Abstract
The melon fruit fly, Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) is distributed widely in temperate, tropical, and sub-tropical regions of the world. It has been reported to damage 81 host plants and is a major pest of cucurbitaceous vegetables, particularly the bitter gourd (Momordica charantia), muskmelon (Cucumis melo), snap melon (C. melo var. momordica), and snake gourd (Trichosanthes anguina). The extent of losses vary between 30 to 100%, depending on the cucurbit species and the season. Its abundance increases when the temperatures fall below 32° C, and the relative humidity ranges between 60 to 70%. It prefers to infest young, green, soft-skinned fruits. It inserts the eggs 2 to 4 mm deep in the fruit tissues, and the maggots feed inside the fruit. Pupation occurs in the soil at 0.5 to 15 cm below the soil surface. Keeping in view the importance of the pest and crop, melon fruit fly management could be done using local area management and wide area management. The melon fruit fly can successfully be managed over a local area by bagging fruits, field sanitation, protein baits, cue-lure traps, growing fruit fly-resistant genotypes, augmentation of biocontrol agents, and soft insecticides. The wide area management program involves the coordination of different characteristics of an insect eradication program (including local area options) over an entire area within a defensible perimeter, and subsequently protected against reinvasion by quarantine controls. Although, the sterile insect technique has been successfully used in wide area approaches, this approach needs to use more sophisticated and powerful technologies in eradication programs such as insect transgenesis and geographical information systems, which could be deployed over a wide area. Various other options for the management of fruit fly are also discussed in relation to their bio-efficacy and economics for effective management of this pest.
Keywords: host range, distribution, sterile insect technique, insect-transgenesis, management
Introduction
The dipteran family Tephritidae consists of over 4000 species, of which nearly 700 species belong to Dacine fruit flies (Fletcher, 1987). Nearly 250 species are of economic importance, and are distributed widely in temperate, sub-tropical, and tropical regions of the world (Christenson and Foote, 1960). The first report on melon fruit flies was published by Bezzi (1913), who listed 39 species from India. Forty-three species have been described under the genus Bactrocera including cucurbitae, dorsalis, zonatus, diversus, tau, oleae, opiliae, kraussi, ferrugineus, caudatus, ciliatus, umbrosus, frauenfeldi, occipitalis, tryoni, neohumeralis, opiliae, jarvisi, expandens, tenuifascia, tsuneonsis, latifrons, cucumis, halfordiae, cucuminatus, vertebrates, frontalis, vivittatus, amphoratus, binotatus, umbeluzinus, brevis, serratus, butianus, hageni, scutellaris, aglaia, visendus, musae, newmani, savastanoi, diversus, and minax, from Asia, Africa, and Australia (Syed, 1969; Cavalloro, 1983; Drew and Hooper, 1983; Munro, 1984; Fletcher, 1987). Amongst these, Bactrocera cucurbitae (Coquillett) is a major threat to cucurbits (Shah et al., 1948). Senior-White (1924) listed 87 species of Tephritidae in India. Amongst these, the genus, Bactrocera (Dacus) causes heavy damage to fruits and vegetables in Asia (Nagappan et al., 1971).
For cucurbits, especially bitter gourd, Momordica charantia Linn., the melon fruit fly damage is the major limiting factor in obtaining good quality fruits and high yield (Srinivasan, 1959; Lall and Singh, 1969; Mote, 1975; Rabindranath and Pillai, 1986). It prefers young, green, and tender fruits for egg laying. The females lay the eggs 2 to 4 mm deep in the fruit pulp, and the maggots feed inside the developing fruits. At times, the eggs are also laid in the corolla of the flower, and the maggots feed on the flowers. A few maggots have also been observed to feed on the stems (Narayanan, 1953). The fruits attacked in early stages fail to develop properly, and drop or rot on the plant. Since, the maggots damage the fruits internally, it is difficult to control this pest with insecticides. Therefore, there is a need to explore alternative methods of control, and develop an integrated control strategy for effective management of this pest. The available information on the melon fruit fly has been reviewed in this manuscript to explore the possibilities for successful management of this pest in cucurbits.
Distribution
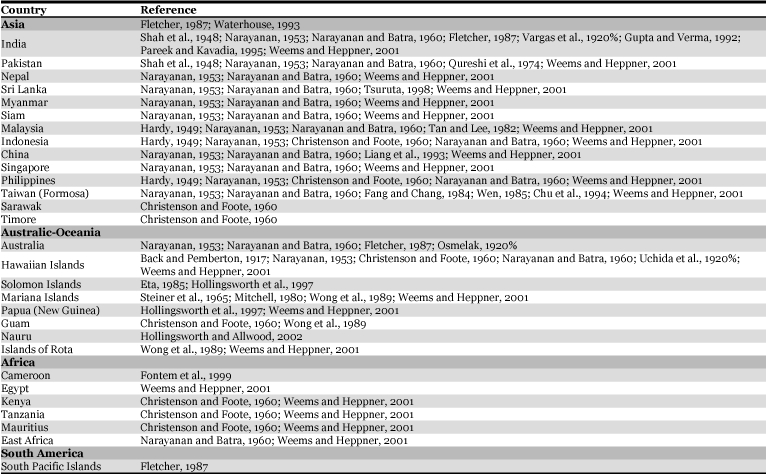
The melon fruit fly is distributed all over the world, but India is considered as its native home (Table 1). It was discovered in Solomon Islands in 1984, and is now widespread in all the provinces, except Makira, Rennell-Bellona and Temotu (Eta, 1985). In the Commonwealth of the Northern Mariana Islands, it was detected in 1943 and eradicated by sterile-insect release in 1963 (Steiner et al., 1965; Mitchell, 1980), but re-established from the neighboring Guam in 1981 (Wong et al., 1989). It was detected in Nauru in 1982 and eradicated in 1999 by male annihilation and protein bait spraying, but was re-introduced in 2001 (Hollingsworth and Allwood, 2002). Although it is found in Hawaii, it is absent from the continental United States (Weems and Heppner, 2001).
Table 1.
Geographic distribution of melon fruit fly, Bactrocera cucurbitae.
Host range
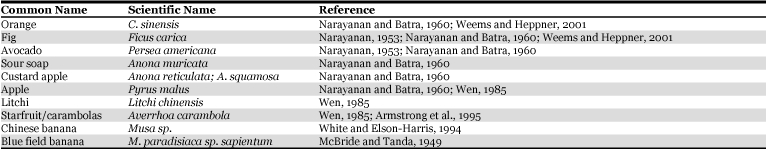
Melon fruit fly damages over 81 plant species (Table 2). Based on the extensive surveys carried out in Asia and Hawaii, plants belonging to the family Cucurbitaceae are preferred most (Allwood et al. 1999). Doharey (1983) reported that it infests over 70 host plants, amongst which, fruits of bitter gourd (Momordica charantia), muskmelon (Cucumis melo), snap melon (Cucumis melo var. momordica) and snake gourd (Trichosanthes anguina and T. cucumeria) are the most preferred hosts. However, White and Elson-Harris (1994) stated that many of the host records might be based on casual observations of adults resting on plants or caught in traps set in non-host plant species. In the Hawaiian Islands, melon fruit fly has been observed feeding on the flowers of the sunflower, Chinease bananas and the juice exuding from sweet corn. Under induced oviposition, McBride and Tanda (1949) reported that broccoli (Brassica oleracea var. capitata), dry onion (Allium cepa), blue field banana (Musa paradisiaca sp. sapientum), tangerine (Citrus reticulata) and longan (Euphoria longan) are doubtful hosts of B. cucurbitae. The melon fly has a mutually beneficial association with the Orchid, Bulbophyllum patens, which produces zingerone. The males pollinate the flowers and acquire the floral essence and store it in the pheromone glands to attract con-specific females (Hong and Nishida, 2000).
Table 2.
Host range of melon fruit fly, Bactrocera cucurbitae.
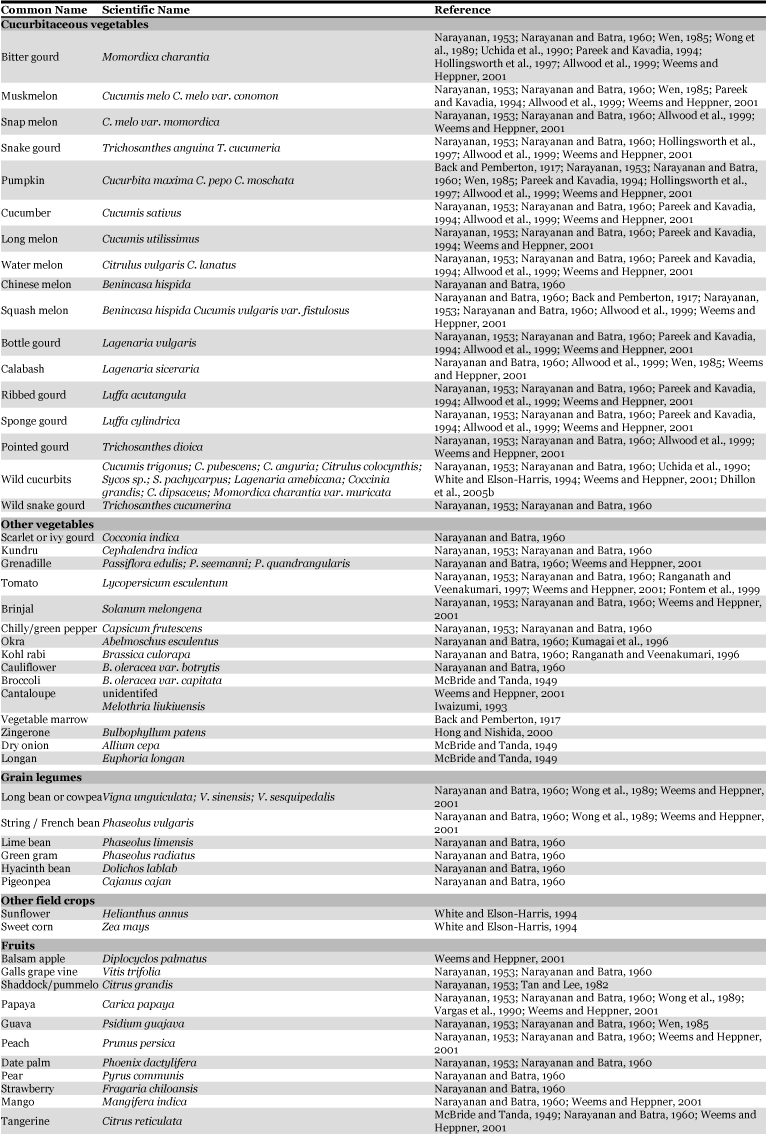
Table 2, con't.
Nature and extent of damage
Maggots feed inside the fruits, but at times, also feed on flowers, and stems. Generally, the females prefer to lay the eggs in soft tender fruit tissues by piercing them with the ovipositor. A watery fluid oozes from the puncture, which becomes slightly concave with seepage of fluid, and transforms into a brown resinous deposit. Sometimes pseudo-punctures (punctures without eggs) have also been observed on the fruit skin. This reduces the market value of the produce. In Hawaii, pumpkin and squash are heavily damaged even before fruit set. The eggs are laid into unopened flowers, and the larvae successfully develop in the taproots, stems, and leaf stalks (Weems and Heppner, 2001). Miyatake et al.(1993) reported < 1% damage by pseudo-punctures by the sterile females in cucumber, sponge gourd and bitter gourd. After egg hatching, the maggots bore into the pulp tissue and make the feeding galleries. The fruit subsequently rots or becomes distorted. Young larvae leave the necrotic region and move to healthy tissue, where they often introduce various pathogens and hasten fruit decomposition. The vinegar fly, Drosophilla melanogaster has also been observed to lay eggs on the fruits infested by melon fly, and acts as a scavenger (Dhillon et al., 2005b). The extent of losses vary between 30 to 100%, depending on the cucurbit species and the season. Fruit infestation by melon fruit fly in bitter gourd has been reported to vary from 41 to 89% (Lall and Sinha, 1959; Narayanan and Batra, 1960; Kushwaha et al., 1973; Gupta and Verma, 1978; Rabindranath and Pillai, 1986). The melon fruit fly has been reported to infest 95% of bitter gourd fruits in Papua (New Guinea), and 90% snake gourd and 60 to 87% pumpkin fruits in Solomon Islands (Hollingsworth et al., 1997). Singh et al. (2000) reported 31.27% damage on bitter gourd and 28.55% on watermelon in India.
Life Cycle
The melon fruit fly remains active throughout the year on one or the other host. During the severe winter months, they hide and huddle together under dried leaves of bushes and trees. During the hot and dry season, the flies take shelter under humid and shady places and feed on honeydew of aphids infesting the fruit trees. The lower developmental threshold for melon fruit fly was recorded as 8.1° C (Keck, 1951). The lower and upper developmental thresholds for eggs were 11.4 and 36.4° C (Messenger and Flitters, 1958). The accumulative day degrees required for egg, larvae, and pre-egg laying adults were recorded as 21.2, 101.7, and 274.9 day degrees, respectively (Keck, 1951). This species actively breeds when the temperature falls below 32.2° C and the relative humidity ranges between 60 to 70%. Fukai (1938) reported the survival of adults for a year at room temperature if fed on fruit juices. In general, its life cycle lasts from 21 to 179 days (Fukai, 1938; Narayanan and Batra, 1960). Development from egg to adult stage takes 13 days at 29° C in Solomon Islands (Hollingsworth et al., 1997). High temperature, long period of sunshine, and plantation activity influence the B. cucurbitae abundance in the North-eastern Taiwan (Lee et al., 1992). Bhatia and Mahto (1969) reported that the life cycle is completed in 36.3, 23.6, 11.2, and 12.5 days at 15, 20, 27.5, and 30° C, respectively. There are 8 to 10 generations in a year (White and Elson-Harris, 1994; Weems and Heppner, 2001).
The egg incubation period on pumpkin, bitter gourd, and squash gourd has been reported to be 4.0 to 4.2 days at 27 ± 1° C (Doharey, 1983), 1.1 to 1.8 days on bitter gourd, cucumber and sponge gourd (Gupta and Verma, 1995), and 1.0 to 5.1 days on bitter gourd (Koul and Bhagat, 1994; Hollingsworth et al., 1997). The larval period lasts for 3 to 21 days (Renjhan, 1949; Narayanan and Batra, 1960; Hollingsworth et al., 1997), depending on temperature and the host. On different cucurbit species, the larval period varies from 3 to 6 days (Chawla, 1966; Chelliah, 1970; Doharey, 1983; Koul and Bhagat, 1994; Gupta and Verma, 1995). Egg viability and larval and pupal survival on cucumber have been reported to be 91.7, 86.3, and 81.4%, respectively; while on pumpkin these were 85.4, 80.9, and 73.0%, respectively, at 27 ± 1° C (Samalo et al., 1991).
The full-grown larvae come out of the fruit by making one or two exit holes for pupation in the soil. The larvae pupate in the soil at a depth of 0.5 to 15 cm. The depth up to which the larvae move in the soil for pupation, and survival depend on soil texture and moisture (Jackson et al., 1998; Pandey and Misra, 1999). Doharey (1983) observed that the pupal period lasts for 7 days on bitter gourd and 7.2 days on pumpkin and squash gourd at 27 ± 1° C. In general, the pupal period lasts for 6 to 9 days during the rainy season, and 15 days during the winter (Narayanan and Batra, 1960). Depending on temperature and the host, the pupal period may vary from 7 to 13 days (Hollingsworth et al., 1997). On different hosts, the pupal period varies from 7.7 to 9.4 days on bitter gourd, cucumber, and sponge gourd (Gupta and Verma, 1995), and 6.5 to 21.8 days on bottle gourd (Koul and Bhagat, 1994; Khan et al., 1993).
The males of the B. cucurbitae mate with females for 10 or more hours, and sperm transfer increases with the increase in copulation time. Egg hatchability is not influenced by mating duration (Tsubaki and Sokei, 1988). Yamagishi and Tsubaki (1990) observed that no sperms were transferred during the first 0.5 h of copulation. Sperm transfer increased to nearly 6400 until 4 h, and thereafter, the number of sperms remained almost unchanged up to 8 h of copulation. The pre-oviposition period of flies fed on cucumbers ranged between 11 to 12 days (Back and Pemberton, 1917; Hollingsworth et al., 1997). Pre-oviposition and oviposition periods range between 10 to 16.3, and 5 to 15 days, respectively, and the females live longer (21.7 to 32.7 days) than the males (15.0 to 28.5 days) (Koul and Bhagat, 1994). The adults survive for 27.5, 30.71 and 30.66 days at 27 ± 1° C on pumpkin, squash gourd and bitter gourd, respectively (Doharey, 1983). Khan et al. (1993) reported that the males and females survived for 65 to 249 days and 27.5 to 133.5 days respectively. The pre-mating and oviposition periods lasted for 4 to 7 days and 14 to 17 days, respectively. The females survived for 123 days on papaya in the laboratory (24° C, 50% RH and LD 12: 12) (Vargas et al., 1992), while at 29° C they survived for 23.1 to 116.8 days (Vargas et al., 1997). Mean single generation time is 71.7 days, net reproductive rate 80.8 births per female, and the intrinsic rate of increase is 0.06 times (Vergas et al. 1992). Yang et al. (1994) reported the net reproductive rate to be 72.9 births per female.
Bactrocera cucurbitae strains were selected for longer developmental period and larger body size on the basis of pre-oviposition period, female age at peak fecundity, numbers of eggs at peak fecundity, total fecundity, longevity of males and females, age at first mating, and number of life time matings (Miyatake, 1995). However, longer developmental period was not necessarily associated with greater fecundity and longevity (Miyatake, 1996). The peak larval, pre-oviposition, and oviposition periods were observed to be 6.48 versus 6.89, 14.0 versus 20.0, and 32 versus 62 days, respectively after nine and 24 generations of mass rearing and selection under laboratory conditions (Miyatake, 1997; 1998a). The egg hatchability and larval-pupal survival were 81.3 versus 89%, and 75.8 versus 77.2% after nine and 24 generations of mass rearing and selection. Miyatake (1998b) reported that males show heritable variation in pre-mating period, while no such effects were observed in the females. The population of B. cucurbitae mass reared for a long time has a shorter pre-mating period than the population reared for short-term. A genetic trade-off has been observed between early-fecundity and longevity. The mass reared population has a negative genetic correlation between early-fecundity and longevity indicating antagonistic pleiotropy. The selected strain had lower and early fecundity than the non-selected strain (Soemori and Nakamori, 1981; Kamikado et al., 1987; Kakinohana and Yamagishi, 1991 and Miyatake, 1997). Therefore, it may be interesting to examine the mating ability of the males of the selected strain, because the effectiveness of the sterile-male release technique depends on the mating ability of the sterile males released into the eco-system. The genetic trade-off between behavioral traits should be taken into account along with life history during mass rearing programs, which might result in significant pre-mating isolation in the melon fly populations (Miyatake, 1998a; Miyatake and Shimizu, 1999).
Strategies for integrated management of melon fruit fly
The fruits of cucurbits, of which the melon fly is a serious pest, are picked up at short intervals for marketing and self-consumption. Therefore, it is difficult to rely on insecticides as a means of controlling this pest. In situations where chemical control of melon fruit fly becomes necessary, one has to rely on soft insecticides with low residual toxicity and short waiting periods. Therefore, keeping in view the importance of the pest and crop, the melon fruit fly management could be done using local area management or wide area management.
Local area management
Local area management means the minimum scale of pest management over a restricted area such as at field level/crop level/village level, which has no natural protection against reinvasion. The aim of local area management is to suppress the pest, rather than eradicate it. Under this management option a number of methods such as bagging of fruits, field sanitation, protein baits and cue-lure traps, host plant resistance, biological control, and soft insecticides, can be employed to keep the pest population below economic threshold in a particular crop over a period of time to avoid the crop losses without health and environmental hazards, which is the immediate concern of the farmers.
Bagging of fruit.
Bagging of fruits on the tree (3 to 4 cm long) with 2 layers of paper bags at 2 to 3 day intervals minimizes fruit fly infestation and increases the net returns by 40 to 58% (Fang, 1989a, b; Jaiswal et al., 1997). Akhtaruzzaman et al. (1993) suggested cucumber fruits should be bagged at 3 days after anthesis, and the bags should be retained for 5 days for effective control. It is an environmentally safe method for the management of this pest.
Field sanitation.
The most effective method in melon fruit fly management uses primary component- field sanitation. To break the reproduction cycle and population increase, growers need to remove all unharvested fruits or vegetables from a field by completely burying them deep into the soil. Burying damaged fruits 0.46 m deep in the soil prevents adult fly eclosion and reduces population increase (Klungness et al., 2005).
Monitoring and control with parapheromone lures/cue-lure traps.
The principal of this particular technique is the denial of resources needed for laying by female flies such as protein food (protein bait control) or parapheromone lures that eliminate males. There is a positive correlation between cue-lure trap catches and weather conditions such as minimum temperature, rainfall, and minimum humidity. The sex attractant cue-lure traps are more effective than the food attractant tephritlure traps for monitoring the B. cucurbitae in bitter gourd (Pawar). Methyl eugenol and cue-lure traps have been reported to attract B. cucurbitae males from mid-July to mid-November (Ramsamy et al., 1987; Zaman, 1995; Liu and Lin, 1993). A leaf extract of Ocimum sanctum, which contain eugenol (53.4%), beta-caryophyllene (31.7%) and beta-elemene (6.2%) as the major volatiles, when placed on cotton pads (0.3 mg) attract flies from a distance of 0.8 km (Roomi et al., 1993). Thus, melon fruit fly can also be controlled through use of O. sanctum as the border crop sprayed with protein bait (protein derived from corn, wheat or other sources) containing spinosad as a toxicant. Cue-lure traps have been used for monitoring and mass trapping of the melon fruit flies in bitter gourd (Paw et al. 1991; Permalloo et al., 1998; Seewooruthun et al., 1998). A number of commercially produced attractants (Flycide® with 85% cue-lure content; Eugelure® 20%; Eugelure® 8%; Cue-lure® 85% + naled; Cue-lure® 85% + diazinon; Cue-lure® 95% + naled) are available on the market, and have been found to be effective in controlling this pest (Iwaizumi et al., 1991). Chowdhury et al. (1993) captured 2.36 to 4.57 flies/ trap/ day in poison bait traps containing trichlorfon in bitter gourd. The use of male lure cearlure B1® (Ethylcis-5-Iodo-trans-2-methylcyclohexane-1-carboxylate) have been found to be 4-9 times more potent than trimedlure® for attracting medfly, Ceratitis capitata males (Mau et al., 2003b), and thus could be tried for male annihilation strategies of melon fruit fly areawide control programs. A new protein bait GF-120 Fruit Fly Bait® containing spinosad as a toxicant have been found to be effective in the areawide management of melon fruit fly in Hawaii (Prokopy et al., 2003, 2004). The GF-120 Fruit Fly Bait® would be highly effective, when applied to sorghum plants surrounding cucumbers against protein-hungry melon flies, but would be less effective in preventing protein-satiated females from arriving on cucumbers. Maize can also be used as a border crop for melon fruit fly attraction through application of protein bait (Dhillon, personal observations). Although, the protein baits, parapheromone lures, cue-lures, and baited traps have been successful for the monitoring and control of melon fruit fly, the risk is the immigration of protein-satiated females. The risk of immigration of already-satiated females could principally be managed by increasing the distance these satiated immigrants must travel (Stonehouse et al., 2004).
Biological control.
There are no reports on the successful use of bio-control agents against the melon fruit fly. Srinivasan (1994) reported Opius fletcheri Silv. to be a dominant parasitoid of B. cucurbitae, but the efficacy of this parasitoid has not been tested under field conditions in India. The parasitization of B. cucurbitae by O. flatcheri has been reported to vary from 0.2 to 1.9% in M. charantia fields in Honolulu at Hawaii (Wong et al., 1989). Similar level of parasitization (<3%) was also reported from northern India by Nishida (1963). However, Willard (1920), Newell et al. (1952), and Nishida (1955) have reported parasitization at levels of 80, 44, and 37%, respectively, from Hawaii. Thus, there is a need to reevaluate the parasitization potential of O. flatcheri before its exploitation as biocontrol agent for the management of B. cucurbitae. More recently, a new parasitoid, Fopius arisanus has also been included in the IPM program of B. cucurbitae at Hawaii (Wood, 2001). A Mexican strain of the nematode, Steinernema carpocapsae Weiser (Neoaplectana carpocapsae), has been reported to cause 0 to 86% mortality to melon fruit fly after an exposure of 6 days to 5000 to 5,000,000 nematodes/cup in the laboratory, and an average of 87.1% mortality under field conditions when applied at 500 infective juveniles/cm2 soil (Lindegren, 1990). Sinha (1997) reported that culture filterate of the fungus, Rhizoctonia solani Kuhn, to be an effective bio-agent against B. cucurbitae larvae. While, the fungus, Gliocladium virens Origen, has been reported to be an effective against B. cucurbitae (Sinha and Singh 1998). Culture filtrates of the fungi R. solani, Trichoderma viridae Pers., and G. virens affected the oviposition and development of B. cucurbitae adversely (Sinha and Saxena, 1999).
The efficacy of most of these bio-agents is unclear under field conditions. Therefore, there is a need to evaluate the efficacy of these bio-control agents against B. cucurbitae for practical use in integrated pest management programs.
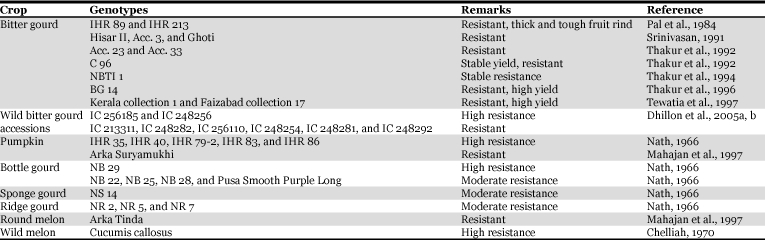
Host plant resistance.
Host plant resistance is an important component in integrated pest management programs. It does not cause any adverse effects to the environment, and no extra cost is incurred to the farmers. Unfortunately success in developing high yielding and fruit fly-resistant varieties has been limited. The sources of resistance to fruit fly are listed in Table 3. There is a distinct possibility of transferring resistance genes in the cultivated genotypes from the wild relatives of cucurbits for developing varieties resistant to melon fruit fly through wide hybridization.
Table 3.
Sources of resistance to melon fruit fly, Bactrocera cucurbitae.
Chemical Control.
Chemical control of the melon fruit fly is relatively ineffective. However, insecticides such as malathion, dichlorvos, phosphamidon, and endosulfan are moderately effective against the melon fly (Agarwal et al., 1987). Bhatnagar and Yadava (1992) reported malathion (0.5%) to be more effective than carbaryl (0.2%) and quinalphos (0.2%) on bottle gourd, sponge gourd, and ridge gourd. The application of molasses + malathion (Limithion 50 EC) and water in the ratio of 1: 0.1: 100 provides good control of melon fly (Akhtaruzzaman et al., 2000). Application of either 0.05% fenthion or 0.1% carbaryl at 50% appearance of male flowers, and again at 3 days after fertilization is helpful in reducing the melon fly damage (Srinivasan, 1991). Gupta and Verma (1982) reported that fenitrothion (0.025%) in combination with protein hydrolysate (0.25%) reduced fruit fly damage to 8.7 % as compared to 43.3 % damage in untreated control. Application of carbofuran granules at 1.5 kg a.i./ ha at the time of sowing, vining, and flowering gave 83.35% protection to bitter gourd against B. cucurbitae (Thomas and Jacob, 1990). Dicrotophos (at 600g a.i.) and trichlorfon (at 1920g a.i./ ha) has been found to give good control of B. cucurbitae in muskmelon (Chughtai and Baloch, 1988). Formathion is more effective than trichlorfon (Talpur et al., 1994). Diflubenzuron has also been reported to be effective in controlling the melon fly (Mishra and Singh, 1999). Reddy (1997) reported triazophos to be the most effective insecticide against this pest on bitter gourd. Highest yield and lowest damage were observed in pumpkin when treated with carbofuran at 1.5 kg a.i./ ha at 15 days after germination (Borah, 1998). An extract of Acorus calamus (0.15%) reduced the adult longevity from 119.2 days to 26.6 days when fed continuously with sugar mixed with extract (at 1 ml/g sugar) (Nair and Thomas, 1999). Neem oil (1.2 %) and neem cake (4.0 %) have also been reported to be as effective as dichlorvos (0.2 %), (Ranganath et al., 1997).
Wide area management
Wide area management is not a unitary concept, but incorporates a number of related but distinct methods including local area management. The methods used for a wide area management approach include male-sterile insect release, insect transgenesis, and quarantine control techniques in combination with available local area management options. The aim of wide area management is to coordinate and combine different characteristics of an insect eradication program over an entire area within a defensible perimeter. The area must be subsequently protected against reinvasion by quarantine controls, for example, by pest eradication on isolated islands. The USDA-ARS areawide IPM programs of melon fruit fly started in 1999 in collaboration with the Hawaiian State Department of Agriculture and University of Hawaii, using the environmentally sound strategies such as field sanitation, male annihilation with male lures and attractants, protein bait sprays/traps, augmentative releases of biological control agents (Fopius arisanus and Psyttalia fletcheri), and sterile insect release. It has proved to be economically viable, environmentally sensitive, sustainable, and has suppressed fruit flies below economic thresholds with the minimum use of organophosphate and carbamate insecticides (Wood, 2001; Mau et al., 2003b; Vargas et al., 2003; Klungness et al., 2005). An IPM program that used field sanitation, protein bait applications, male annihilation, and release of sterile flies and parasites reduced fruit fly infestation from 30 to 40% to less than 5%, and cut organophosphate pesticide use by 75 to 90% (Vargas, 2004).
The recent wide area management program eradication program of B. cucurbitae in Seychelles demonstrated a three tier model including a) initial population reduction using bait sprays, b) elimination of reproduction using parapheromone lure blocks to eradicate males and thus prevent oviposition by females, and c) intensive surveying by traps and fruit inspection, until it can be certain that the pest is entirely eradicated (Mumford, 2004). Although, the sterile insect technique has been successfully used in area-wide approaches, the wide area management needs more sophisticated and powerful technologies in their eradication program, such as insect transgenesis, which could be deployed over wide-area and is less susceptible to immigrants. Above all, the use of the geographical information system has been used as a tool to mark site-specific locations of traps, host plants roads, land use areas and fruit fly populations within a specified operational grid (Mau et al., 2003a).
Male-sterile technique.
In this technique, sterile males are released in the fields for mating with the wild females. Sterilization is accomplished through irradiation, chemo-sterilization, or by genetic manipulation. In sterile insect programs the terms ‘sterility’ or sterile insect' refer to the transmission of dominant lethal mutations that kill the progeny. The females either do not lay eggs or lay sterile eggs. Ultimately, the pest population can be eradicated by maintaining a barrier of sterile flies. A sterile insect program is species specific, and is considered an ecologically safe procedure and has been successfully used in area-wide approaches to suppress or eradicate pest insects in entire regions such as the pink bollworm, Pectinophora gossypiella in California (Walters et al., 2000), the tsetse fly, Glossina austeni in Zanzibar (Vreysen, 2001), the New World screwworm, Cochliomyia hominivorax in North and Central America (Wyss, 2000), and various tephritid fruit fly species in different parts of several continents (Klassen et al., 1994). Chemo-sterilization (by exposing the flies to 0.5 g tepa in drinking water for 24 h) and gamma irradiation are the only widely tested and accepted male-sterile techniques against melon fly (Gojrati and Keiser 1978; Odani et al., 1991). Nakamori et al. (1993) found in Okinawa that frequent and intensive release of sterile flies did not increase the ratio of sterile to wild flies in some areas, suggesting that it is important to identify such areas for eradication of this pest. Eradication of this pest has already been achieved through sterile-male release in Kikaijma Islands in 1985, Amami-oshima in 1987, Tokunoshima, and the Okierabu-jima and Yoron-jima Islands in 1989 (Sekiguchui, 1990; Anonymous, 1991a, Anonymous, 1991b; Yoshizawa, 1997). In the Mediterranean fruit fly (medfly), Ceratitis capitata, release of sterile males increased the effectiveness of the sterile insect program (Hendrichs et al., 2005). The use of male-sterile and male annihilation techniques has successfully eradicated the melon fly from Japan for over 24 years (Shiga, 1992; Liu, 1993). However, the suppression of B. cucurbitae reproduction through male annihilation with cue-lure may be problematic. Matsui et al. (1990) reported that no wild tephritids were caught with cue-lure traps after intensification of distribution of cue-lure strings, but the mating rates of mature females did not decrease as compared to those on control islands. Conventional sterilization based on ionizing radiation causes chromosome fragmentation without centromeres, where the chromosome fragments will not be transmitted correctly to the progeny, and can have adverse effects on viability and sperm quality, resulting in reduced competitiveness of sterilized individuals (Hilbrook and Fujimoto 1970; Hooper and Katiyar, 1971; Mayer et al., 1998; Cayol et al., 1999)
Transgene based, embryo-specific lethality system.
Although, the sterile insect technique can be used successfully to suppress economically important pest species, conventional sterilization by ionizing radiation reduces insect fitness, which can result in reduced competition of the sterilized insects (Horn and Wimmer, 2003). A transgene-based, female-specific expression method of a conditional dominant lethal gene (Atkinson et al., 2001; Handler, 2001; Horn et al., 2002), has been well tested in Drosophila melanogaster, and might be transferable to other insect pest species (Heinrich and Scott, 2000; Thomas et al., 2000; Horn and Wimmer, 2003). Thus, the transgene based, dominant embryo lethality system can generate large numbers of competitive and vigorous sterile males, and can be used successfully in a sterile insect program.
Quarantine.
The import and export of infested plant material from one area or country to other non-infested places is the major mode of the spread of insect-pests. The spread of the melon fly can be blocked through tight quarantine and treatment of fruits at the import/export ports. Cold treatment at 1.1 ± 0.6° C for 12 days disinfested Hawaiian starfruit, Averrhoa carambola, of tephritid eggs and larvae (Armstrong et al., 1995). Heat treatment of avocado fruits infested with eggs and larvae of B. cucurbitae for 40° C for 24 h reduced the estimated surviving population by 99.5 to 100% (Yang 1996). Import controls carried out in airports in France since 1993 on tropical fruits have revealed the presence of 12 non-European and one European species of Tephritidae, (Bayart et al., 1997).
Conclusion
Keeping in view the importance of the pest and crop, the melon fruit fly can be managed or suppressed locally at the growers fields using any of the option combinations available including, bagging of fruits, field sanitation, cue-lure traps, spray of protein baits with toxicants, growing fruit fly-resistant genotypes, augmentative releases of biological control agents, and soft insecticides. On the other hand, the incorporation of a number of different techniques including the sterile insect technique, transgene based embryo-specific lethality system, and quarantine, in addition to the available local area management options, could be exploited for better results in wide area management of melon fruit fly. The local area management aims mainly at suppression, rather than eradication. Use of wide area management to coordinate and combine different parts of an insect eradication program over an entire area, within a defensible perimeter, can subsequently protect against reinvasion by quarantine controls. The use of a geographical information system could also be used as an IPM tool to mark site-specific locations of traps, host plants roads, land use areas and fruit fly populations within a specified operational region. Although, sterile insect programs have been successfully used in area-wide approaches, more sophisticated and powerful technologies should be used in their eradication program such as insect transgenesis, which could be deployed over wide areas.
References
- Agarwal ML, Sharma DD, Rahman O. Melon fruit fly and its control. Indian Horticulture. 1987;32:10–11. [Google Scholar]
- Akhtaruzzaman M, Alam MZ, Ali-Sardar MM. Efficiency of different bait sprays for suppressing fruit fly on cucumber. Bulletin of the Institute of Tropical Agriculture, Kyushu University. 2000;23:15–26. [Google Scholar]
- Akhtaruzzaman M, Alam MZ, Ali-Sardar MM. Suppressing fruit fly infestation by bagging cucumber at different days after anthesis. Bangladesh Journal of Entomology. 1999;9:103–112. [Google Scholar]
- Anonymous. Melon fly, Dacus cucurbitae (Bactrocera cucurbitae), eradicated from Okinawa Islands. FAO Plant Protection Bulletin. 1991a;39:118. [Google Scholar]
- Anonymous. Biology and control of fruit flies. Food and Fertilizer Technology Center Newsletter. 1991b;94:8–10. [Google Scholar]
- Armstrong JW, Silva ST, Shishido VM. Quarantine cold treatment for Hawaiian carambola fruit infested with Mediterranean fruit fly, melon fly, or oriental fruit fly (Diptera: Tephritidae) eggs and larvae. Journal of Economic Entomology. 1995;88:683–687. [Google Scholar]
- Atkinson PW, Pinkerton AC, O'Brochta DA. Genetic transformation systems in insects. Annual Review of Entomology. 2001;46:317–346. doi: 10.1146/annurev.ento.46.1.317. [DOI] [PubMed] [Google Scholar]
- Atwal AS. 1986 Agricultural Pests of India and South-East Asia. Kalyani Publishers, New Delhi, India. [Google Scholar]
- Back EA, Pemberton CE. 1917 The melon fly in Hawaii. USDA, Washington, DC Bulletin. 491:64. pp. [Google Scholar]
- Bayart JD, Phalip M, Lemonnier R, Gueudre F. Fruit flies. Results of four years of import control on fruits in France. Phytoma. 1997;49:20–25. [Google Scholar]
- Bezzi M. Indian Tephritids (fruit flies) in the collection of the Indian Museum, Calcutta. Memoirs of the Indian Museum. 1913;3:153–175. [Google Scholar]
- Bhatia SK, Mahto Y. Influence of temperature on the speed of development of melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae) Indian Journal of Agricultural Sciences. 1969;40:821–828. [Google Scholar]
- Bhatnagar KN, Yadava SRS. An insecticidal trial for reducing the damage of some cucurbitaceous fruits due to Dacus cucubitae Coq. Indian Journal of Entomology. 1992;54:66–69. [Google Scholar]
- Borah RK. Evaluation of an insecticide schedule for the control of red pumpkin beetle and melon fruit fly on red pumpkin in the hills zone of Assam. Indian Journal of Entomology. 1998;60:417–419. [Google Scholar]
- Cavalloro R. 1983 Fruit Flies of Economic Importance. In: Cavalloro R. editor. CEC/IOBC Symposia, Athens, Greece 1982, 642. pp. Rotterdam: Balkema, Germany. [Google Scholar]
- Cayol JP, Vilardi J, Rial E, Vera MT. New indices and method to measure the sexual compatibility and mating performance of Ceratitis capitata (Diptera: Tephritidae) laboratory-reared strains under field cage conditions. Journal of Economic Entomology. 1999;92:140–145. [Google Scholar]
- Chang LY, Yen CC. Selection of food attractants to the melon fly, Dacus cucurbitae Coquillett, and supplementary effect of yellow insect adhesive paper. Chinese Journal of Entomology. 1995;15:35–47. [Google Scholar]
- Chawla SS. Some critical observations on the biology of melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae) Research Bulletin of Punjab University (Science) 1966;17:105–109. [Google Scholar]
- Chelliah S. Host influence on the development of melon fly, Dacus cucurbitae Coquillett. Indian Journal of Entomology. 1970;32:381–383. [Google Scholar]
- Chowdhury MK, Malapert JC, Hosanna MN. Efficiency of poison bait trap in controlling fruit fly, Dacus cucurbitae in bitter gourd. Bangladesh Journal of Entomology. 1993;3:91–92. [Google Scholar]
- Christenson LD, Foote RH. Biology of fruit flies. Annual Review of Entomology. 1960;5:171–192. [Google Scholar]
- Chu YI, Lee KT, Tseng YH. Occurrence of melon and Oriental fruit fly in Republic of Naru. Plant Protection Bulletin, Taipei. 1994;36:131–140. [Google Scholar]
- Chughtai CG, Baloch VK. Insecticidal control of melon fruit fly. Pakistan Journal of Entomological Research. 1988;9:192–194. [Google Scholar]
- Dhillon MK, Naresh JS, Ram Singh, Sharma NK. Evaluation of bitter gourd (Momordica charantia L.) genotypes to melon fruit fly, Bactrocera cucurbitae (Coquillett) Indian Journal of Plant Protection. 2005a;33:55–59. [Google Scholar]
- Dhillon MK, Naresh JS, Ram Singh, Sharma NK. Influence of physico-chemical traits of bitter gourd, Momordica charantia L. on larval density and resistance to melon fruit fly, Bactrocera cucurbitae (Coquillett) Journal of Applied Entomology. 2005b;129:393–399. [Google Scholar]
- Doharey KL. Bionomics of fruit flies (Dacus spp.) on some fruits. Indian Journal of Entomology. 1983;45:406–413. [Google Scholar]
- Drew RAI, Hooper GHS. Population studies of fruit flies (Diptera: Tephritidae) in South-East Queensland. Oecologia. 1983;56:153–159. doi: 10.1007/BF00379685. [DOI] [PubMed] [Google Scholar]
- Ellwood AJ, Chinajariyawong A, Drew RAI, Hamacek EL, Hancock DL, Hengsawad C, Jinapin JC, Jirasurat M, Kong Krong C, Kritsaneepaiboon S, Leong CTS, Vijaysegaran S. Host plant records for fruit flies (Diptera: Tephritidae) in South-East Asia. Raffies Bulletin of Zoology (Supplement) 1999;7:1–92. [Google Scholar]
- Eta CR. 1985 Eradication of the melon fly from Shortland Islands (special report). Solomon Islands Agricultural Quarantine Service, Annual Report. Ministry of Agriculture and Lands, Honiara. [Google Scholar]
- Fang MN, Chang CP. The injury and seasonal occurrence of melon fly, Dacus cucurbitae Coquillett, in central Taiwan (Tephritidae: Diptera) Plant Protection Bulletin Taiwan. 1984;29:45–51. [Google Scholar]
- Fang MN. Studies on using different bagging materials for controlling melon fly on bitter gourd and sponge gourd. Bulletin of Taichung District Agriculture Improvement Station. 1989a;25:3–12. [Google Scholar]
- Fang MN. A non-pesticide method for the control of melon fly. Special Publication of Taichung District Agriculture Improvement Station. 1989b;16:193–205. [Google Scholar]
- Fletcher BS. The biology of Dacine fruit flies. Annual Review of Entomology. 1987;32:115–144. [Google Scholar]
- Fontem DA, Gumedzoe MYD, Nono WR. Biological constraints in tomato production in the Western highlands of Cameroon. Tropicaltura. 1999;16/17:89–92. [Google Scholar]
- Fukai K. Studies on the possibility of life of the Formosa melon fly in Japan. Nojikairyo-shiryo. 1938;134:147–213. [Google Scholar]
- Gojrati HAN, Keiser I. 1974 Spermatogenesis and oogenesis of Ceratitis capitata Wieldemann, Dacus dorsalis Hendel and Dacus cucurbitae Coquillett when sexually sterilized with Tepa after adult emergence. In: Proceedings of the Symposium on the Sterility Principle for Insect Control, 22–26 July 1974, pp. 325–328.International Atomic Energy Agency and Food and Agriculture Organization, Innsbruck, Vienna, Austria. [Google Scholar]
- Gupta D, Verma AK. Population fluctuations of the maggots of fruit flies (Dacus cucurbitae Coquillett and D. tau Walker) infesting cucurbitaceous crops. Advances in Plant Science. 1992;5:518–523. [Google Scholar]
- Gupta D, Verma AK. Host specific demographic studies of the melon fruit fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae) Journal of Insect Science. 1995;8:87–89. [Google Scholar]
- Gupta JN, Verma AN. Screening of different cucurbit crops for the attack of the melon fruit fly, Dacus cucurbitae Coq. (Diptera: Tephritidae) Haryana Journal of Horticulture Science. 1978;7:78–82. [Google Scholar]
- Gupta JN, Verma AN. Effectiveness of fenitrothion bait sprays against melon fruit fly, Dacus cucurbitae Coquillett in bitter gourd. Indian Journal of Agricultural Research. 1982;16:41–46. [Google Scholar]
- Handler AM. A current prospective on insect gene transformation. Insect Biochemical and Molecular Biology. 2001;31:111–128. doi: 10.1016/s0965-1748(00)00159-4. [DOI] [PubMed] [Google Scholar]
- Hardy DE, Adachi MS. Studies on the fruit flies of the Philippines Islands, Indonesia and Malaya. Part I. Dacini (Diptera: Tephritidae) Pacific Science. 1954;8:147–204. [Google Scholar]
- Hardy DE. Studies in Hawaiian fruit flies (Diptera: Tephritidae) Proceedings of the Entomological Society of Washington. 1949;51:181–205. [Google Scholar]
- Heinrich JC, Scott MJ. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proceedings, National Academy of Science, USA. 2000;97:8229–8232. doi: 10.1073/pnas.140142697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrichs J, Franz G, Rendon P. Increased effectiveness and applicability of the sterile insect technique through male-only releases for control of Mediterranean fruit flies during fruiting seasons. Journal of Applied Entomology. 1995;119:371–377. [Google Scholar]
- Holbrook FR, Fujimoto MS. Mating competitiveness of unirradiated and irradiated Mediterranean fruit flies. Journal of Economic Entomology. 1970;63:1175–1176. [Google Scholar]
- Hollingsworth R, Vagalo M, and Tsatsia F. 1997 Biology of melon fly, with special reference to the Solomon Islands. In: Allwood AJ and Drew RAI editors. Management of fruit flies in the Pacific. Proceedings of Australian Country Industrial Agricultural Research. 76:140–144. [Google Scholar]
- Hollingsworth R, Allwood AJ. 2002 Melon fly. In: SPC Pest Advisory Leaflets, pp. 1–2. [Google Scholar]
- Hong KT, Nishida R. Mutual reproductive benefits between a wild orchid, Bulbophyllum patens and Bactrocera fruit flies via a floral synomone. Journal of Chemical Ecology. 2000;26:533–546. [Google Scholar]
- Hooper GHS, Katiyar KP. Competitiveness of gamma-sterilized males of the Mediterranean fruit flies. Journal of Economic Entomology. 1971;64:1068–1071. doi: 10.1093/jee/64.5.1068. [DOI] [PubMed] [Google Scholar]
- Horn C, Schmid BGM, Pogoda FS, Wimmer EA. Fluorescent transformation markers for insect transgenesis. Insect Biochemical and Molecular Biology. 2002;32:1221–1235. doi: 10.1016/s0965-1748(02)00085-1. [DOI] [PubMed] [Google Scholar]
- Horn C, Wimmer EA. A transgene-based, embryo-specific lethality system for insect pest management. Nature Biotechnology. 2003;21:64–70. doi: 10.1038/nbt769. [DOI] [PubMed] [Google Scholar]
- Iwaizumi R, Kumagai M, Katsumata S. Research on infestation in several kinds of fruits by the melon fly, Bactrocera cucurbitae (Coquillett) and the Oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) Research Bulletin Plant Protection Service Japan. 1994;30:93–97. [Google Scholar]
- Iwaizumi R, Sawaki M, Kobayashi K, Maeda C, Toyokawa Z, Ito M, Kawakami T, Matsui M. A comparative experiment on the attractiveness of the several kinds of the cue-lure toxicants to the melon fly, Dacus cucurbitae (Coquillett) Research Bulletin Plant Protection Service Japan. 1991;27:75–78. [Google Scholar]
- Jackson CG, Long JP, Klungness LM. Depth of pupation in four species of fruit flies (Diptera: Tephritidae) in sand with and without moisture. Journal of Economic Entomology. 1998;91:138–142. [Google Scholar]
- Jaiswal JP, Gurung TB, and Pandey RR. 1997 Findings of melon fruit fly control survey and its integrated management 1996/97, Kashi, Nepal. Lumle Agriculture Research Centre Working Paper 97/53, pp. 1–12. [Google Scholar]
- Jang EB. Systems approach to quarantine security: post harvest application of sequential mortality in the Hawaiian grown ‘Sharwil’ avocado system. Journal of Economic Entomology. 1996;89:950–956. [Google Scholar]
- Kakinohana H, Yamagishi M. 1991 The mass production of the melon fly techniques and problems. In: Kawasaki K, Iwahashi O, Kaneshiro K editors. Proceedings of the International Symposium on Biology and Control of Fruit Flies 1991, pp. 1–10.Okinawa, Japan. [Google Scholar]
- Kamikado T, Chishaki N, Kamiwada H, Tanaka A. Mass rearing of the melon fly, Dacus cucurbitae Coquillett, by the sterile insect release method. I. Changes in the amount of eggs laid and longevity of mass reared adults. Proceedings of the Association of Plant Protection Kyushu. 1987;33:164–166. [Google Scholar]
- Keck CB. Effect of temperature on development and activity of the melon fly. Journal of Economic Entomology. 1951;44:1001–1002. [Google Scholar]
- Khan L, Haq MU, Mohsin AU, Inayat-Tullah C. Biology and behavior of melon fruit fly, Dacus cucurbitae Coq. (Diptera: Tephritidae) Pakistan Journal of Zoology. 1993;25:203–208. [Google Scholar]
- Khan L, Inayatullah C, Manzoor UH. Control of melon fly, Dacus cucurbitae (Diptera: Tephritidae) on melon in Pakistan. Tropical Pest Management. 1992;38:261–264. [Google Scholar]
- Klassen W, Lindquist DA, and Buyckx EJ. 1994 Overview of the joint FAO/IAEA Division's involvement in fruit fly sterile insect technique programs. In: Calkins CO, Klassen W, Liedo P editors. Fruit Flies and the Sterile Insect Technique, pp. 3–26.CRC Press, Boca Raton, Florida. [Google Scholar]
- Klungness LM, Jang EB, Mau RFL, Vargas RI, Sugano JS, Fujitani E. New approaches to sanitation in a cropping system susceptible to tephritid fruit flies (Diptera: Tephritidae) in Hawaii. Journal of Applied Science and Environmental Management. 2005;9:5–15. [Google Scholar]
- Koul VK, Bhagat KC. Biology of melon fruit fly, Bactrocera (Dacus) cucurbitae Coquillett (Diptera: Tephritidae) on bottle gourd. Pest Management and Economic Zoology. 1994;2:123–125. [Google Scholar]
- Kumagai M, Tsuchiya T, Katsumata H. Larval development of Bactrocera dorsalis (Hendel) and Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) in okra. Research Bulletin Plant Protection Service Japan. 1996;32:95–98. [Google Scholar]
- Kushwaha KS, Pareek BL, Noor A. Fruit fly damage in cucurbits at Udaipur. Udaipur University Research Journal. 1973;11:22–23. [Google Scholar]
- Lall BS, Singh BN. Studies on the biology and control of melon fly, Dacus cucurbitae (Coq.) (Diptera: Tephritidae) Labdev Journal of Science and Technology. 1969;7B:148–153. [Google Scholar]
- Lall BS, Sinha SN. On the biology of the melon fly, Dacus cucurbitae (Coq.) (Diptera: Tephritidae) Science & Culture. 1959;25:159–161. [Google Scholar]
- Lee LWY, Hwang YB, Cheng CC, Chang JC. Population fluctuation of the melon fly, Dacus cucurbitae, in northeastern Taiwan. Chinese Journal of Entomology. 1992;12:285–292. [Google Scholar]
- Liang GQ, Hancock DL, Xu W, Liang F. Notes on the Dacine of Southern China (Diptera: Tephritidae) Journal of Australian Entomological Society. 1993;32:137–40. [Google Scholar]
- Lindegren JE. 1990 Field suppression of three fruit fly species (Diptera: Tephritidae) with Steinernema carpocapsae. In: Proceedings 5th International Colloquium on Invertebrate Pathology and Microbial Control 20-24 August 1990, Adelaide, Australia, 1-. 223. pp. [Google Scholar]
- Liu YC, Lin JS. The response of melon fly, Dacus cucurbitae Coquillett to the attraction of 10% MC. Plant Protection Bulletin Taipei. 1993;35:79–88. [Google Scholar]
- Liu YC. 1993 Pre-harvest control of Oriental fruit fly and melon fly. Plant Quarantine in Asia and the Pacific, Report of APO Study Meeting, 17–26 March 1992, Taipei, Taiwan Republic of China, pp. 73–76. [Google Scholar]
- Mahajan V, Mukherjee SC, Shaw SS. Use resistant vegetable varieties: A best alternative to tackle diseases and insect pests. Farmer and Parliament. 1997;33:7–8. 29–30. [Google Scholar]
- Mathew MP, Rekha CR, Gopalakrishnan TR. New host of the melon fly, Bactrocera cucurbitae (Coq) Insect Environment. 1999;5:12–13. [Google Scholar]
- Matsui M, Nakamori H, Kohama T, Nagamine Y. The effect of male annihilation on a population of wild melon flies, Dacus cucurbitae Coquillett (Diptera: Tephritidae) in Northern Okinawa. Japanese Journal of Applied Entomology and Zoology. 1990;34:315–317. [Google Scholar]
- Mau RFL, Jang EB, Vargas RI, Chan CM, Chou M, Sugano JS. Implementation of a Geographical Information System With Integrated Control Tactics For Area wide Fruit Fly Pest Management. Proceedings of Meeting on Areawide Control for Fruit Flies. 2003a;5:23–33. [Google Scholar]
- Mau RFL, Sugano JS, and Jang EB. 2003b Farmer education and organization in the hawaii areawide fruit fly pest management program. Recent Trends on Sterile Insect Technique and Area-wide Integrated Pest Management: Economic Feasibility, Control Projects, Farmer Organization and Dorsalis Complex Control Study, pp. 47–57.Research Institute of Subtropics, Okinawa, Japan. [Google Scholar]
- Mayer DG, Atzeni MG, Stuart MA, Anaman KA, Butler DG. Mating competitiveness of irradiated flies for screwworm fly eradication campaigns. Preventive Veterinary Medicine. 1998;36:1–9. doi: 10.1016/s0167-5877(98)00078-6. [DOI] [PubMed] [Google Scholar]
- Messenger PS, Flitters NE. Effect of constant temperature environments on the egg stage of three species of Hawaiian fruit flies. Annals of the Entomological Society of America. 1958;51:109–119. [Google Scholar]
- Mishra PN, Singh MP. Studies on the ovicidal action of diflubenzuron on the eggs of Dacus (Bactrocera) cucurbitae Coq. damaging cucumber. Annals of Plant Protection Science. 1999;7:94–6. [Google Scholar]
- Mitchell WC. Verification of the absence of Oriental fruit and melon fruit fly following an eradication program in the Mariana Islands. Proceedings of the Hawaiian Entomological Society. 1980;23:239–243. [Google Scholar]
- Miyatake T, Irabu T, Higa R. Oviposition punctures in cucurbit fruits and their economic damage caused by the sterile female melon fly, Bactrocera cucurbitae Coquillett. Proceedings of the Association of Plant Protection Kyushu. 1993;39:102–105. [Google Scholar]
- Miyatake T, Shimizu T. Genetic correlations between life history and behavioral traits can cause reproductive isolation. Evolution. 1999;53:201–208. doi: 10.1111/j.1558-5646.1999.tb05345.x. [DOI] [PubMed] [Google Scholar]
- Miyatake T. Two-way artificial selection for developmental period in Bactrocera cucurbitae (Diptera: Tephritidae) Annals of the Entomological Society of America. 1995;88:848–855. [Google Scholar]
- Miyatake T. Comparison of adult life history traits in lines artificially selected for long and short larval and pupal developmental periods in the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae) Applied Entomology and Zoology. 1996;31:335–343. [Google Scholar]
- Miyatake T. Genetic trade-off between early fecundity and longevity in Bactrocera cucurbitae (Diptera: Tephritidae) Heredity. 1997;78:93–100. doi: 10.1038/sj.hdy.6880900. [DOI] [PubMed] [Google Scholar]
- Miyatake T. Genetic changes of life history and behavioral traits during mass rearing in the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae) Research on Population Ecology. 1998a;40:301–310. [Google Scholar]
- Miyatake T. Genetic variation in pre-mating period of the mass-reared melon fly, Bactrocera cucurbitae (Diptera: Tephritidae) Applied Entomology and Zoology. 1998b;33:29–33. [Google Scholar]
- Mote UN. Control of fruit fly (Dacus cucurbitae) on bitter gourd and cucumber. Pesticides. 1975;9:36–37. [Google Scholar]
- Mumford JD. 2004 Economic analysis of area-wide fruit fly management. In: Barnes B, Addison M, editors. Proceedings of the 6th International Symposium on Fruit Flies of Economic Importance, Stellenbosch, South Africa, 6-10 May 2002. Infruitec Press, Stellenbosch, South Africa. [Google Scholar]
- Munro KH. A taxonomic treatise on the Decidae (Tephritoidea: Diptera) of Africa. Entomological Memories of the Department of Agriculture, Republic of South Africa. 1984;61:1–313. [Google Scholar]
- Nagappan K, Kamalnathan S, Santharaman T, Ayyasamy MK. Insecticidal trials for the control of the melon fruit fly, Dacus cucurbitae Coq. infesting snake gourd, Trichosanthes anguina. Madras Agriculture Journal. 1971;58:688–690. [Google Scholar]
- Nair S, Thomas J. Effect of Acorus calamus L. extracts on the longevity of Bactrocera cucurbitae Coq. Insect Environment. 1999;5:27. [Google Scholar]
- Nakamori H, Shiga M, Kinjo K. Characteristics of hot spots of melon fly, Bactrocera (Dacus) cucurbitae Coquillett (Diptera: Tephritidae) in sterile fly release areas in Okinawa Island. Japanese Journal of Applied Entomology and Zoology. 1993;37:123–128. [Google Scholar]
- Narayanan ES, Batra HN. 1960 Fruit Flies and Their Control. Indian Council of Agricultural Research, New Delhi, India. 1–68.pp. [Google Scholar]
- Narayanan ES. Seasonal pests of crops. Indian Farming. 1953;3(4):8–11. 29–31. [Google Scholar]
- Nath P. Varietal resistance of gourds to the fruit fly. Indian Journal of Horticulture. 1966;23:69–78. [Google Scholar]
- Newell IW, Mitchell WC, Rathburn FL. Infestation norms for Dacus cucurbitae in Momordica balsamina and seasonal differences in activity of the parasite, Opius fletcheri. Proceedings of the Hawaiian Entomological Society. 1952;148:497–508. [Google Scholar]
- Nishida T. Natural enemies of the melon fly, Dacus cucurbitae Coq. in Hawaii. Journal of Economic Entomology. 1955;48:171–178. [Google Scholar]
- Nishida T. 1963 Zoogeographical and ecological studies of Dacus cucurbitae (Diptera: Tephritidae) in India. Technical Bulletin No. 54. Hawaiian Agricultural Station, University of Hawaii, Hawaii. [Google Scholar]
- Odani Y, Sakurai H, Teruya T, Ito Y, Takeda S. Sterilizing mechanism of gamma-radiation in the melon fly, Dacus cucurbitae. Research Bulletin of Faculty of Agriculture Gifu University. 1991;56:51–57. [Google Scholar]
- Osmelak JA. Australia-major pests of major vegetable crops. Quarterly Newsletter-Asia and Pacific Plant Protection Community. 1990;33:9–12. [Google Scholar]
- Pal AB, Srinivasan K, Doijode SD. Sources of resistance to melon fruit fly in bitter gourd and possible mechanisms of resistance. SABRAO Journal. 1984;16:57–69. [Google Scholar]
- Pandey MB, Misra DS. Studies on movement of Dacus cucurbitae maggot for pupation. Shashpa. 1999;6:137–144. [Google Scholar]
- Pareek BL, Kavadia VS. Relative preference of fruit fly, Dacus cucurbitae Coquillett on different cucurbits. Indian Journal of Entomology. 1994;56:72–75. [Google Scholar]
- Pareek BL, Kavadia VS. Screening of muskmelon varieties against melon fruit fly, Dacus cucurbitae Coquillett under field conditions. Indian Journal of Entomology. 1995;57:417–420. [Google Scholar]
- Pawar DB, Mote UN, Lawande KE. Monitoring of fruit fly population in bitter gourd crop with the help of lure trap. Journal of Research, Maharashtra Agricultural Universities. 1991;16:281. [Google Scholar]
- Permalloo S, Seewooruthun SI, Joomaye A, Soonnoo AR, Gungah B, Unmole L, and Boodram R. 1998 An area wide control of fruit flies in Mauritius. In: Lalouette JA, Bachraz DY, Sukurdeep N, Seebaluck BD editors. Proceedings of the Second Annual Meeting of Agricultural Scientists, 12-13 August 1997, pp. 203–210.Food and Research Council, Reduit, Mauritius. [Google Scholar]
- Prokopy RJ, Miller NW, Pinero JC, Barry JD, Tran LC, Oride LK, Vargas RI. Effectiveness of GF-120 Fruit Fly Bait spray applied to border area plants for control of melon flies (Diptera: Tephritidae) Journal of Economic Entomology. 2003;96:1485–1493. doi: 10.1603/0022-0493-96.5.1485. [DOI] [PubMed] [Google Scholar]
- Prokopy RJ, Miller NW, Pinero JC, Oride L, Perez N, Revis HC, Vargas RI. Hoe effective is GF-120 fruit fly bait spray applied to border area sorghum plants for control of melon flies (Diptera: Tephritidae) Florida Entomologist. 2004;87:354–360. [Google Scholar]
- Qureshi ZA, Ashraf M, Bughio AR. Relative abundance of Dacus cucurbitae and Dacus ciliatus in common hosts. Pakistan Journal of Science and Industrial Research. 1974;17:123–124. [Google Scholar]
- Rabindranath K, Pillai KS. Control of fruit fly of bitter gourd using synthetic pyrethroids. Entomon. 1986;11:269–272. [Google Scholar]
- Raju PM, Ali M, Velasco-Negueruela A, Perez-Alonso MJ. Volatile constituents of the leaves of Ocimum sanctum L. Journal of Essential Oils Research. 1999;11:159–161. [Google Scholar]
- Ramsamy MP, Rawanansham T, Joomaye A. Studies on the control of Dacus cucurbitae Coquillett and Dacus d'emmerezi Bezzi (Diptera: Tephritidae) by male annihilation. Revue Agricole et Sucriere de ltle Mauriee. 1987;66:1–3. [Google Scholar]
- Ranganath HR, Suryanarayana MA, Veenakumari K. Management of melon fly (Bactrocera (Zeugodacus) cucurbitae in cucurbits in South Andaman. Insect Environment. 1997;3:32–33. [Google Scholar]
- Ranganath HR, Veenakumari K. Tomato (Lycopersicon esculentum Miller): a confirmed host of the melon fly, Bactrocera (Zeugodacus) cucurbitae Coquillett. Insect Environment. 1996;2:3. [Google Scholar]
- Reddy AV. Evaluation of certain new insecticides against cucurbit fruit fly (Dacus cucurbitae Coq.) on bitter gourd. Annals of Agricultural Research. 1997;18:252–254. [Google Scholar]
- Renjhan PL. On the morphology of the immature stages of Dacus (Strumeta) cucurbitae Coq. (the melon fruit fly) with notes on its biology. Indian Journal of Entomology. 1949;11:83–100. [Google Scholar]
- Roomi MW, Abbas T, Shah AH, Robina S, Qureshi AA, Hussain SS, Nasir KA. Control of fruit flies (Dacus spp.) by attractants of plant origin. Anzeiger fur Schadlingskunde, Aflanzenschutz, Umwdtschutz. 1993;66:155–7. [Google Scholar]
- Samalo AP, Beshra RC, Satpathy CR. Studies on comparative biology of the melon fruit fly, Dacus cucurbitae Coq. Orissa Journal of Agricultural Research. 1991;4:1–2. [Google Scholar]
- Seewooruthun SI, Sookar P, Permalloo S, Joomaye A, Alleck M, Gungah B, and Soonnoo AR. 1998 An attempt to the eradication of the Oriental fruit fly, Bactrocera dorsalis (Hendel) from Mauritius. In: Lalouette JA, Bachraz DY, Sukurdeep N, Seebaluck BD editors. Proceedings of the Second Annual Meeting of Agricultural Scientists, 12-13 August 1997, pp. 181–187.Food and Research Council, Reduit, Mauritius. [Google Scholar]
- Sekiguchui Y. Eradication of the melon fly (Dacus cucurbitae) from Amani Islands of Japan. Quarterly Newsletter-Asia and Pacific Plant Protection Community. 1990;33:19–20. [Google Scholar]
- Senior-White R. 1924 Trypetidae. Catalogue of Indian Insects IV. 1–33.pp. Government of India, Calcutta, India. [Google Scholar]
- Shah MI, Batra HN, Ranjhen PL. Notes on the biology of Dacus (Strumeta) ferrugineus Fab. and other fruit flies in the North-West Frontier Province. Indian Journal of Entomology. 1948;10:249–266. [Google Scholar]
- Shiga M. Future prospects for the eradication of fruit flies. Technical Bulletin of Food and Fertilizer Technology Center. 1992;128:1–12. [Google Scholar]
- Singh SV, Mishra A, Bisan RS, Malik YP, Mishra A. Host preference of red pumpkin beetle, Aulacophora foveicollis and melon fruit fly, Dacus cucurbitae. Indian Journal of Entomology. 2000;62:242–246. [Google Scholar]
- Sinha P, Saxena SK. Effects of culture filtrates of three fungi in different combinations on the development of Dacus cucurbitae in vitro. Indian Phytopathology. 1998;51:361–362. [Google Scholar]
- Sinha P, Saxena SK. Effect of culture filtrates of three fungi in different combinations on the development of the fruit fly, Dacus cucurbitae Coq. Annals of Plant Protection Service. 1999;7:96–9. [Google Scholar]
- Sinha P. Effects of culture filtrates of fungi on mortality of larvae of Dacus cucurbitae. Journal of Environmental Biology. 1997;18:245–248. [Google Scholar]
- Soemori H, Nakamori H. Production of successive generations of a new strain of the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae) and reproductive characteristics in mass rearing. Japanese Journal of Applied Entomology and Zoology. 1981;25:229–235. [Google Scholar]
- Srinivasan K. 1991 Pest management in cucurbits – An overview of work done under AICVIP. In: Group Discussion of Entomologists Working in the Coordinated Projects of Horticultural Crops, 28–29 January 1991, pp. 44–52.Central Institute of Horticulture for Northern Plains, Lucknow, Uttar Pradesh, India. [Google Scholar]
- Srinivasan K. 1994 Recent trends in insect pest management in vegetable crops. In: Dhaliwal GS, Arora R editors. Trends in Agricultural Insect Pest Management, pp. 345–372.Commonwealth Publishers, New Delhi, India. [Google Scholar]
- Srinivasan PM. Guard your bitter gourd against the fruit fly. Indian Farming. 1959;9:8. [Google Scholar]
- Steiner LF, Harris EJ, Mitchell WC, Fujimoto MS, Christenson LD. Melon fly eradication by over flooding with sterile flies. Journal of Economic Entomology. 1965;58:519–522. [Google Scholar]
- Stonehouse JM, Mumford JD, and Verghese A. 2004 Returns to scale in pest suppression and eradication: Issues for the wide-area management of fruit flies in India. In: Subramanyam B, Ramamurthy VV, Singh VS editors. Proceedings of the National Symposium on Frontier Areas of Entomological Research, November 5-7 2003, pp. 151–158.Indian Agricultural Research Institute, New Delhi, India. [Google Scholar]
- Syed RA. 1969 Studies on the ecology of some important species of fruit flies and their natural enemies in West Pakistan. CIBC, Commonwealth Agriculture Bureau, Farnham Royal, Slough, UK, 12. pp. [Google Scholar]
- Talpur MA, Rustamani MA, Hussain T, Khan MM, Katpar PB. Relative toxicity of different concentrations of Dipterex and Anthio against melon fly, Dacus cucurbitae Coq. on bitter gourd. Pakistan Journal of Zoology. 1994;26:11–12. [Google Scholar]
- Tan KH, Lee SL. Species diversity and abundance of Dacus (Diptera: Tephritidae) in five ecosystems of Penang, West Malaysia. Bulletin of Entomological Research. 1982;72:709–716. [Google Scholar]
- Tewatia AS, Dhankhar BS, Dhankhar SK. Growth and yield characteristics of melon fruit fly resistant and highly susceptible genotypes of bitter gourd- A note. Haryana Journal of Horticultural Science. 1997;25:253–255. [Google Scholar]
- Thakur JC, Khattra AS, Brar KS. Comparative resistance to fruit fly in bitter gourd. Haryana Journal of Horticultural Science. 1992;21:285–288. [Google Scholar]
- Thakur JC, Khattra AS, Brar KS. Stability analysis for economic traits and infestation of melon fruit fly (Dacus cucurbitae) in bitter gourd (Momordica charantia) Indian Journal of Agricultural Sciences. 1994;64:378–381. [Google Scholar]
- Thakur JC, Khattra AS, Brar KS. Correlation studies between economic traits, fruit fly infestation and yield in bitter gourd. Punjab Vegetable Growers. 1996;31:37–40. [Google Scholar]
- Thomas C, Jacob S. Bioefficacy and residue dynamics of carbofuran against the melon fruit fly, Dacus cucurbitae Coq. infesting bitter gourd, Momordica charantia L. in Kerala. Journal of Entomological Research. 1990;14:30–34. [Google Scholar]
- Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287:2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- Tsubaki Y, Sokei Y. Prolonged mating in the melon fly, Dacus cucurbitae (Diptera: Tephritidae): Competition for fertilization by sperm loading. Research on Population Ecology. 1988;30:343–352. [Google Scholar]
- Tsuruta K. Pictoral key to Dacine fruit flies associated with economic plants in Sri Lanka. Research Bulletin of Plant Protection Service Japan. 1998;34:23–35. [Google Scholar]
- Uchida GK, Vargas RI, Beardsley JW, Liquido NJ. Host stability of wild cucurbits for melon fly, Dacus cucurbitae Coquillett, in Hawaii, with notes on their distribution and taxonomic status. Proceedings of the Hawaiian Entomological Society. 1990;30:37–52. [Google Scholar]
- Vargas RI, Jang EB, and Klungness LM. 2003 Area-wide pest management of fruit flies in hawaiian fruits and vegetables. In: Recent Trends on Sterile Insect Technique and Area-wide Integrated Pest Management, p. 37-46. Research Institute of Subtropics, Okinawa, Japan. [Google Scholar]
- Vargas RI, Stark JD, Nishida T. Population dynamics, habitat preference and seasonal distribution patterns of Oriental fruit fly and melon fly (Diptera: Tephritidae) in an agricultural area. Environmental Entomology. 1990;19:1820–1828. [Google Scholar]
- Vargas RI, Stark JD, and Nishida T. 1992 Ecological framework for integrated pest management of fruit flies in papaya orchards. In: Ooi PAC, Lim GS, Teng PS editors. Proceedings of the third International Conference on Plant Protection in the Tropics 20-23 March 1990, pp. 64–69.Malaysian Plant Protection Society, Genting Highlands, Kuala Lumpur, Malaysia. [Google Scholar]
- Vargas RI, Walsh WA, Kanehira D, Jang EB, Armstrong JW. Demography of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperatures. Annals of the Entomological Society of America. 1997;90:162–168. [Google Scholar]
- Vargas RI. 2004 Area-wide Integrated Pest Management for Exotic Fruit Flies in Hawaii. p. 12. In: FLC Awards Program. The FLC-TPWG National Meeting, 3–6 May 2004, San Diego, California. [Google Scholar]
- Vreysen MJ. Principles of area-wide integrated tsetse fly control using the sterile insect technique. Medicine for Tropics. 2001;61:397–411. [PubMed] [Google Scholar]
- Walters ML, Staten RT, and Roberson RC. 2000 Pink bollworm integrated management using sterile insects under field trial conditions, Imperial Valley, California. In: Tan KH editor. Area-Wide Control of Fruit Flies and Other Insect Pests, pp. 201–206.Penerbit Universiti Sains Malaysia, Penang, Malaysia. [Google Scholar]
- Waterhouse DF. 1993 The major arthropod pests and weeds of agriculture in Southeast Asia. In: ACIAR Monograph 21, pp. 141. Australian Center for International Agricultural Research, Canberra, Australia. [Google Scholar]
- Weems HV Jr, Heppner JB. 2001 Melon fly, Bactrocera cucurbitae Coquillett (Insecta: Diptera: Tephritidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry, and T.R. Fasulo, University of Florida. University of Florida Publication EENY- 199. [Google Scholar]
- Wen HC. Field studies on melon fly (Dacus cucurbitae) and attractant experiment in southern Taiwan. Journal of Agricultural Research China. 1985;34:228–235. [Google Scholar]
- White IM, Elson-Harris MM. 1994 Fruit Flies of Economic Significance: Their Identification and Bionomics. Commonwealth Agriculture Bureau International, Oxon, UK. 1–601.pp. [Google Scholar]
- Willard HF. Opius fletcheri as a parasite of the melon fly in Hawaii. Journal of Agricultural Research. 1920;6:423–438. [Google Scholar]
- Wong TTY, Cunningham RT, Mcinnis DO, Gilmore JE. Seasonal distribution and abundance of Dacus cucurbitae (Diptera: Tephritidae) in Rota, Commonwealth of the Mariana Islands. Environmental Entomology. 1989;18:1079–1082. [Google Scholar]
- Wood M. Forcing exotic, invasive insects into retreat: new IPM program targets Hawaii's fruit flies. Agricultural Research Washington. 2001;49:11–13. [Google Scholar]
- Wyss JH. 2000 Screwworm eradication in the Americas-overview. In: Tan KH editor. Area-Wide Control of Fruit Flies and Other Insect Pests, pp. 79–86.Penerbit Universiti Sains Malaysia, Penang, Malaysia. [DOI] [PubMed] [Google Scholar]
- Yamagishi M, Tsubaki Y. Copulation duration and sperm transfer in the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae) Applied Entomology and Zoology. 1990;25:517–519. [Google Scholar]
- Yang PJ, Carey JR, Dowell RV. Tephritid fruit flies in China: Historical background and current status. Pan-Pacific Entomologist. 1994;70:159–167. [Google Scholar]
- Yoshizawa O. Successful eradication programs on fruit flies in Japan. Research Bulletin of Plant Protection Service Japan. 1997;33:10. [Google Scholar]
- Zaman M. Assessment of the male population of the fruit flies through kairomone baited traps and the association of the abundance levels with the environmental factors. Sarhad Journal of Agriculture. 1995;11:657–670. [Google Scholar]


