Abstract
In an effort to uncover genes associated with ovary activation in honey bee workers, the extent to which eight candidate genes co-varied in their expression with experimentally-induced changes in worker reproductive state was examined. Groups of caged, queenless workers narcotized with CO2 on consecutive days early in adult life showed a significantly lower level of ovary activation than did groups of untreated workers. This same experimental treatment, by contrast, is known to accelerate ovary activation and induce egg laying in virgin honey bee queens – an observation that suggests that CO2 narcosis has contrasting effects in queen versus worker ovary activation. Experimentally-induced changes to worker reproductive state were associated with changes in gene expression. Vitellogenin, an egg yolk precursor, and transferrin, an iron transporter, were two transcripts found to be significantly down-regulated as a function of the ovary-inhibiting treatment. CO2 narcosis did not effect the expression of six other genes selected as putative markers for processes that may underlie ovary activation. The show that the expression of vitellogenin and transferrin is correlated with ovary activation in workers, and may therefore be part of the gene network involved in the regulatory control of functional sterility in honeybees.
Keywords: anaesthesia, genes for sterility, reproductive division of labor, social insects, quantitative PCR, transferrin, worker sterility, vitellogenin
Introduction
Like many other species of social insect, the honey bee Apis mellifera L., is characterized by extreme reproductive division of labour. The vast majority of workers are functionally sterile, and never activate their ovaries throughout their life (Barron et al., 2001), leaving the production of eggs to the queen. Uncovering the genes associated with worker sterility in social insects will provide an important step forward in our understanding of a major evolutionary transition (Maynard Smith and Szathmàry, 1995), namely, the transition from individual organisms to integrated societies, and our best example yet of kin-selected ‘genes for altruism’ (sensu Dawkins 1976): genes that reduce an individual's direct reproductive success while enhancing the reproductive success of relatives.
All worker honeybees develop into adults with vestigial ovaries. Although some or all wild type workers of intermediate age are able to subsequently activate their ovaries in response to a period of protracted queenlessness, and lay eggs, queenright workers almost never do so, retaining their ovaries in a vestigial state. An important exception is the selectively-bred ‘anarchistic’ line of honeybees maintained at The University of Sydney in which about 40% of 10 day-old workers have functionally activated ovaries and lay eggs at high frequency (Barron et al., 2001). This mutant line demonstrates that worker sterility has a genetic basis (Oldroyd et al., 1994). Furthermore, the patterns of inheritance of the anarchistic phenotype show that there are a small number of genes that regulate worker ovary activation in response to social cues (Oldroyd and Osborne, 1999). Given the strong genetic component to the regulation of ovary activation in honeybees, the gene-expression profiles of workers with and without activated ovaries should reveal genes associated with the regulatory control of worker sterility.
For reasons that are not well understood, the ovaries of unmated (virgin) queens can be experimentally activated, as if mated, by subjecting queens to double CO2 narcosis (Mackensen, 1947). This phenomenon is routinely exploited to induce oviposition in unmated but artificially inseminated queens (Laidlaw and Page, 1997). This idiosyncratic response has facilitated the physiological study of queen egg production (Engels et al., 1976; Engels and Ramamurty, 1976; Engels, 1987). However, few studies have applied this technique to the study of worker egg production (Harris and Harbo, 1990; Harris et al., 1996), and no study has yet examined locus-specific changes in gene-expression associated with ovary activation, in queens or workers. The effects of CO2 narcosis in queen honeybees indicate that the treatment triggers the up-regulation of genes that in turn activate the queen's ovaries. This is particularly evident in the case of vitellogenin, an egg protein, whose synthesis is dramatically stimulated in queen abdomens following CO2 treatment (Engels et al., 1976). Genes directly associated with ovary activation in queens, like vitellogenin for example, are prime candidates for genes associated with ovary activation in normally sterile workers.
We speculated that the genes associated with ovary activation in queens in response to CO2 narcosis might also be the genes associated with ovary activation in workers. To test this hypothesis we exposed groups of caged workers to double CO2 narcosis and compared rates of ovary activation and levels of gene expression against groups of control (un-narcotized) workers. Differential ovary activation and gene expression in response to CO2 exposure occurred. These findings are discussed in the context of the regulation of ovary activation in queens and workers.
Materials and Methods
Experimental treatment
The general approach was to use groups of caged queenless workers for comparing rates of ovary activation and levels of gene expression. To this end, we incubated emerging sealed brood from wild type colonies at 35° C overnight. The following morning, newly-emerged workers were collected and groups of 30 were transferred into wire-mesh cages (approx. 14 × 10 × 7 cm) fitted with a piece of beeswax foundation comb (6 cm × 3 cm), water and food (45% honey; 10% water; 45% royal jelly, Lifetime Health Products, Australia). After two (Experiment 1) or four (Experiment 2) days of incubation (∼35° C, 40% RH), workers were narcotized at room temperature by placing whole cages into a larger plastic container and flushing the container with compressed CO2 until the workers were immobilized. The workers were maintained in the narcotised state for 10 minutes. The following day, workers were narcotised again, for 3 minutes (after Engels et al., 1976). In each case, workers were completely anaesthetized. Then, at various time intervals following the double narcosis treatment (4-hrs, 24-hrs, 48-hrs, 96-hrs), workers were collected from incubation by snap-freezing them in liquid N2. Thus, collections were single pairs of cages sampled at each collection interval.
Bee abdomens were later dissected according to Dade (1977) and assigned a numerical score reflecting the state of ovary activation: 0 (ovaries thin and lacking defined ova), 1 (ovaries slightly thickened but still lacking defined ova), 2 (ovaries thick with clearly defined ova), 3 (ovaries thick with a least one fully mature ova). For each collection, differences in levels of ovary activation between treated and control cages were evaluated using t-tests for equality of mean ovary score between cages, as well as X2 tests of the proportions of each ovary activation class between treatments.
RNA extraction and cDNA synthesis
Total RNA was extracted from the abdomens of eight workers per treated and untreated cage. A combined Trizol/Qiagen protocol was used to extract RNA under standard conditions so that it was suitable for quantifying levels of mRNA. Briefly, frozen tissues were homogenized in 1.5 ml Eppendorf tubes, containing 50–70 µl of Trizol reagent (Invitrogen, www.invitrogen.com), using disposable pestles from Sigma (Sigma-Aldrich, www.sigmaaldrich.com) attached to a hand-held engraving device (Arlec, Super Tool, www.arlec.com.au/). One abdomen was used per tube. Following the homogenization step the volume was adjusted to 500 µl with Trizol and then mixed with 100 µl of chloroform. The RNA-containing buffer phase was recovered after spinning for 10 min at 10,000 × g, mixed with an equal volume of 70% ethanol and applied on a Qiagen (www.qiagen.com) RNeasy column. The remaining steps were carried out according to Qiagen's protocols. Standardised aliquots of individual RNA extractions were pooled by treatment prior to cDNA synthesis. We used pooled rather than individual samples to examine gene expression in order to minimize inter-individual variations, as described in other studies (Grozinger et al., 2003; Tian et al., 2004). Reverse transcription was performed according to Invitrogen's protocol using 5 µl of the pooled total RNA. The cDNA synthesis primer was T(20)MN (M=A,G, or C; N=A, G, C, or T).
Selection of candidate markers for ovarian activation in the honeybee
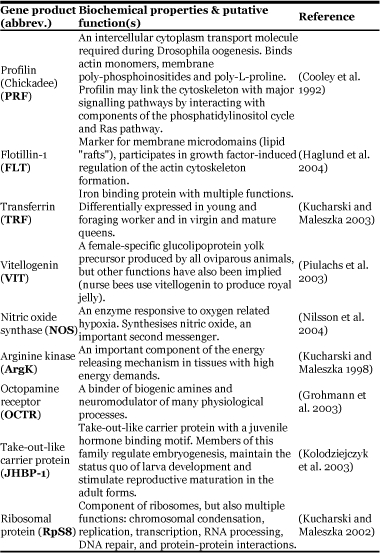
The genes selected for transcriptional evaluation in this study encode transcripts known, or that were suspected, to be involved either in ovarian development (vitellogenin, transferrin, profilin, octopamine receptor) or in signalling pathways critical for cellular growth or cellular differentiation (flotillin, nitric oxide synthase, take-out-like). Arginine kinase was also selected because it is an important component of energy transfer and its differential expression in the honey bee has already been reported (Kucharski and Maleszka, 1998). Selection of these candidate markers was based on: 1) their functional gene descriptions at Interactive Fly (www.sdbonline.org) or Online Mendelian Inheritance in Man (www.ncbi.nlm.nih.gov) and 2) the availability of an annotated gene sequence in the honey bee (http://www.hgsc.bcm.tmc.edu/projects/honeybee/). Table 1 provides more details regarding the biochemical and functional properties of these candidate genes.
Table 1.
Description of genes used for transcriptional evaluation
Quantitative PCR
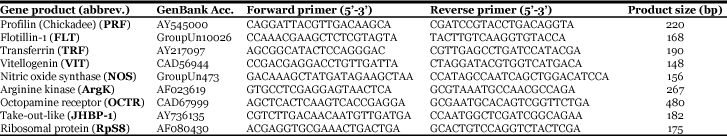
Target sequences for PCR were selected from cDNAs and ESTs using the genomic scaffolds at the Baylor College of Medicine. To eliminate genomic DNA amplification we manually designed primers that either spaned intron/exon junctions or amplified exon sequences separated by long introns. Our primers were 40-60% GC-rich and contained 20-24 bases to maximize reaction efficacy. All primers were experimentally tested in an Eppendorf gradient cycler to determine the optimal annealing temperature and to ensure that only one band of correct size was produced. These gene-specific primers are listed in Table 2.
Table 2.
Sequences of gene-specific primers used for qRT-PCR assays
The relative amount of specific cDNA template between treated and control samples was quantified using real time quantitative PCR (qRT-PCR). Taq polymerase (0.2 units) from Promega (www.promega.com) was used in 20 µl reactions (in triplicate) containing 2 mM MgCl2, 200 µM of each dNTP, 0.25 µM each of forward and reverse primer, and cDNA template equivalent to approximately 25 ng of total RNA. For every reaction, an RNA sample without reverse transcriptase was included to control for genomic DNA contamination. Product formation was monitored by the inclusion of SYBR Green I (Fisher Biotech, www.fishersci.com) at a final dilution of 1:40,000. Thermocycling was conducted in a RotorGene 3000 Thermal Cycler (Corbett Research, www.corbettresearch.com) for 35 cycles consisting of denaturation for 30 s at 94° C, annealing at 60° C for 30 s, extension at 72° C for 30 s, and fluorescence acquisition at 84° C for 15 s. Cycling was preceded by a 15 min 95° C activation step. Specificity of amplification was confirmed through a melt curve analysis of final PCR products by ramping the rotor temperature from 55° C to 99° C at 0.2° C s-1 with fluorescence acquired after every 1° C increase.
Estimation of Changes in Transcript Abundance
The amplification efficiencies of primer pairs used in qRT-PCR assays varied by less than 10% compared with that of the normalizer (ribosomal protein S8; Table 1), which was ∼1.85. These values were determined by the slope of the curve generated by amplification of serially diluted cDNA over at least three orders of magnitude (data not shown). Accordingly, all primer pairs were nominated as having approximately equal efficiencies and, under this simplifying assumption, fold changes in relative transcript abundance were calculated using the method of Livak and Schmittgen (2001).
Results
CO2 narcosis and ovary activation - Experiment 1
Very young workers, bees narcotised on days 2 and 3 and examined just 4 hrs after treatment, had relatively low mean ovary scores, with no individual scored as having well-defined ova (i.e. all scores ≤ 1). Neither the mean score (Figure 1A) nor the categorical distribution of ovary activation scores (X2 1= 0.069, P = 0.8) differed between treated and control cages. At 24 hrs post-treatment a general increase to mean score was observed in both treated and control groups. However, this increase did not differ between treatments X2 1= 0.271, P = 0.6, (Fig.1).
Figure 1. Effects of CO2 on ovary activation of worker honeybees A).
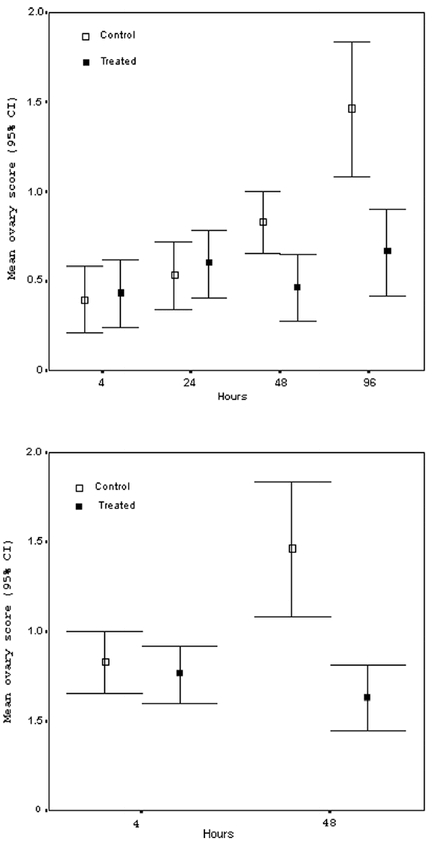
In Experiment 1 Apis mellifera workers were narcotized on days 2 and 3 of adult life, and their ovaries examined after 4 hrs, 24 hrs, 48 hrs, and 96 hrs. The mean ovary score of narcotized bees was significantly lower than controls at 48 hrs (t-test, P = 0.005) and 96 hrs (t-test, P = 0.003). B) In Experiment 2 workers were narcotized on days 4 and 5 of adult life, and their ovaries examined after 4 hrs and 48 hrs. The mean ovary score of narcotized bees was significantly lower than controls at 48 hrs (t-test, P < 0.001).
By 48 hrs post-treatment, however, an effect was apparent. Control workers showed significantly greater levels of ovary activation than did treated workers, X2 2= 7.735, P = 0.021 (Fig. 1A). Finally, bees examined 96 hrs post-treatment showed the greatest difference ovary activation between treated and control cages X2 3= 10.764, P = 0.013 (Fig. 1A), again with the control group showing significantly higher levels of ovary activation.
CO2 narcosis and ovary activation - Experiment 2
For bees narcotised 4 and 5 days post emergence, the pattern was similar. At 4 hrs after treatment bees had low and similar ovary scores, X2 2= 1.077, P = 0.6, while at 48 hrs following treatment controls showed significantly greater ovary activation than did treated workers X2 3= 16.831, P = 0.001 (Fig. 1B).
Quantitative real-time PCR
Figure 2 shows the responsiveness of eight candidate genes to CO2 treatment. Bees narcotised on days 4 and 5 (Experiment 2) and collected 48 hrs later were used as the basis for examining gene expression differences. For all eight genes, the level of expression was calculated for treated bees relative to non-treated controls. Thus, genes whose relative expression was estimated at ‘1’ were deemed to show no expression difference between treated and control groups, whereas genes significantly above or below this value, as evidenced by their 95% confidence intervals, were considered to be over- or under-expressed in treated workers, respectively. Genes encoding profilin (aka chickadee; PRF), flotillin (FLT), nitric oxide synthase (NOS), arginine kinase (ArgK), octopamine receptor (OCTR), and take-out-like carrier protein (JHBP-1) showed no consistent difference in expression (Figure 2), even though differences in ovary activation were now apparent among the workers. Therefore these genes do not appear to be associated with experimentally induced differences in ovary activation, at least not by this assay. In contrast, the genes encoding vitellogenin (VIT) and transferrin (TRF) were down-regulated (∼ 4-5 fold; Fig. 2) in treated relative to control groups. The expression of these two genes is therefore associated with differences in functional ovary activation among workers.
Figure 2. Relative quantification of gene expression.
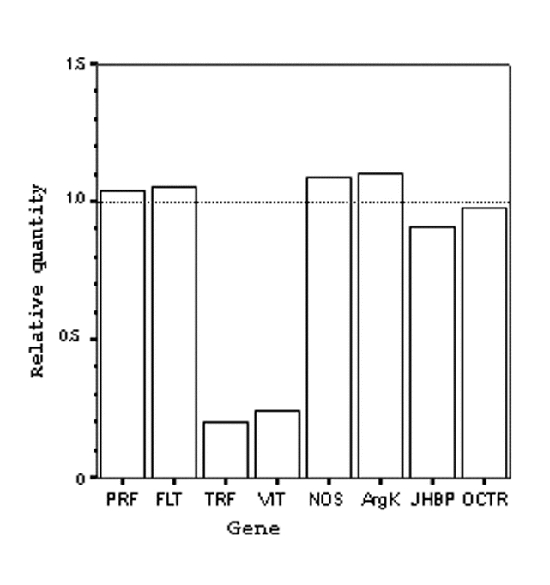
The expression levels of eight candidate genes in Apis mellifera as estimated using real-time quantitative PCR. Relative quantities are normalized to an endogenous reference gene (S8, see text). Note that vitellogenin (VIT) and transferrin (TRF) are down-regulated following CO2-induced changes in reproductive state. Relative quantities represent an average from three assays of a single group of 8 individuals.
Discussion
CO2-induced changes in ovary activation
Our study confirms that double CO2 narcosis inhibits ovary activation in workers (Biedermann, 1964; Harris and Harbo, 1990; Harris et al., 1996; Kropàcovà et al., 1968). This was true regardless of whether workers were narcotised when young (on days 2 and 3; Fig. 1A) or slightly older (on days 4 and 5; Fig. 1B), and all narcotised groups had mean ovary scores of less than ‘1’. In contrast, Mackensen (1947) showed that double CO2 narcosis of virgin queens actually accelerated ovary activation and egg laying. It is well known that in the absence of mating or narcosis, queens will not activate their ovaries or initiate oviposition for many weeks, if ever. Thus the contrasting effects of narcosis on ovary activation in queens versus workers provides a potentially powerful model system for studying the molecular mechanisms that regulate worker sterility in honeybees, and potentially other social insects.
Molecular correlates to ovary activation in worker honeybees
This study shows that abdominal expression of vitellogenin and transferrin is reduced in narcotised workers compared to controls. This suggests that these two genes may be part of the network that regulates functional sterility in worker honeybees.
The process of ovary activation probably involves a hierarchy of events and hundreds of genes (Bownes, 1986), some of which may not be directly associated with the synthesis of ovarian tissue per se. For example, oocyte development depends on the synthetic activities of other, non-oocytic cells. For most insects, yolk is synthesised outside of the ovary and secondarily imported into the oocyte (Raikhel and Dadhialla, 1992). Oocytes then take up the yolk proteins, such as vitellogenin, from the extracellular fluid by receptor-mediated endocytosis (Raikhel and Dadhialla, 1992). Transferrin too can be incorporated via this mechanism into developing eggs, and likely functions to mediate iron-uptake (Kucharski and Maleszka, 2003; Kurama et al., 1995). By contrast, genes encoding proteins that are essential for the regulation of the actin cytoskeleton (i.e. flotillin) or linking the cytoskeleton with major signalling pathways (i.e. profilin) are expressed directly within the ovarian tissue and are tightly regulated during development (Cooley et al., 1992). By sampling whole abdomens in our assay genes involved in different functional categories related to egg production are potentially detected.
Of the eight candidate genes examined, six did not change their expression with changes in reproductive state. These genes represent different, but overlapping functional categories: receptor/sensing molecules (octopamine receptor, take-out-like carrier protein), signalling cascades (nitric oxide synthase, profilin, take-out-like), structural dynamics (profilin, flotillin), and energy transfer (arginine kinase). Broadly, these genes can be regarded as developmental regulators (Table 1), but either they are not differentially expressed between CO2-treated and control worker groups, or their differential expression was not detected by the assay.
Two genes, vitellogenin and transferrin, which are both involved in oocyte packaging, did show differences in expression (> 4-fold; Fig. 2) following the double CO2 treatment. For vitellogenin, this response was temporally consistent with previous observations on like-treated virgin queens (Engels et al., 1976). Unlike queens, however, the change in worker vitellogenin expression was not conditional on bees being re-introduced into host colonies (Engels et al., 1976). Instead, workers responded to CO2 as they matured within cages, albeit as a group member rather than as lone individuals. More notably, the direction of the transcriptional response is opposite to that known for queens: workers decrease, rather than increase, vitellogenin synthesis in the abdomen following CO2 narcosis. This contrasting pattern of transcription in queens versus workers in response to CO2 treatment parallels the physiological pattern concerning ovary activation itself, and again accentuates the kin-selected difference in reproductive potential between these two castes.
Vitellogenin is a prerequisite for ovary activation in honeybees (Engels et al., 1990). Besides its proximate importance to egg production, however, vitellogenin is also used to synthesize proteinaceous royal jelly in the hypopharyngeal glands in the heads of workers. Nurse-age workers feed this jelly to dependent but related larvae, a behaviour that constitutes a form of kin-selected alloparental care. Amdam et al. (2003) speculate that a key adaptation to eusociality by honeybees was the diversion of vitellogenin from its primary role as a yolk protein to a secondary role in the production of brood food. As a consequence, vitellogenin is expected to be functionally associated with colony-level traits such as alloparental care and reproductive division of labour (Amdam et al., 2004). Our finding that vitellogenin synthesis is down-regulated in abdomens of ovary-deactivated workers implies that this molecule is actively linked to the reproductive status of individual workers. In these experiments caged bees were not exposed to larvae and thus it is not certain whether they would show a concomitant up-regulation of vitellogenin in their heads associated with nursing and the production of royal jelly. Nontheless, these results, together with available knowledge from queens, suggests that there is a general link between CO2 exposure, vitellogenin synthesis, and caste-specific ovary activation in honeybees. Vitellogenin is a promising new candidate component in the regulatory pathway that controls functional sterility in workers, a pathway of great theoretical significance that has never been empirically deconstructed.
The relationship between transferrin and ovary activation is less clear because transferrin is truly multifunctional in the honey bee (Kucharski and Maleszka, 2003). However, its co-regulation with vitellogenin in the current study, and it's selective incorporation into eggs during oogenesis of Sarcophaga (Kurama et al., 1995) and Riptortus (Hirai et al., 2000), suggests that transferrin may have an important role in the activation of worker ovaries. Transferrin's likely function is to provide essential iron ions to developing oocytes and embryos (Hirai et al., 2000), and may also play a defensive role by sequestering iron away from pathogens that have entered the egg (Weinberg, 1984). Our finding that transferrin is down-regulated in ovary deactivated workers implies that, like vitellogenin, it is intimately associated with ovary activation in honey bee workers. It seems less likely that transferrin would have played an active role in the evolution of honey bee alloparental care or the reproductive division of labour, but nonetheless its known function and observed kinetics suggest it too could be a component in the regulatory pathway that controls functional sterility in workers.
Speculation on regulation of ovary activation
The mechanism by which CO2 affects ovary activation via molecular intermediates in workers and queens is unknown, but there is some evidence to suggest that honeybees are sensitive to CO2 and that the gas is an important exogenous factor that modulates several aspects of social life. Firstly, honeybees are equipped with sensitive CO2 receptors on their antennae (Strange and Diesendorf, 1973). Workers use these antennal receptors to tightly regulate CO2 concentrations within their colonies (Seeley, 1974). Second, CO2 narcosis is known to affect honey bee foraging (Ebadi et al., 1980), hoarding (Mardan and Rinderer 1980), fanning behaviour (Seeley, 1974), sound production (Schneider and Gary, 1984), and some age-related polyethisms (Heran, 1952; Ribbands, 1950). Combined, these studies suggest that CO2 may be an important factor in the modulation of honeybee task specialization. Third, note that honeybee queens are kin-selected for extremely high fecundity and react to narcosis by accelerating reproductive development (Engels et al., 1976; Engels and Ramamurty, 1976; Mackensen, 1947), whereas workers are selected for low fecundity or sterility and react to narcosis by retarding reproductive development (Biedermann, 1964: Harris and Harbo, 1990; Harris et al., 1996; Kropàcovà et al., 1968; the present study). This contrasting effect of CO2 on queen versus worker reproduction suggests a caste-specific response to CO2 and, more generally, it suggests that CO2 differentially affects the regulatory mechanism underpinning honeybee reproductive division of labor.
One possibility is that exposure to CO2 affects the level of neurosecretions in worker brains (Harris et al., 1996). Levels of dopamine and serotonin, for example, co-vary with changes in worker ovarian development (Harris and Woodring, 1995) and even with CO2-induced changes in ovarian development (Harris et al., 1996). It is possible therefore that CO2 triggers the regulatory mechanism that controls worker sterility by affecting, for example, the level of dopamine or serotonin, which are putative up-stream components in the regulatory pathway that controls ovary activation in workers. A second or dual possibility is that CO2 affects the titre of juvenile hormone (JH) in the hemolymph (Bühler et al., 1983), which in turn affects age-related behavior in workers, possibly including reproduction (Robinson et al., 1991). Note that, unlike for insects generally, JH has an inverse relationship with vitellogenin in honey bee workers: high JH titre turns off vitellogenin synthesis (Pinto et al., 2000). Thus, JH and vitellogenin are causally linked, and CO2 may stimulate the regulatory mechanism that normally controls worker sterility by causing an increase in JH, which causes a decrease in vitellogenin, which presumably retards ovary activation, as has observed been here.
Whatever the initial changes are that narcosis causes within the nervous system, and that ultimately gives rise to observed differences in ovary activation, we can begin to infer down-stream regulatory components via their differential expression on ovary activation or deactivation, in particular as a consequence of CO2 treatment. This approach will give a first indication of molecules important to the maintenance of worker sterility.
Concluding remarks
Gene expression involves a cascade of events, from transcription factor transactivation to RNA processing and maturation. The candidate genes approach (Fitzpatrick et al., 2005) used in this study is based on molecules that are probably involved in relatively down-stream events and therefore may not represent molecules that initially trigger the process of ovary activation or deactivation, in particular as a consequence of CO2 treatment. Nonetheless, by identifying molecular components of regulatory pathways via their differential expression, we can begin to describe the molecular circuitry of reproductive regulation. Subsequent comparative studies of honey bee castes and of other insects for which regulatory mechanisms are much better understood (Bownes, 1986) will then be possible. An association between rate of vitellogenin and transferrin transcription and worker ovary activation has not previously been demonstrated, and it is suggested that these two proteins are part of the network involved in the regulation of worker ovary functional activation, and thus involved in the regulation of functional sterility of workers.
Acknowledgments
The authors would like to thank Julianne Lim for technical assistance, and Andrew B Barron and Shelley ER Hoover for useful discussion. Support for this work was provided by a grant from the Australian Research Council to BPO and RM and by ARC Special Centre for the Molecular Genetics and Development (RM). PK was supported by grants from the Thailand Research Fund and the Royal Golden Jubilee Ph.D. Program for RGJ No. 4.C.CU/44/B.1
References
- Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1799–1902. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honeybees. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron AB, Oldroyd BP, Ratnieks FLW. Worker reproduction in honey-bees (Apis) and the anarchic syndrome: a review. Behavioral Ecology and Sociobiology. 2001;50:199–208. [Google Scholar]
- Biedermann MZ. Neurosekretion bei Arbeiterinnen und Königinnen von Apis mellifica L. unter natürlichen und experimentellen Bedingungen. Zeitschrift für Wissenschaftliche Zoologie. 1964;170:256–308. [Google Scholar]
- Bownes M. Expression of the genes coding for vitellogenin (yolk protein) Annual Review of Entomology. 1986;31:507–531. [Google Scholar]
- Bühler A, Lanzrein B, Wille H. Influence of temperature and carbon dioxide concentration on juvenile hormone titer and dependent parameters of adult worker honeybees (Apis mellifera L) Journal of Insect Physiology. 1983;29:885–893. [Google Scholar]
- Cooley L, Verheyen E, Ayers K. Chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992;69:173–184. doi: 10.1016/0092-8674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- Dade HA. 1977 Anotomy and Dissection of the Honeybee. International Bee Research Association, London. [Google Scholar]
- Dawkins R. 1976 The Selfish Gene. Oxford Univ. Press, Oxford, UK. [Google Scholar]
- Ebadi R, Gary NE, Lorenzen K. Effects of carbon dioxide and low temperature narcosis on honeybees, Apis mellifera. Environmental Entomology. 1980;9:144–147. [Google Scholar]
- Engels W, Goncalves LS, Engels E. Effects of carbon dioxide on vitellogenin metabolism in unmated queen honeybees. Journal of Apicultural Research. 1976;15:3–10. [Google Scholar]
- Engels W, Ramamurty RS. Initiation of oogenesis in allectomized virgin honey bee queens by carbon dioxide treatment. Journal of Insect Physiology. 1976;22:1427–1432. [Google Scholar]
- Engels W. 1987 Reproduction and caste development in social bees. In: Eder J, Rembold H (eds.), Chemistry and Biology of Social Insects . Verlag J. Peperny, München, pp. 275–281. [Google Scholar]
- Engels W, Kaatz H, Zillikens A, Simoes ZLP, Trube A, Braun R, and Dittrich F. 1990 Honey bee reproduction: vitellogenin and caste-specific regulation of fertility. In: Eder J, Rembold H (eds.), Chemistry and Biology of Social Insects . Verlag J. Peperny, München, pp. 275–281. [Google Scholar]
- Fitzpatrick MJ, Ben-Shahar Y, Smid HM, Vet LEM, Robinson GE, Sokolowski MB. Candidate genes for behavioural ecology. Trends in Ecology & Evolution. 2005;20:96–104. doi: 10.1016/j.tree.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Grohmann L, Blenau W, Erber J, Ebert PR, Strunker T, and Baumann A. 2003 Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. Journal of Neurochemistry. 725–735. [DOI] [PubMed] [Google Scholar]
- Grozinger CM, Sharabash NM, Whitfield CW, Robinson GE. Pheromone-mediated gene expression in the honey bee brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14519–14525. doi: 10.1073/pnas.2335884100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K, Ivankovic-Dikic I, Shimokawa N, Kruh GD, Dikic I. Recruitment of Pyk2 and Cbl to lipid rafts mediates signals important for actin reorganization in growing neurites. Journal of Cell Science. 2004;117:2557–2568. doi: 10.1242/jcs.01148. [DOI] [PubMed] [Google Scholar]
- Harris JW, Harbo JR. Suppression of ovary development of worker honeybees by association with workers treated with carbon dioxide. Journal of Apicultural Research. 1990;29:187–193. [Google Scholar]
- Harris JW, Woodring J. Elevated brain dopamine levels associated with ovary development in queenless worker honeybees (Apis mellifera L) Comparative Biochemistry and Physiology. 1995;111C:271–279. [Google Scholar]
- Harris JW, Woodring J, Harbo JR. Effects of carbon dioxide on levels of biogenic amines in the brains of queenless worker and virgin queen honeybees (Apis mellifera) Journal of Apicultural Research. 1996;35:69–78. [Google Scholar]
- Heran H. Untersuchungen über den Temperatursinn der Honigbiene (Apis mellifera) unter besonderer Berücksichtigung der Wahrnehmung strahlender Wärme. Zeitschrift fur Vergleichende Physiologie. 1952;34:179–206. [Google Scholar]
- Hirai M, Watanabe D, Chinzei Y. A juvenile hormone-repressible transferrin-like protein from the bean bug, Riptortus clavatus : cDNA sequence analysis and protein identification during diapause and vitellogenesis. Archives of Insect Biochemistry and Physiology. 2000;44:17–26. doi: 10.1002/(SICI)1520-6327(200005)44:1<17::AID-ARCH3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Kolodziejczyk R, Kochman M, Bujacz G, Dobryszycki P, Ozyhar A, Jaskolski M. Crystallization and preliminary crystallographic studies of juvenile hormone-binding protein from Galleria mellonella haemolymph. Acta Crystallographica Section D - Biological Crystallography. 2003;59:519–521. doi: 10.1107/s0907444902022904. [DOI] [PubMed] [Google Scholar]
- Kropàcovà S, Haslbachova H, Novàk VL. Development of honeybee ovaries as affected by narcosis and injections of certain substances. Sborn. Vys. zemed. v. Brne. 1968;16:537–543. [Google Scholar]
- Kucharski R, Maleszka R. Arginine kinase is highly expressed in the compound eye of the honey bee, Apis mellifera. Gene. 1998;211:343–349. doi: 10.1016/s0378-1119(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Kucharski R, Maleszka R. Evaluation of differential gene expression during behavioural development in the honeybee using microarrays and northern blots. Genome Biology. 2002;3:7.1–7.9. doi: 10.1186/gb-2002-3-2-research0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski R, Maleszka R. Transcriptional profiling reveals multifunctional roles for transferrin in the honeybee, Apis mellifera. Journal of Insect Science. 2003;3:1–8. doi: 10.1093/jis/3.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurama T, Kurata S, Natori S. Molecular characterization of an insect transferrin and its selective incorporation into eggs during oogenesis. European Journal of Biochemistry. 1995;228:229–235. [PubMed] [Google Scholar]
- Laidlaw HH, Page REJ. 1997 Queen Rearing and Bee Breeding. Wicwas Press, Cheshire, USA. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mackensen O. Effect of carbon dioxide on initial oviposition of artificially inseminated and virgin queen bees. Journal of Economic Entomology. 1947;40:344–349. doi: 10.1093/jee/40.3.344. [DOI] [PubMed] [Google Scholar]
- Mardan M, Rinderer TE. Effects of carbon dioxide and cold anesthesia on the hoarding behavior of the honeybee. Journal of Apicultural Research. 1980;19:149–153. [Google Scholar]
- Maynard Smith J, Szathmàry E. 1995 The Major Transitions in Evolution. W. H. Freeman, New York, NY. [Google Scholar]
- Nilsson I, Shibuya M, Wennstrom S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Experimental Cell Research. 2004;299:476–485. doi: 10.1016/j.yexcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Oldroyd BP, Smolenski AJ, Cornuet JM, Crozier RH. Anarchy in the beehive. Nature. 1994;371:749. [Google Scholar]
- Oldroyd BP, Osborne KE. The evolution of worker sterility in honeybees: the genetic basis of failure of worker policing. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:1335–1339. [Google Scholar]
- Pinto LZ, Bitondi MMG, Simoes ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. Journal of Insect Physiology. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Piulachs MD, Guidugli KR, Barchuk AR, Cruz J, Simoes ZLP, Belles X. The vitellogenin of the honey bee, Apis mellifera : structural analysis of the cDNA and expression studies. Insect Biochemistry and Molecular Biology. 2003;33:459–465. doi: 10.1016/s0965-1748(03)00021-3. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Dadhialla TS. Accumulation of yolk proteins in insect oocytes. Annual Review of Entomology. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- Ribbands CR. Changes in the behavior of honey-bees following their recovery from anaesthesia. Journal of Experimental Biology. 1950;27:302–310. doi: 10.1242/jeb.27.3.302. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Strambi C, Strambi A, Feldlaufer MF. Comparison of juvenile hormone and ecdysteroid haemolymph titers in adult worker and queen honey bee (Apis mellifera) Journal of Insect Physiology. 1991;37:929–935. [Google Scholar]
- Schneider SS, Gary NE. Quacking - a sound produced by worker honeybees after exposure to carbon dioxide. Journal of Apicultural Research. 1984;23:25–30. [Google Scholar]
- Seeley T. Atmospheric carbon dioxide regulation in honey-bee (Apis mellifera) colonies. Journal of Insect Physiology. 1974;20:2301–2305. doi: 10.1016/0022-1910(74)90052-3. [DOI] [PubMed] [Google Scholar]
- Strange G, Diesendorf D. The response of the honeybee antennal CO2-receptors to N2O and Xe. Journal of Comparative Physiology. 1973;86:139–158. [Google Scholar]
- Tian HS, Vinson SB, Coates CJ. Differential gene expression between alate and dealate queens in the red imported fire ant, Solenopsis invicta Buren (Hymenoptera : Formicidae) Insect Biochemistry and Molecular Biology. 2004;34:937–949. doi: 10.1016/j.ibmb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Iron withholding: A defense against infection and neoplasia. Physiological Reviews. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]


