Abstract
Once mated, the optimal strategy for females of the monandrous butterfly, Pararge aegeria, is to avoid male contact and devote as much time as possible to ovipositing, as there is little advantage for females to engage in multiple matings. In other butterfly species the presence of males during egg laying has been shown to affect aspects of oviposition behavior and it has been suggested that repeated interference from males has the potential to reduce reproductive output. The aim of this study was to assess the effects of male presence during oviposition on reproductive output and behavior of a population of P. aegeria obtained from Madeira Island, Portugal, and maintained in the laboratory. Two experiments were performed where females were housed individually in small cages. Experiment 1 examined how social factors influenced the egg laying behavior of females. To do this the presence or absence of males was manipulated and egg size and number was measured over the first 14 days of oviposition. It was observed that when males were present during oviposition females made a trade-off between egg size and number. Experiment 2 examined how social factors affected oviposition site choice. Again, male presence/absence was manipulated, but in this experiment where the female laid her egg in relation to host quality was scored, and the size of the egg laid was measured. In the absence of males females selectively positioned their larger eggs on good quality host plants. However, selective oviposition was no longer observed when females were in the presence of males. We suggest that P. aegeria females from the Madeira Island population are adapted for a flexible oviposition strategy, governed by external cues, allowing a trade-off between egg size and number when the time available for egg laying is limiting.
Keywords: male presence, egg size-number trade-off, host plant choice
Introduction
The choices that a female makes at oviposition can greatly affect the survival and development of her offspring (Awmack and Leather, 2002; Fox and Czesak, 2000; Réale and Roff, 2002; Thompson and Pellymyr, 1991). At oviposition a female may vary her egg laying in response to many factors including; her own state, e.g. the quantity of nutritional reserves, the environmental conditions she encounters, e.g. host quality (Heimpel and Rosenheim, 1995), in response to trade-offs between clutch size and adult survival (Roff, 1992), and current and future reproduction (Rosenheim, 1999).
One frequently studied reproductive trade-off is that of propagule size versus propagule number. The classic Smith & Fretwell (1974) model predicts a negative relationship between propagule size and number, and suggests that for each species a particular combination must exist to maximize fitness. Many studies have since shown that in unpredictable environments selection will favor a bet-hedging reproductive behavior where a range of propagule sizes and numbers are produced to increase the lifetime reproductive success (Clutton-Brock, 1991; Fox and Czesak, 2000; Roff, 1992; Stearns, 1992). Studies on various insect species demonstrate that larger offspring should be produced when conditions for offspring growth and survival are poor (Awmack and Leather, 2002; Carriére and Roff, 1995; Fischer et al., 2003a; Fischer et al., 2003b; Fox et al., 1997; Leather and Burnand, 1987; Rotem et al., 2003; Savalli and Fox, 2002; Takakura, 2004). However, the model of Mangel et al. (1994) predicts that mothers should choose larger clutch sizes, thereby producing small less fecund offspring, when their offspring inhabit a poor environment. Therefore, the time when mothers should choose larger offspring over smaller offspring (and vice versa) is still subject to debate.
Some insect species adjust the size-number trade-off of their offspring according to the quality of the hosts that they encounter. The time available for females to search for optimal oviposition sites has the potential to greatly affect the decision-making process of females. Behavioral plasticity during oviposition enables females to increase the rate at which hosts are found when time is limited. However, the strategies employed to increase host encounter rate may result in a decline in the quality of hosts on which eggs are laid (Papaj, 2000). Time limitation therefore also has the potential to affect this trade-off. At high male densities, repeated male harassment of females has the potential to reduce the time available for oviposition, and may result in a reduction in the reproductive success of females (Boisseau, 1996).
Preliminary data collected by Boisseau (1996) suggested that the presence of males during the first week of oviposition may cause Pararge aegeria (L) females to be engaged in a high level of activity, thus minimizing the time available for oviposition and oviposition resting. Although further studies are required to confirm this finding, this observation may provide an explanation for the cryptic post-copulatory behavior of P. aegeria females, with active avoidance of males (Wickman and Wiklund, 1983). Other butterfly species also show active avoidance of males once mated (Baguette et al., 1996; Odendaal et al., 1989; Shapiro, 1970; Wickman, 1986). This post-copulation behavior may have evolved to reduce the potential cost (to the offspring) of the differing mating strategies between the two sexes. Although previous studies of other butterfly species have shown that high male density affects oviposition behavior (Baguette et al., 1996; Odendaal et al., 1989; Ohtani, 1985; Shapiro, 1970; Wickman, 1986) few have studied the effect of male presence on egg laying itself.
Female lepidoptera, particularly capital breeders (Tammaru and Haukioja, 1996), are known to lay larger eggs at the start of the oviposition period and gradually lay smaller eggs over time as their larval derived resources become depleted (Begon and Parker, 1986; Boggs, 1986; Harvey, 1977; Karlsson, 1987; Karlsson and Wiklund, 1985; Torres-Vila and Rodriguez-Molina, 2002; Wellington, 1965; Wickman and Karlsson, 1987; Wiklund and Karlsson, 1984; Wiklund and Persson, 1983). Although this trend has frequently been observed in butterfly species, as yet it is unknown whether female butterflies are able to adjust their clutch size in response to the environment or the social conditions they encounter.
This study aims to examine the combined effect of the environmental condition host quality, and of the social factor male presence, on the oviposition behavior and the egg size number trade-off. Two experiments were performed where individual females were housed in small cages either in the presence or absence of males during oviposition. In the first experiment we examined how social factors influenced both the number and size of eggs that females lay. In the second experiment the effect of social factors on oviposition site choice, and the size of the egg laid, was measured. At oviposition, females in the presence of males were expected to change their egg laying and either lay a larger number of small eggs, or lay a smaller number of large eggs.
Materials and Methods
Study organism
In 2000 at Portela on Madeira Island, Portugal, 55 eggs of P. aegeria were collected along two transects, one egg was removed from each host-plant Brachypodium sylvaticum (Beauv., 1762), and brought to the Manchester Metropolitan University butterfly house for rearing. After eclosion, these stock adults (n = 30, m: f sex ratio = 1:1) were used and the colony was maintained over 5 generations in a flight cage 1.25 × 3.90 × 1.80m. Adults were fed daily with a 10% honey solution via 5 artificial flowers (for design see Cory and Goulson, 1993), distributed at random in the flight cage. Honey supplies were replenished daily. Ten B. sylvaticum plants were made available for each generation of females to lay their eggs. These plants were distributed randomly throughout the flight cage. Photoperiod (Nylin et al., 1995) and temperature (Sibly et al., 1997) are known to affect the development of P. aegeria larvae. A 12:12h LD cycle, a temperature of 21 2° C and a humidity of 50 10% were therefore strictly maintained for the whole of the growth period (i.e. from egg stage to adult stage). Lighting in the butterfly house was provided at a frequency of 50/60 Hz by 8 ceiling lamps.
Factors that are known to affect female fecundity were controlled. Parental age has been shown to affect fecundity (LaMunyon and Eisner, 1993; Svärd and Wiklund, 1989; Wedell and Cook, 1998; Wiklund and Persson, 1983) therefore, for the first mating, all of the males and females were randomly selected and introduced on the first day of eclosion where they were both willing and able to mate. The age of all of the non-virgin males used were within 1 or 2 days of each other.
Female reproductive output is strongly dependent on the resources accumulated in the larval stage (Boggs, 1981) therefore all of the females used in this study were reared singly and provided with food ad libitum to avoid food shortages. At the end of the experiment, a random subset of females from each treatment (in total n = 19) was selected and their left forewing was measured (mm) using digital calipers. This measurement was taken as an overall indicator of body size.
Host-plants
The larval host-plants, B. sylvaticum, were grown from commercially produced seed, and sown in 4-inch pots containing soil-based compost. To reduce variation in quality, all of the plants were reared under identical conditions at a temperature of 23 ± 2° C and a humidity of 45 ± 10 %. Light was provided at an intensity of 7000 Lux over a 16:8h LD cycle. Plants were watered daily, but never fertilized. All of the plants were used when they had between 50 and 70 blades. Each host plant was only used once, so that one plant was assigned at random to an individual female and used in the experimental procedures described below.
Experimental procedures
Experiment 1: The effect of social factors on oviposition
To determine how the presence of males during oviposition affects egg laying pattern and behavior of individual females, 62 newly emerged virgin males and virgin females were placed together in pairs in a cage (0.5m × 0.5m × 0.5m) along with a B. sylvaticum plant and an artificial flower (after Cory and Goulson, 1993). This flower was re-filled with a 10% honey solution, once daily, to provide the adult butterflies with a permanent food source.
In the first treatment group (n = 31), the adult pair were left until the first egg was laid. When the first egg was laid, the male was removed from the cage and the female was left to lay the rest of her eggs, unharassed, over 14 days of oviposition. The number of eggs laid in total was recorded. All of the eggs from each female were kept until hatching to ensure that mating had been successful.
In the second treatment group (n = 31), the adult pair were left until the first egg was laid, but on the day of first egg laying, instead of removing the male, an extra virgin male was placed in the cage. To maintain harassment levels throughout the oviposition period, the males were replaced every 2 days with 2 new non-virgin males.
On the first day of oviposition, for each female, 3 eggs were selected at random and their diameter was measured using a micrometer. Repeatability tests were performed on egg size to determine how much within-day variation existed in the daily samples collected for each individual. Using these three egg measurements, the repeatability (t), or the proportion of variation due to differences between, rather than within females in egg size, was calculated using ANOVA (Becker, 1992). Due to the highly repeatable nature of the eggs laid (Boake, 1989; Falconer and Mackay, 1996) by each individual (t = 0.54 ± 0.04, n = 62), for the remainder of the oviposition period only one of the eggs collected, per day, per individual, was used for diameter measurements. These daily egg measurements were then used to calculate mean egg size, per individual, over the whole 14 days of oviposition.
Experiment 2: The effect of social factors on host selection
To determine how the presence of males during oviposition affects the selection of egg laying sites, two treatment groups were again created, as described above. In the first treatment group, females were alone during their oviposition period (n = 14). In the second treatment group, females were kept with 2 males throughout their oviposition period (n = 14). In this experiment, each day of oviposition (n = 14 days) the plant was removed from the cage and inspected for eggs. Each egg on the plant was removed from each blade of grass and the health of the blade was recorded. Health of blade was taken as a measure of oviposition site choice and was measured as either; 100% (green blade, no brown blotches or wilting) 50% (less than 50% of the blade is wilting or brown) or 0% (blade of grass is dead, totally brown and wilted). For each female, half of the eggs laid in each of the oviposition categories were kept and their size was recorded. Egg size was recorded daily by measuring the diameter using a micrometer.
Statistical analysis
The total egg laying behavior of 90 females was examined in experiments 1 and 2. The total number of eggs laid was observed to range between 1 and 269. Egg size was observed to range between 0.600 and 1.120 mm. All of the mean egg size and mean total egg number values calculated per individual were included in analyses. We confirmed the validity of including all data by testing with and without the extreme values. Although the slope changes, neither the pattern nor the statistical significance was affected. All the data were therefore included as they appeared to be biologically meaningful.
Experiment 1: The effect of social factors on oviposition
A least squares regression was performed to investigate the existence of a trade-off in mean egg size and total number of eggs laid, and to determine whether the presence of males during oviposition may affect any such trade-off. Thus, for each treatment our null hypothesis was the same, that there is no relationship between egg size and number. All statistical procedures were performed using Systat 9.0.
Experiment 2: The effect of social factors on host selection
To test whether females alter their egg-laying behavior in response to both the size of the egg they have to lay and the social conditions that they encounter, we scored where the female laid her egg in relation to the choice of the blade of grass as described above. Females alight at an oviposition site and within 2 seconds deposit an egg (personal observation). This would suggest that eggs are pre-packaged on arrival, and that egg production is not completed after the oviposition site is chosen. For this reason, blade choice was assigned the dependent variable. The dependent variable, blade choice was categorical, with 3 categories, while egg size was measured on a continuous scale and social condition measured as 2 categories, male absence (treatment 1) and male presence (treatment 2). Given the nature of these data, regression with categorical dependent variable, or logistic regression (Wilkinson et al., 1996), was used to test the hypothesis. Distance-weighted least-squares (DWLS) nonparametric curve fitting was used to visualize the relationship between egg size and blade choice (Wilkinson et al., 1996).
Results
Experiment 1: The effect of social factors on oviposition
To examine how the presence or absence of males during oviposition affects the reproductive output of individual females the relationship between mean egg size and the total number of eggs laid was examined. A least squares regression was performed for both groups of females to investigate the existence of a trade-off in mean egg size and the total number of eggs laid. For females on their own during oviposition, a negative relationship between egg number and egg size was observed but this relationship was non-significant (F = 0.797, d.f. = 1, 23, P = 0.381). For females in the presence of males during oviposition, a significant negative relationship between egg size and number was observed (F = 10.333, d.f. = 1, 24, P = 0.0037). In the presence of males, females make a trade-off between egg size and number, laying a larger number of small eggs (Figure 1).
Fig. 1.
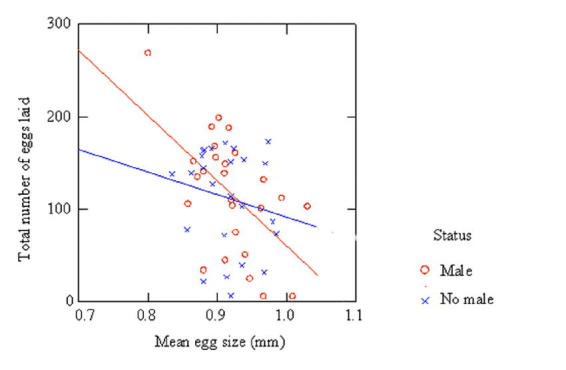
A least squares regression to determine the relationship between mean egg size (mm) and total number of eggs laid. Regression for individual females where males were present during oviposition; F = 10.333, d.f = 1, 24, P = 0.0037. Regression for individual females that were on their own during oviposition; F = 0.797, d.f = 1, 23, P = 0.381.
Experiment 2: The effect of social factors on host selection
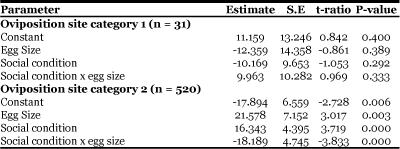
Laying behavior on completely healthy blades (oviposition site choice, category 3, n = 146) was compared to laying on blades with some damage (category 2), and to laying on completely damaged blades (category 1). For logistic regression analysis we considered oviposition site choice category 3 to be the referent sample, and categories 1 and 2 were evaluated with respect to it (Sokal et al., 1995).
Category 2 oviposition sites had substantially lower odds of possessing large eggs, or eggs that were laid by females that were alone during oviposition. Females were much more likely to lay eggs on completely healthy blades if they are laying large eggs (Table 1, estimate = 21.578, t = 3.017, P = 0.003) or when unharassed (Table 1, estimate = 16.343, t = 3.719, P < 0.001). Furthermore, the effect of harassment interacts with egg size, such that when females were in the presence of males they do not choose oviposition sites according to the size of the egg they have to lay (Table 1, interaction estimate = −18.189, t = −3.833, P < 0.001). A similar pattern was not observed for category 1 oviposition sites, but this may be due to the sample of eggs laid at these sites being small (Table 1). There was, however, a significant overall effect of egg size and male presence on the choice of oviposition site (log-likelihood of constants only model = 35.458, df = 6, Chi-square P < 0.001).
Table 1.
A summary of logistic regression results relating oviposition site choice (category 1, 2 or 3) to egg size and social condition.
These effects can be visualized with non-parametric DWLS regression (Figure 2). When harassed, female behavior was unaffected by the size of the egg she is laying. In contrast, if the female is free from harassment, the larger the egg the more likely the female was to lay an egg on a healthy blade.
Fig. 2.
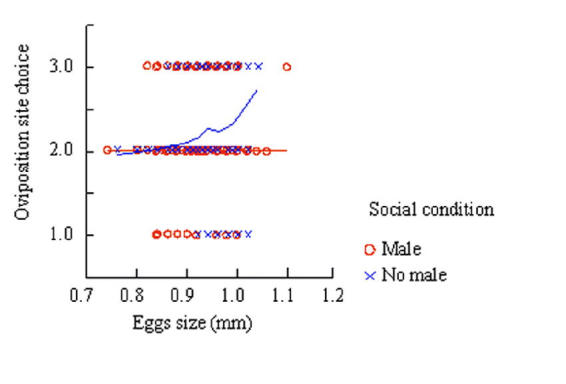
Non parametric DWLS (distance weighted least squares) regression to show if the presence of males during oviposition prevents females from selectively laying their large eggs at healthy oviposition sites. A non-parametric regression was used to visualize the relationship between the categorical dependent variable and the continuous independent variable without constraining the nature of the pattern that would be seen. The dependent variable, oviposition site choice was categorical, with 3 categories, where 1 is a low quality site and 3 is a high quality site. Egg size was measured on a continuous scale. Social condition measured as 2 categories, male presence (red data points) and male absence (blue data points).
Discussion
Females in the absence of males during oviposition were observed to adjust the quality of their host plant selection according to the size of the egg that they laid, laying larger eggs on high quality hosts (cf. Papaj, 2000). However, females that were time limited due to the presence of males during oviposition made a trade-off between egg size and number, laying a larger number of small eggs. This suggests that Madeira Island populations of P. aegeria follow the Smith & Fretwell model (1974) and have a negative relationship between egg size and number when time is limiting. Further, these females were not selective about host quality and did not position their large eggs at high quality sites. Under time-constrained conditions, therefore, female P. aegeria maximize their host encounter rate by laying a larger number of small eggs and by being less selective about host quality. This finding would add support to the model of Mangel et al. (1994).
Wiklund and Persson (1983) examined the oviposition strategy of laying gradually smaller eggs over time and suggested that females could maximally increase their fecundity by 25% if they laid small eggs only, and questioned why P. aegeria females were not selected for high fecundity. The results from this study suggest that by adopting an intermediate strategy between high fecundity (many smaller eggs) and low fecundity (fewer larger eggs), females are more flexible, and can adapt their oviposition strategy according to external cues. When time to search for suitable oviposition sites was limiting (i.e. in the presence of males), the selection of egg laying sites was not biased by egg size, and females showed no active selection. These results add support to preliminary data collected by McClellan (2000) who found that time constraints during oviposition, as a result of male harassment, resulted in an increase in the frequency of egg clumping. Under time constrained conditions, therefore, females adopt a high fecundity oviposition strategy to maximize the host plant encounter rate. Conversely, when the time available to search for optimal oviposition sites was unrestricted by external pressures, females adopted a low fecundity oviposition strategy. This low fecundity strategy is beneficial because each large egg is deposited at an optimal oviposition site, thus maximizing offspring survival (Minkenberg et al., 1992).
Other studies hypothesized that larvae hatching from large eggs would have increased fitness, and Torres-Vila & Rodriguez-Molina (2002) have recently found that for Lobesia botrana, large larvae had greater starvation resistance than small larvae from small eggs. But studies with P. aegeria have not, so far, found differences between large and small larvae for larval mortality, larval development, starvation resistance or subsequent pupal weight (Karlsson and Wiklund, 1985; Wiklund and Persson, 1983). This would suggest that there may be another, as yet unknown, benefit from laying large eggs explaining our finding that females are more selective about where they lay their large eggs.
The mechanism by which the females in this study were able to ‘control’ the size of the eggs that they laid is unclear, and therefore requires further examination. Torres-Vila and Rodriguez-Molina (2002) found that water stressed female L. botrana significantly reduced the size of their eggs, and argued that even though females eclose with a large complement of mature eggs (termed pro-ovigeny; Rosenheim, 1999), water ingestion between mating and the start of oviposition was crucial for increasing the size of the eggs produced. Male presence during oviposition is known to increase the activity levels of P. aegeria females (Boisseau, 1996). This increased activity may cause females to become dehydrated, and this may be physiologically responsible for the smaller egg sizes observed for females kept at high male density. We suggest, therefore, that water regulation may be important over the whole oviposition period.
Additional studies are required to determine the particular nature of the stress imposed by male presence. In addition, examination of the affect of other social factors (e.g. the presence of multiple females) on oviposition behavior is recommended.
Acknowledgments
Thanks to Katherine McClellan, Sarah Blain and Lisa Hall for their help with the collection of the egg data and for their contribution to design of the methodology. Richard Preziosi, Nina Wedell and Casper Breuker provided helpful comments on an earlier version of the manuscript. Maggi Gapper and Janet Bunter are thanked for their technical support and maintenance of the plant stocks.
References
- Awmack CS, Leather SR. Host-plant quality and fecundity in herbivorous insects. Annual Review of Entomology. 2002;47:817–844. doi: 10.1146/annurev.ento.47.091201.145300. [DOI] [PubMed] [Google Scholar]
- Baguette M, Convie I, Neve G. Male density affects female spatial behavior in the butterfly Proclossiana-eunomia. Acta Oecologica. 1996;17:225–232. [Google Scholar]
- Becker WA. 1992 Manual of quantitative genetics. McNaughton & Gunn Inc., Ann Arbor. [Google Scholar]
- Begon M, Parker GA. Should egg size and clutch size decrease with age? Oikos. 1986;47:293–302. [Google Scholar]
- Boake CRB. Repeatability: its role in evolutionary studies of mating behavior. Evolutionary Ecology. 1989;3:173–182. [Google Scholar]
- Boggs CL. Selection pressures affecting male nutrient investment at mating in heliconiine butterflies. Evolution. 1981;35:931–940. doi: 10.1111/j.1558-5646.1981.tb04959.x. [DOI] [PubMed] [Google Scholar]
- Boggs CL. Reproductive strategies of female butterflies: variation in and constraints on fecundity. Ecological Entomology. 1986;11:7–15. [Google Scholar]
- Boisseau SD. 1996 Reproductive patterns of the speckled wood butterfly Pararge aegeria: factors determining variations in fecundity and longevity with a note on interspecific competition with Pararge xiphia, on Madeira. Unpublished MSc thesis. Manchester Metropolitan University. [Google Scholar]
- Carriére Y, Roff DA. The evolution of offspring size and number: a test of the Smith-Fretwell model in three species of crickets. Oecologia. 1995;102:389–396. doi: 10.1007/BF00329806. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. 1991 The evolution of parental care. Princeton University Press, Princeton. [Google Scholar]
- Cory JS, Goulson D. Flower constancy and learning in foraging preferences of the green veined butterfly Pieris napi. Ecological Entomology. 1993;18:315–320. [Google Scholar]
- Falconer DS, Mackay T. 1996 Introduction to Quantitative Genetics. 4th edn. Longman Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Bot ANM, Brakefield PM, Zwaan B.J. Fitness consequences of temperature-mediated egg size plasticity in a butterfly. Functional Ecology. 2003a;17:803–810. [Google Scholar]
- Fischer K, Brakefield PM, Zwaan BJ. Plasticity in butterfly egg size: Why larger offspring at lower temperatures? Ecology. 2003b;84:3138–3147. [Google Scholar]
- Fox CW, Czesak ME. Evolutionary ecology of progeny size in arthropods. Annual Review of Entomology. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341. [DOI] [PubMed] [Google Scholar]
- Fox CW, Thakar MS, Mousseau TA. Egg size plasticity in a seed beetle: an adaptive maternal effect. American Naturalist. 1997;149:149–163. [Google Scholar]
- Harvey GT. Mean weight and rearing performance of successive egg clusters of eastern spruce budworm (Lepidoptera: Tortricidae) Canadian Entomologist. 1977;109:487–496. [Google Scholar]
- Heimpel GE, Rosenheim JA. Dynamic host feeding by the parasitoid Aphytis melinus: the balance between current and future reproduction. Journal of Animal Ecology. 1995;64:153–167. [Google Scholar]
- Karlsson B. Variation in egg weight, oviposition rate and reproductive reserves with female age in a natural population of the speckled wood butterfly, Pararge aegeria. Ecological Entomology. 1987;12:473–476. [Google Scholar]
- Karlsson B, Wiklund C. Egg weight variation in relation to egg mortality and starvation endurance of newly hatched larvae in some satyrid butterflies. Ecological Entomology. 1985;10:205–211. [Google Scholar]
- LaMunyon CW, Eisner T. Post copulatory sexual selection in an arctiid moth (Utetheisa ornatrix) Proceedings of the National Academy of Science U.S.A. 1993;90:4689–4692. doi: 10.1073/pnas.90.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leather SR, Burnand AC. Factors affecting life-history parameters of the pine beauty moth, Panolis flammea (D&S): the hidden costs of reproduction. Functional Ecology. 1987;1:331–338. [Google Scholar]
- Mangel M, Rosenheim JA, Alder FR. Clutch size, offspring performance and intergenerational fitness. Behavioural Ecology. 1994;5:412–417. [Google Scholar]
- McClellan KE. 2000 The effects of male harassment on female ovipositioning in the speckled wood butterfly (Pararge aegeria). Unpublished BSc (Hons) thesis. Manchester Metropolitan University. [Google Scholar]
- Minkenberg OPJM, Tatar M, Rosenheim JA. Egg load as a source of variability in insect foraging and oviposition behavior. Oikos. 1992;65:134–142. [Google Scholar]
- Nylin S, Wickman P-O, Wiklund C. Life-cycle regulation and life history plasticity in the speckled wood butterfly: are reaction norms predictable? Biological Journal of the Linnean Society. 1995;55:143–157. [Google Scholar]
- Odendaal FJ, Turchin P, Sturmitz FR. Influence of host -plant density and male harassment on the distribution of female Euphydryas anicia (Nymphalidae) Oecologia. 1989;78:283–288. doi: 10.1007/BF00377167. [DOI] [PubMed] [Google Scholar]
- Ohtani T. The adult behavior of the Japanese Cabbage White (Lepidoptera, Pieridae) in the field. Tyo to Ga. 1985;36:43–76. [Google Scholar]
- Papaj DR. Ovarian dynamics and host use. Annual Review of Entomology. 2000;45:423–448. doi: 10.1146/annurev.ento.45.1.423. [DOI] [PubMed] [Google Scholar]
- Réale D, Roff DA. Quantitative genetics of oviposition behavior and interactions among oviposition traits in the sand cricket. Animal Behaviour. 2002;64:397–406. [Google Scholar]
- Roff DA. 1992 The evolution of life histories: theory and analysis. Chapman & Hall, New York. [Google Scholar]
- Rosenheim JA. The relative contributions of time and eggs to the cost of reproduction. Evolution. 1999;53:376–385. doi: 10.1111/j.1558-5646.1999.tb03773.x. [DOI] [PubMed] [Google Scholar]
- Rotem K, Agrawal AA, Laima K. Parental effects in response to variation in food quality: adaptive plasticity across generations? Ecological Entomology. 2003;28:211–218. [Google Scholar]
- Savalli UM, Fox CW. Proximate mechanisms influencing egg size plasticity in the seed beetle Stator limbatus (Coleoptera: Bruchidae) Arthropod Biology. 2002;95:724–734. [Google Scholar]
- Shapiro AM. The role of sexual behavior in density-related dispersal of pierid butterflies. American Naturalist. 1970;104:367–372. [Google Scholar]
- Smith CC, Fretwell SD. The optimal balance between size and number of offspring. American Naturalist. 1974;108:499–506. [Google Scholar]
- Sibly RM, Winokur L, Smith RH. Interpopulation variation in phenotypic plasticity in the speckled wood butterfly, Pararge aegeria. Oikos. 1997;78:323–330. [Google Scholar]
- Sokal RR, Rohlf FJ. 1995 Biometry: the principles and practice of statistics in biological research. 3d ed. WH Freeman & Company, USA. [Google Scholar]
- Stearns SC. 1992 The evolution of life histories. Oxford University Press, Oxford. [Google Scholar]
- Svärd L, Wiklund C. Mass and production rates of ejaculates in relation to monandry/polyandry in butterflies. Behavioural Ecology and Sociobiology. 1989;24:395–402. [Google Scholar]
- Takakura KI. Variation in egg size within and among generations of the bean weevil, Bruchidius dorsalis (Coleoptera, Bruchidae): Effects of host plant quality and paternal nutritional investment. Annals of the Entomologcal Society of America. 2004;97:346–352. [Google Scholar]
- Tammaru T, Haukioja E. Capital breeders and income breeders among lepidoptera – Consequences to population dynamics. Oikos. 1996;77:561–564. [Google Scholar]
- Thompson JN, Pellymyr O. Evolution of oviposition behavior and host preference in lepidoptera. Annual Review of Entomology. 1991;36:65–89. [Google Scholar]
- Torres-Vila LM, Rodriguez-Molina MC. Egg size variation and its relationship with larval performance in the Lepidoptera: the case of the European grapevine moth Lobesia botrana. Oikos. 2002;99:272–283. [Google Scholar]
- Wedell N, Cook PA. Determinants of paternity in a butterfly. Proceedings of the Royal Society of London Series B. 1998;265:625–630. [Google Scholar]
- Wellington WG. Some maternal influences on progeny quality in the Western Tent caterpillar Malacosoma plunale (Dyar) Canadian Entomologist. 1965;97:1–14. [Google Scholar]
- Wickman P-O. Courtship solicitation by females of the small heath butterfly Coenonympha pamphilus (Lepidoptera: Satyridae) and their behavior in relation to male territories before and after copulation. Animal Behaviour. 1986;34:153–157. [Google Scholar]
- Wickman P-O, Karlsson B. Changes in egg colour, egg weight, and oviposition rate with the number of eggs laid by wild females of the small heath butterfly Coenonympha pamphilus. Ecological Entomology. 1987;12:109–114. [Google Scholar]
- Wickman P-O, Wiklund C. Territorial defence and its seasonal decline in the speckled wood butterfly (Pararge aegeria) Animal Behaviour. 1983;31:1206–1216. [Google Scholar]
- Wiklund C, Karlsson B. Egg size variation in satyrid butterflies: adaptive vs. historical, “Bauplan”, and mechanistic explanations. Oikos. 1984;43:391–400. [Google Scholar]
- Wiklund C, Persson B. Fecundity, and the relation of egg weight variation to offspring fitness in the speckled wood butterfly Pararge aegeria, or why don't butterfly females lay more eggs? Oikos. 1983;40:53–63. [Google Scholar]
- Wilkinson L, Blank G, and Gruber C. 1996 Desktop data analysis with Systat. Prentice Hall Publishers, New Jersey. [Google Scholar]


