Abstract
Seawater and plankton samples were collected over a period of 17 months from November 1998 to March 2000 along the coast of Peru. Total DNA was extracted from water and from plankton grouped by size into two fractions (64 μm to 202 μm and >202 μm). All samples were assayed for Vibrio cholerae, V. cholerae O1, V. cholerae O139, and ctxA by PCR. Of 50 samples collected and tested, 33 (66.0%) were positive for V. cholerae in at least one of the three fractions. Of these, 62.5% (n = 32) contained V. cholerae O1; ctxA was detected in 25% (n = 20) of the V. cholerae O1-positive samples. None were positive for V. cholerae O139. Thus, PCR was successfully employed in detecting toxigenic V. cholerae directly in seawater and plankton samples and provides evidence for an environmental reservoir for this pathogen in Peruvian coastal waters.
Cholera continues to be an important and devastating disease, especially in those regions of the world where it is endemic. Before its reemergence in Peru and throughout Latin America in 1991, the disease had been absent from the Americas for nearly 100 years. There has been much speculation as to the cause of this reemergence and whether there has always been an environmental reservoir for Vibrio cholerae in Latin America. Since 1991, seasonal patterns of cholera outbreaks have been well documented in Central and South America, with the largest numbers of cases occurring during the warm, summer months (January to March) (7, 21).
In 1977, Colwell et al. (3) first hypothesized that coastal waters were an important reservoir of V. cholerae. V. cholerae has since been detected in seawater and other environmental sources around the world, both in areas where cholera is endemic and in cholera-free areas (4, 12, 13, 14, 16). Despite its ubiquity, the ability to determine the presence of this bacterial species in the environment with a degree of efficiency has been hindered by the culture techniques relied upon for detection. Under certain environmental conditions, V. cholerae has been shown to enter a viable but nonculturable state that can result in significant underestimation of the total V. cholerae population (23). Techniques employing microscopy, with either direct or indirect fluorescent-antibody staining, have been developed and provide important data on the occurrence of viable but nonculturable V. cholerae O1 and O139. However, it is obvious that the labeled antibody approach to detect all ∼200 serogroups of V. cholerae is not feasible (24). Furthermore, V. cholerae non-O1 and non-O139 strains can acquire genes for toxin production by transduction and, therefore, have been hypothesized to be the source of new epidemic and pandemic clones, the toxigenic O139 serogroup having arisen from recombination with a toxigenic O1 strain(s) (5). Currently, neither traditional culture methods nor the direct fluorescent-antibody assay (DFA) can detect the presence of the cholera toxin directly in the field. While most environmental V. cholerae strains lack the genes required to produce cholera toxin (19), the possibility of genetic exchange in the environment and the potential emergence of new toxigenic clones highlight the importance of including ctxA in environmental screening.
PCR and other molecular detection methods offer a useful alternative to culturing and microscopy, especially for environmental samples. Recently, species-specific oligonucleotide probes for V. cholerae have been used in colony blot hybridization (14, 15, 20). This approach has resulted in higher total counts than traditional methods because nonselective media can be used. However, colony blot probing is limited to culturable cells. PCR primers have now been developed that allow for specific detection of a range of targets (species, serogroup, toxin, etc.) in any given sample (22). While these results cannot provide direct evidence that such cells are viable, they do make possible rapid assessment of the potential total population. We report here a PCR method for direct detection of V. cholerae serogroups O1 and O139 and the gene coding for cholera toxin production (ctxA) in environmental samples. Seawater and plankton samples were collected along the coast of Peru for analysis. The objective of the research was to develop an understanding of the ecology of V. cholerae, as well as the occurrence and distribution of the toxigenic O1 and O139 subpopulations. This information, based on direct detection of the toxin gene (ctxA) in the environment, will allow development of a predictive model for cholera epidemics.
Sample collection.
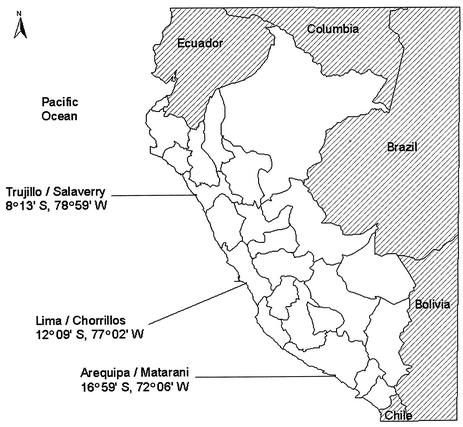
Three stations (Fig. 1) were sampled monthly from a 1,600-km coastline transect between Trujillo and Arequipa from November 1998 to March 2000. Additional bimonthly samples were collected from October to December 2000, just prior to the time of the annual outbreaks of cholera in Peru. From the total sample collection, 50 samples were selected, from which three fractions (water, plankton of >202 μm, and plankton of 64 to 202 μm) were obtained. Approximately 100 ml of seawater was filtered onto 0.2-μm-pore-size polycarbonate membranes, and the filters were stored in 1 to 5 ml of distilled water at −20°C. One to five milliliters of each plankton fraction, without diluent, was also stored at −20°C.
FIG. 1.
Map of sampling stations along the coast of Peru.
DNA extraction.
Total genomic DNA was extracted according to the method described by Rivera et al. (19a) using CTAB (cetyltrimethylammonium bromide) and phenol-chloroform-isopropyl alcohol. DNA was precipitated with a 0.6× volume of isoamyl alcohol, and the pellet was washed with 70% ethanol. DNA was resuspended in a total volume of 100 μl, quantitated by UV spectrophotometry, and stored at −20°C.
Plankton samples were thawed and vigorously mixed by vortexing, after which aliquots (∼2 ml) were extracted using DNeasy tissue kits (Qiagen, Valencia, Calif.) with a modification of the manufacturer's protocol. To accommodate a larger sample volume, three 700-μl sample aliquots were passed through the column for a total of 2.1 ml. DNA was eluted with two additions of 50 μl of sterile filtered (0.02-μm-pore-size filter) Milli-Q water, quantitated with UV spectrophotometry, and stored at −20°C.
PCR.
PCR was performed with the samples sequentially. All 150 samples (three fractions times 50 samples) were tested for the presence of V. cholerae. Positive samples were subsequently analyzed for the presence of O1- and/or O139-specific sequences (10, 19a). Finally, those samples positive for O1 or O139 were tested for ctxA.
For each PCR, 1 to 5 μl of sample DNA was added to a 20- to 24-μl master mix prepared for each target by using a TaqMaster kit from Eppendorf (Hamburg, Germany). All reaction mixtures included 0.2 mM (each) deoxynucleoside triphosphates, 1.25 μM (each) primers, 1× PCR buffer providing 1.5 mM MgCl2, 0.5× TaqMaster additive, and 0.625 U of Taq polymerase. For each sample, at least three dilutions were tested (undiluted, 1:20, and 1:100) because of the occasional occurrence of inhibitors. Primers for V. cholerae were those described by Chun et al. (2) and targeted to a ∼300-bp region of the 16S-23S intergenic spacer region (Table 1). Two separate primer sets were used to target both V. cholerae O1 and V. cholerae O139 (wbeO and rfb genes) (10, 19a, 25) (Table 1). Two separate primer sets were also used to target the ctxA gene of the CTX element (6, 10) (Table 1). For O1, O139, and ctxA targets, where two primer sets were employed, when either set resulted in a positive signal the sample was considered positive for the respective target. Universal primers for the 16S rRNA gene were used as a control test for inhibition in all samples (1).
TABLE 1.
Primers and PCR conditions for specific amplification of V. cholerae, V. cholerae O1, V. cholerae O139, and the ctxA genea
| Target | Primer sequence | Amplicon length (bp) | Annealing temp (°C) | Extension time (min) | Reference |
|---|---|---|---|---|---|
| 16S rDNA | 5′ CAG CMG CCG CGG TAA TWC 3′ | 888 | 60 | 0.5 | 1 |
| 5′ ACG GGC GGT GTG TRC 3′ | |||||
| V. cholerae ITS | 5′ TTA AGC STT TTC RCT GAG AAT G 3′ | ∼300 | 60 | 0.5 | 4 |
| 5′ AGT CAC TTA ACC ATA CAA CCC G 3′ | |||||
| V. cholerae O1 | 5′ CAA CAG AAT AGA CTC AAG AA 3′ | 647 | 50 | 1.0 | 19a |
| 5′ TAT CTT CTG ATA CTT TTC TAC 3′ | |||||
| V. cholerae O1 | 5′ GTT TCA CTG AAC AGA TGG G 3′ | 192 | 55 | 1.0 | 10 |
| 5′ GGT CAT CTG TAA GTA CAA C 3′ | |||||
| V. cholerae O139 | 5′ TTA CCA GTC TAC ATT GCC 3′ | 741 | 55 | 1.5 | 19a |
| 5′ CGT TTC GGT AGT TTT TCT GG 3′ | |||||
| V. cholerae O139 | 5′ AGC CTC TTT ATT ACG GGT GG 3′ | 449 | 55 | 1.0 | 10 |
| 5′ GTC AAA CCC GAT CGT AAA GG 3′ | |||||
| ctxA | 5′ CGG GCA GAT TCT AGA CCT CCT G 3′ | 564 | 60 | 1.0 | 6 |
| 5′ CGA TGA TCT TGG AGC ATT CCC AC 3′ | |||||
| ctxA | 5′ ACA GAG TGA GTA CTT TGA CC 3′ | 308 | 55 | 1.0 | 10 |
| 5′ ATA CCA TCC ATA TAT TTG GGA G 3′ |
All reactions included a 1-min initial denaturation step at 94°C, followed by 35 cycles of denaturation (94°C for 45 s), annealing for 45 s, and extension at 72°C. A final extension at 72°C for 5 min was included in all reactions. Specific annealing temperatures and extension times are listed. rDNA, ribosomal DNA; ITS, intergenic spacer region.
Reaction conditions for each target are described in Table 1. PCR products were run on 1.4% agarose gels and stained with ethidium bromide, and the bands were visualized with a UV transilluminator (UV Products Inc., Upland, Calif.). Images were captured with a Kodak EDAS 290 digital imaging system (Eastman Kodak Co., New Haven, Conn.).
Analysis.
Samples were scored by the presence or absence of specific targets and statistically analyzed by using Fisher's exact or Cochran's Q test for matched samples. In all cases, significance was determined at P values of ≤0.05.
Environmental detection.
Total DNA extraction provided sufficient template for sequential analysis of the microbial community and permitted a narrowing down to the toxigenic strains of epidemic V. cholerae (Table 2). As few as 100 cells of V. cholerae in 250 ml of seawater can be detected by using the sample concentration, DNA extraction, and PCR protocols described here (19a). While the presence of nonviable cells cannot be excluded with the use of PCR, this approach offers a rapid assessment of environmental samples with a higher level of sensitivity than traditional methods, notably culture (A. I. Gil, E. K. Lipp, I. N. G. Rivera, V. Louis, A. Huq, C. F. Lanata, D. N. Taylor, E. Russek-Cohen, N. Choopun, R. B. Sack, and R. R. Colwell, submitted for publication).
TABLE 2.
Occurrence of V. cholerae-specific amplicons at stations sampled in this study a
| Station | No. of V. cholerae-positive samples/total (%) | No. of V. cholerae O1-positive samples/total (%) | No. of ctxA-positive samples/total (%) |
|---|---|---|---|
| Arequipa | 6/9 (66.7) | 5/6 (83.3) | 1/5 (20.0) |
| Lima | 21/29 (72.4) | 11/20 (55.0)b | 3/11 (27.3) |
| Trujillo | 6/12 (50.0) | 4/6 (66.7) | 1/4 (25.0) |
Samples were considered positive if any fraction, i.e., seawater or either of the plankton size fractions, was positive.
One sample missing.
The V. cholerae-specific intergenic spacer region was successfully amplified in 33 of the 50 samples in at least one fraction (66%). PCR for O1 and O139 targets yielded 27 positive amplicons for the V. cholerae O1 serogroup and none for O139. Five of the V. cholerae O1-positive samples were also positive for ctxA (Table 2).
Results from this work showed a higher level of detection of toxigenic V. cholerae O1 than those of a concurrent study using traditional detection methods (culture and DFA with direct viable counts) (Gil et al., submitted) (Table 3). Gil et al. (submitted) found that only 5.9 and 14.3% of samples were positive for V. cholerae O1 by culture and DFA, respectively. Furthermore, no data were obtained on the presence of ctxA in these samples by the methods used (Gil et al., submitted). Despite the difference in detection frequencies, the general trends in both studies followed similar patterns.
TABLE 3.
Method agreement between culture for V. cholerae O1 bacterial counts (Gil et al., submitted) and direct PCR for total V. cholerae and V. cholerae O1 bacterial countsa
| PCR result | No. of samples negative for V. cholerae O1 by culture | No. of samples positive for V. cholerae O1 by culture | Total no. of samples |
|---|---|---|---|
| V. cholerae O1 | |||
| Negative | 8 | 5 | 13 |
| Positive | 17 | 3 | 20 |
| Total | 25 | 8 | |
| V. cholerae | |||
| Negative | 17 | 0 | 17 |
| Positive | 25 | 8 | 33 |
| Total | 42 | 8 |
A sample was considered positive if any one of the three fractions was positive.
Seasonality.
V. cholerae detection in coastal Peru followed ambient temperature increases and coincided with and/or preceded annual outbreaks of cholera in the summer months (January to March) (Table 4). In particular, detection of DNA from toxigenic V. cholerae O1 peaked during this time, when 20% (2 of 10) of all samples were positive for ctxA and V. cholerae O1; the values ranged from 5.9 to 9.1% (1 of 17 to 1 of 11) for the other seasons. In the temperate Chesapeake Bay estuary, where there are no cases of cholera and only ∼22% of samples were positive for culturable V. cholerae (18), the detection pattern followed a similar seasonal cycle. This pattern of increased frequency of detection in warmer months is consistent with the ecology of V. cholerae and other Vibrio spp. and has been extensively reported elsewhere (8, 9, 10, 17). Interestingly, salinity, which was a dominant factor in the occurrence of V. cholerae in the Chesapeake Bay (18), showed no association with the occurrence of V. cholerae in this study, probably due to the lack of variability in the salinity of the coastal geographical areas sampled (Gil et al., submitted). The median and mean salinities were 35 ppt, and salinities ranged between a one-time low of 31 ppt and a one-time high of 37 ppt.
TABLE 4.
Seasonal distribution of total V. cholerae, V. cholerae O1, and ctxA among each of the fractions sampled
| Target | Seasona | No. of positive samples/total (%) of:
|
||
|---|---|---|---|---|
| Water | Plankton (64 to 202 μm) | Plankton (>202 μm) | ||
| V. cholerae | Summer | 7/10 (70.0) | 5/10 (50.0) | 5/10 (50.0) |
| Fall | 4/12 (33.3) | 2/12 (16.7) | 2/12 (16.7) | |
| Winter | 4/11 (36.4) | 2/11 (18.2) | 4/11 (36.4) | |
| Spring | 10/17 (58.8) | 6/17 (35.3) | 4/17 (23.5) | |
| V. cholerae O1 | Summer | 3/7 (42.9) | 3/5 (60.0) | 4/5 (80.0) |
| Fall | 1/4 (25.0) | 2/2 (100) | 1/2 (50.0) | |
| Winter | 1/4 (25.0) | 0/1 (0.0)b | 3/4 (75.0) | |
| Spring | 4/10 (40.0) | 2/6 (33.3) | 3/4 (75.0) | |
| ctxA | Summer | 2/3 (66.7) | 0/3 (0.0) | 0/4 (0.0) |
| Fall | 1/1 (100) | 0/2 (0.0) | 0/1 (0.0) | |
| Winter | 1/1 (100) | 0c | 0/3 (0.0)c | |
| Spring | 1/4 (25.0) | 0/2 (0.0) | 0/33 (0.0) | |
Summer, January, February, March; Fall, April, May, June; winter, July, August, September; spring, October, November, December.
One sample missing.
No samples were positive for O1; ctxA was not tested.
Distribution between plankton and water.
Total V. cholerae was more consistently isolated from water samples than from plankton (Table 5); however, V. cholerae O1 cells were clearly abundant in the large plankton fraction, which included copepods and other zooplankton, relative to those in water and the smaller plankton size fraction. Unexpectedly, samples containing ctxA were not detected in the plankton. A possible explanation for this observation and the relatively low detection rate of V. cholerae, in general, in the plankton is that extraction of DNA from plankton samples was less efficient than that from water samples, with inhibitors affecting the polymerase activity (19a). This was confirmed by a reduction in amplification of total bacterial DNA (PCR for universal 16S sequences) in the plankton (data not shown).
TABLE 5.
Occurrence of V. cholerae, V. cholerae O1, and ctxA in seawater and plankton samples
| Sample type | No. of V. cholerae- positive samples/total (%) | No. of V. cholerae O1-positive samples/total (%) | No. of ctxA positive samples/total (%) |
|---|---|---|---|
| Water | 25/50 (50.0) | 9/25 (36.0) | 5/9 (55.6) |
| Plankton (64 to 202 μm) | 15/50 (30.0) | 7/14 (50.0)a | 0/7 (0.0) |
| Plankton >202 μm | 15/50 (30.0) | 11/15 (73.3) | 0/11 (0.0) |
One sample missing.
In this study, the occurrence of V. cholerae-positive samples was positively correlated with air temperature (P = 0.01), which showed a distinct seasonal trend. Sea surface temperature variations were less definite (with the exception of the warm El Niño temperatures in the summer of 1998), and a statistically significant relationship with the occurrence of V. cholerae was not observed. We noted positive associations between the occurrence of V. cholerae and both chlorophyll a and total bacterial counts, but the relationships were not statistically significant (P ≈ 0.1) (Gil et al., submitted).
In conclusion, we have shown that direct DNA extraction and application of sequential PCR assays are important tools for the rapid assessment of environmental samples for toxigenic V. cholerae and provide a better level of sensitivity than traditional culture and microscopic methods. With these methods, it is clear that V. cholerae is present in the coastal waters of Peru throughout the year, with numbers of cells increasing in spring and summer, just prior to the annual outbreak of cholera in the coastal cities. V. cholerae O1 cells are especially prevalent in plankton of >202 μm. The data demonstrate that the coastal waters and plankton populations in Peru harbor toxigenic V. cholerae, as has been shown for areas of Bangladesh where cholera is endemic (11). It is highly unlikely that cholera in Peru arrived from other cholera-affected regions of the world. A more plausible explanation is that V. cholerae is autochthonous to Peruvian coastal, brackish, and riverine waters, and it is reasonable to examine those environmental conditions giving rise to both plankton blooms and increases in V. cholerae concentrations. This information will help to develop a predictive model for cholera epidemics and provide an early warning system for conditions conducive to cholera outbreaks.
Acknowledgments
This research was supported by NIH grant no. RO1NRO4527-01A1, NIH/National Institute of Nursing research grant no. 1RO1A139129-01, NASA grant no. NAG2-1195, and NSF grant no. 01200677.
We thank Redwan Huq for his assistance with DNA extraction during a summer internship at the Center of Marine Biotechnology.
REFERENCES
- 1.Amann, R. I., W. Ludweg, and K. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun, J., A. Huq, and R. R. Colwell. 1999. Analysis of 16S-23S rRNA intergenic spacer regions of Vibrio cholerae and Vibrio mimicus. Appl. Environ. Microbiol. 65:2202-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colwell, R. R., J. Kaper, and S. W. Joseph. 1977. Vibrio cholerae and Vibrio parahaemolyticus and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394-396. [PubMed] [Google Scholar]
- 4.Colwell, R. R., R. J. Seidler, J. Kaper, S. W. Joseph, S. Garges, H. Lockman, D. Maneval, H. Bradford, N. Roberts, E. Remmers, I. Huq, and A. Huq. 1981. Occurrence of Vibrio cholerae serotype O1 in Maryland and Louisiana estuaries. Appl. Environ. Microbiol. 41:555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faruque, S. M., M. N. Saha Asadulghani, D. A. Sack, R. B. Sack, Y. Takeda, and G. B. Nair. 2000. The O139 serogroup of Vibrio cholerae comprises diverse clones of epidemic and nonepidemic strains derived from multiple V. cholerae O1 or non-O1 progenitors. J. Infect. Dis. 182:1161-1168. [DOI] [PubMed] [Google Scholar]
- 6.Fields, P. I., T. Popovic, K. Wachsmuth, and Ø. Olsvik. 1992. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J. Clin. Microbiol. 30:2118-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franco, A. A., A. D. Fix, A. Prada, E. Paredes, J. C. Palomino, A. C. Wright, J. A. Johnson, R. McCarter, H. Guerra, and J. G. Morris, Jr. 1997. Cholera in Lima, Peru, correlates with prior isolation of Vibrio cholerae from the environment. Am. J. Epidemiol. 146:1067-1075. [DOI] [PubMed] [Google Scholar]
- 8.Heidelberg, J. F. 1997. Seasonal abundance of bacterioplankton populations of bacteria, gamma proteobacteria, Vibrio/Photobacterium, Vibrio vulnificus, Vibrio cholerae/Vibrio mimicus, and Vibrio cincinnatiensis associated with zooplankton in the Choptank River, Maryland. Ph.D. thesis. University of Maryland, College Park.
- 9.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Seasonality of Chesapeake Bay bacterioplankton species. Appl. Environ. Microbiol. 68:5488-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino, K., S. Yamasaki, A. K. Mukhopadhyay, S. Chakraborty, A. Basu, S. K. Bhattacharya, G. B. Nair, T. Shimada, and Y. Takeda. 1998. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol. Med. Microbiol. 20:201-207. [DOI] [PubMed] [Google Scholar]
- 11.Huq, A., E. B. Small, P. A. West, M. I. Huq, R. Rahman, and R. R. Colwell. 1983. Ecological relationship between Vibrio cholerae and planktonic copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huq, A., R. Sack, and R. R. Colwell. 2001. Cholera and global ecosystems, p. 327-347. In J. Aron and J. Patz (ed.), Ecosystem change and public health: a global perspective. Johns Hopkins University Press, Baltimore, Md.
- 13.Jesudason, M. V., V. Balaji, U. Mukudan, and C. J. Thomas. 2000. Ecological study of Vibrio cholerae in Vellore. Epidemiol. Infect. 124:201-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, S. C. 2001. Vibrio cholerae in recreational beach waters and tributaries of Southern California. Hydrobiologia 460:157-164. [Google Scholar]
- 15.Jiang, S. C., and W. Fu. 2001. Seasonal abundance and distribution of Vibrio cholerae in coastal waters quantified by a 16S-23S intergenic spacer probe. Microb. Ecol. 42:540-548. [DOI] [PubMed] [Google Scholar]
- 16.Kaysner, C. A., C. Abeyta, Jr., M. M. Wekell, A. DePaola, Jr., R. F. Stott, and J. M. Leitch. 1987. Incidence of Vibrio cholerae from estuaries of the United States West Coast. Appl. Environ. Microbiol. 53:1344-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipp, E. K., C. Rodriguez-Palacios, and J. B. Rose. 2001. Occurrence and distribution of the human pathogen Vibrio vulnificus in a subtropical Gulf of Mexico estuary. Hydrobiologia 460:165-173. [Google Scholar]
- 18.Louis, V., E. Russek-Cohen, N. Choopun, I. N. G. Rivera, B. Gangle, S. C. Jiang, A. Rubin, J. A. Patz, A. Huq, and R. R. Colwell. 2003. Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 69:2773-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera, I. N. G., J. Chun, A. Huq, B. Sack, and R. R. Colwell. 2001. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl. Environ. Microbiol. 67:2421-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Rivera, I. N. G., E. K. Lipp, N. Choopun, A. Gil, A. Huq, and R. R. Colwell. Method for DNA extraction and application of multiplex PCR to detect toxigenic V. cholerae O1 and O139 in aquatic ecosystems. Environ. Microbiol., in press. [DOI] [PubMed]
- 20.Robert-Pillot, A., S. Baron, J. Lesne, J.-M. Fournier, and M.-L. Quilici. 2002. Improved detection of Vibrio cholerae in environmental water samples by culture on selective medium and colony hybridization assay with an oligonucleotide probe. FEMS Microbiol. Ecol. 40:39-46. [DOI] [PubMed] [Google Scholar]
- 21.Seas, C., J. Miranda, A. I. Gil, R. Leon-Baru, J. A. Patz, A. Huq, R. R. Colwell, and R. B. Sack. 2000. New insights on the emergence of cholera in Latin America during 1991: the Peruvian experience. Am. J. Trop. Med. Hyg. 62:513-517. [DOI] [PubMed] [Google Scholar]
- 22.Singh, D. V., S. R. Isac, and R. R. Colwell. 2002. Development of a hexaplex PCR assay for rapid detection of virulence and regulatory genes in Vibrio cholerae and Vibrio mimicus. J. Clin. Microbiol. 40:4321-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu, H. S., N. C. Roberts, F. L. Singleton, R. W. Attwell, D. J. Grimes, and R. R. Colwell. 1982. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
- 24.Yamai, S., T. Okitsu, T. Shimada, and Y. Katsube. 1997. Distribution of serogroups of Vibrio cholerae non-O1 non-O139 with specific reference to their ability to produce cholera toxin, and addition of novel serogroups. J. Jpn. Assoc. Infect. Dis. 71:1037-1045. [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki, S., T. Shimizu, K. Hoshino, S. T. Ho, T. Shimada, G. B. Nair, and Y. Takeda. 1999. The genes responsible for O-antigen synthesis of Vibrio cholerae O139 are closely related to those of Vibrio cholerae O22. Gene 237:321-332. [DOI] [PubMed] [Google Scholar]