins from Photorhabdus. World Intellectual Property, Patent WO 98/08932 A1. [Google Scholar]
- Finney DJ. 1971 Probit Analysis, 3rd ed. Cambridge University Press. [Google Scholar]
- Gelman DB, Bell RA, Liska LJ, Hu JS. Artificial diets for rearing Colorado potato beetle, Leptinotarsa decenlineata. Journal of Insect Science. 2001;1:7–14. [PMC free article] [PubMed] [Google Scholar]
- Gelman DB, Blackburn MB, Hu JS, Gerling D. The nymphal-adult molt of the silverleaf whitefly (Bemisia argentifolii): Timing, regulation and progress. Archives of Insect Biochemistry and Physiology. 2002;51:67–79. doi: 10.1002/arch.10051. [DOI] [PubMed] [Google Scholar]
- Guo L, Fatig RO III, Orr GL, Schafer BW, Strickland JA, Sukhapinda K. Photorhabdus luminescens W-14 insecticidal activity consists of at least two similar but distinct proteins. The Journal of Experimental Biology. 1999;274:9836–9842. doi: 10.1074/jbc.274.14.9836. [DOI] [PubMed] [Google Scholar]
- Hurst MR, Glare TR, Jackson TA, Ronson CW. Plasmid located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. Journal of Bacteriology. 2000;182:5127–5138. doi: 10.1128/jb.182.18.5127-5138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancovich JK, Davidson EW, Lavine M, Hendrix DL. Feeding chamber and diet for culture of nymphal Bemisia argentifolii (Homoptera: Aleyrodidae) Journal of Economic Entomology. 1997;90:628–633. [Google Scholar]
- Kiernan JA. 1990 Histological and Histochemical Methods: Theory and Practice. Pergamon Press. [Google Scholar]
- Kinsinger RA, McGaughey WH. Histopathological effects of Bacillus thuringiensis on larvae of the indianmeal moth and the almond moth. Annals of the Entomological Society of America. 1979;72:787–790. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Sergeant M, Ellis D, Ousley M, Jarrett P. Sequence analysis of insecticidal genes from Xenorhabdus nematophilus PMFI296. Applied and Environmental Microbiology. 2001;67:2062–2069. doi: 10.1128/AEM.67.5.2062-2069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Wren BW, Thomson NR, Titball RW, Holden MTG, Prentice MB, Sebaihia M, James KD, Churcher C, Mungall KL, Baker S, Basham D, Bentley SD, Brooks K, Cerdeno-Tarraga AM, Chillingworth T, Cronin A, Davies RM, Davis P, Dougank G, Feltwell T, Hamlin N, Holroyd S, Jagels K, Karlyshev AV, Leather S, Moule S, Oyston PCF, Quail M, Rutherford K, Simmonds M, Skelton J, Stevens K, Whitehead S, Barrell BG. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- Purcell JP, Greenplate JT, Jennings MG, Ryerse JS, Pershing JC, Sims SR, Prinsen MJ, Corbin DR, Tran M, Sammons RD, Stonard RJ. Cholesterol oxidase: a potent insecticidal protein active against boll weevil larvae. Biochemical Biophysical Research Communications. 1993;196:1406–1413. doi: 10.1006/bbrc.1993.2409. [DOI] [PubMed] [Google Scholar]
- Sutter GR, Raun ES. Histopathology of European-corn-borer larvae treated with Bacillus thuringiensis. Journal of Invertebrate Pathology. 1967;9:90–103. [Google Scholar]
- Waterfield N., Dowling A., Sharma S., Daborn P.J., Potter U., ffrench-Constant R.H.. Oral toxicity of Photorhabdus luminescens W14 toxin complexes in Escherichia coli. Applied and Environmental Microbiology. 2001(a);67:5017–5024. doi: 10.1128/AEM.67.11.5017-5024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield N.R., Bowen D. J., Fetherston J.D., Perry R. D., ffrench-Constant R. H.. The toxin complex genes of Photorhabdus : a growing gene family. Trends in Microbiology. 2001(b);9:185–191. doi: 10.1016/s0966-842x(01)01978-3. [DOI] [PubMed] [Google Scholar]
- Waterfield NR, Daborn PJ, ffrench-Constant RH. Genomic islands in Photorhabdus. Trends in Microbiology. 2002;10:541–545. doi: 10.1016/s0966-842x(02)02463-0. [DOI] [PubMed] [Google Scholar]
- Yu CG, Mullins MA, Warren GW, Koziel MG, Estruch JJ. The Bacillus thuringiensis vegetative insecticidal protein Vip3A lyses midgut epithelium cells of susceptible insects. Applied and Environmental Microbiology. 1997;63:532–536. doi: 10.1128/aem.63.2.532-536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


