Abstract
In this paper, we report the cloning and characterization of three Paenibacillus azotofixans DNA regions containing genes involved in nitrogen fixation. Sequencing analysis revealed the presence of nifB1H1D1K1 gene organization in the 4,607-bp SacI DNA fragment. This is the first report of linkage of a nifB open reading frame upstream of the structural nif genes. The second (nifB2H2) and third (nifH3) nif homologues are confined within the 6,350-bp HindIII and 2,840-bp EcoRI DNA fragments, respectively. Phylogenetic analysis demonstrated that NifH1 and NifH2 form a monophyletic group among cyanobacterial NifH proteins. NifH3, on the other hand, clusters among NifH proteins of the highly divergent methanogenic archaea.
Nitrogen fixation-related genes have been highly conserved throughout evolution even though they are widely distributed among eubacteria and archaea (4, 7, 11, 13, 15). In terms of their physical and biochemical properties, the mechanisms of the nitrogen fixation process are very similar among these organisms. The conventional dinitrogenase is composed of an α2β2 tetramer; the α and β subunits are encoded by the nifD and nifK genes, respectively. Also included in the nitrogenase complex is nitrogenase reductase, which is encoded by the nifH gene. In most diazotrophs, the nifHDK genes are contiguous. Sequence and mutational analyses of nitrogen fixation-related genes of various diazotrophs indicate that the arrangement of nif and associated genes differs considerably among these organisms. Examples of organisms with a noncontiguous arrangement of structural nif genes are Frankia sp. strain FaC1, Bradyrhizobium japonicum, and Rhizobium sp. strain Irc78 (2, 14).
Paenibacillus azotofixans ATCC 35681 is a gram-positive, facultatively anaerobic diazotroph that falls into a broad cluster of nitrogen fixers in rRNA group 3; this cluster also includes P. macerans and P. polymyxa (3). Diazotrophic strains of P. azotofixans were shown to possess the ability to fix atmospheric dinitrogen with high efficiency (8, 25, 29). In contrast to the majority of diazotrophs, their ability to fix nitrogen is not affected by the presence of nitrate (29).
PCR amplification of the nifH gene fragment.
The objective of identifying DNA fragments containing nif homologues was achieved by using the 380-bp nifH gene as a homologous probe. Alignment of NifH polypeptide sequences from representative diazotrophs was performed using ClustalX software (9). Based on these sequence alignments, nifH-degenerate oligonucleotides (5′-TAY GGN AAR GGN GGN ATN GGN AA-3′ and 5′-GCR AAN CCN CCR CAN ACN ACR TC-3′) were designed as primers.
Chromosomal DNA (40 ng/ml) was PCR amplified in a 50-μl reaction volume containing 1× PCR buffer (Promega), a 1 mM concentration of each primer, a 0.2 mM concentration of each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 2.0 U of Weiss Taq DNA polymerase (Promega). The following PCR parameters were used: 94°C for 5 min; 30 thermal cycles of 94°C for 30s, 45°C for 30s, and 72°C for 30s; and a final extension step at 72°C for 10 min.
Screening of genomic library and Southern blot analysis.
A genomic library of individual lambda clones from primary recombinants was screened according to standard procedures (28), using the PCR-amplified nifH probe. Following secondary and tertiary screenings, positively hybridized plaques were isolated and their DNA was extracted. The purified DNA was subjected to restriction enzyme digestions (EcoRI, HindIII, and SacI). Southern analysis using the nifH probe revealed the presence of three distinctly different DNA digestion profiles (data not shown), suggesting the existence of three different nif gene-containing DNA regions, which were subsequently gel purified and ligated. Hybridization analysis using the digoxigenin-labeled nifH PCR probe was also performed with genomic DNA digested with the EcoRI and HindIII restriction enzymes (data not shown). The results obtained suggested the presence of more than one copy of the nifH gene in P. azotofixans, in agreement with previous studies by Oliveira et al. (21) and Rosado et al. (26).
Sequence analysis of nifH1, nifH2, nifH3, and other nif genes.
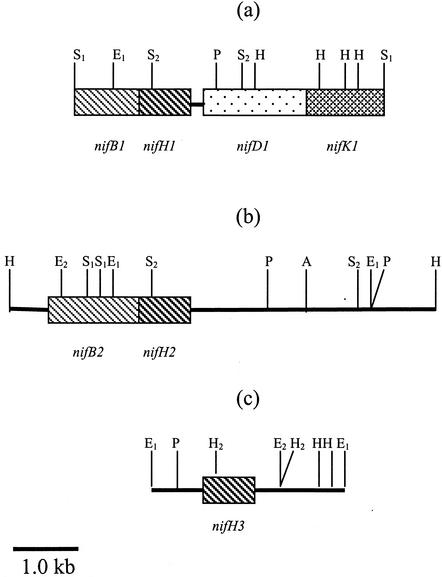
The 4,607-bp SacI fragment contained a 320-amino-acid partial nifB1 coding region, the complete nifH1 and nifD1 open reading frames (ORFs), and the first 387 amino acids of nifK1 (Fig. 1a). These alleles were designated nifB1, nifH1, nifD1, and nifK1, respectively. This is the first report of linkage of a nifB ORF upstream of the conventional structural nif genes. Analysis of the region immediately upstream of the nifH1D1K1 ORFs revealed the presence of potential ribosome binding sites (RBSs; GAAGG, GAGG, and GAGG, respectively) (30) located between 8 and 11 bp from the ATG initiation codon of each ORF. The suggested nifH1 RBS overlaps with the 3′ end of the nifB1 coding region. Examination of the 143-bp nifH1-nifD1 intergenic region revealed the presence of an 11-bp inverted-repeat structure that might have a regulatory function during nifD1K1 transcription. Similar inverted repeats have been described for other diazotrophs (4, 11, 16). Comparison of its amino acid sequences with sequences in the database revealed that this nifD1 ORF has the highest degree of homology with members of the gram-positive, high-G+C-content genus Frankia (69% identity with Frankia alni strain ArI3 and 68% identity with Frankia sp. strain EUIK1). A 4-nucleotide overlap occurs between the 3′ end of nifD1 and the 5′ end of nifK1, an indication of a possible translational coupling phenomenon (22). The sequence of nifK1 was partial from the putative ATG, coding for 387 amino acids, with a putative RBS located 10 bp upstream (within the 3′ end of nifD1).
FIG. 1.
Organization of nif genes in a 4.607-kb SacI fragment from pCQC102 containing the entire nifH1 and nifD1 genes and partial nifB1 and nifK1 genes (a), a 6.350-kb HindIII fragment from pCQC100 containing nifH2 and nifB2 (b), and a 2.840-kb EcoRI fragment from pCQC101 containing nifH3 (c). Restriction enzyme cutting sites are indicated as follows: A, HpaI; H, HindIII; H2, HincII; E1, EcoRI; E2, EcoRV; P, PstI; S1, SacI; and S2, SacII.
A second nifB-nifH cluster (designated nifB2H2) was found in a 6,350-bp HindIII fragment (Fig. 1b). As with NifH1, the protein coding region of NifH2 is 879 nucleotides in length and encodes a predicted 292-amino-acid polypeptide. Interestingly, as in nifH1, the putative RBS (30) for nifH2 is located within the 5′ end of its corresponding nifB2 gene. The nifB2 ORF terminates with a single stop codon, TAA, which is followed by the initiation codon for nifH2 6 bp downstream. Unlike nifB1H1, this nif cluster does not have the nifDK genes within the 3.5-kb region downstream of the nifH2 termination codon. Instead, two potential ORFs that appear to lack any known nif-related function are found. The closest homologies were with various transporter substrate-binding proteins.
The third nifH homologue (nifH3) was found within the 2,840-bp EcoRI fragment (Fig. 1c). No adjacent nifB or nifDK coding regions were found within close proximity. A putative ORF (truncated) that displayed homology to transporter ATP-binding proteins was found approximately 80 bp upstream of the nifH3 start codon.
Amino acid alignment of NifH proteins.
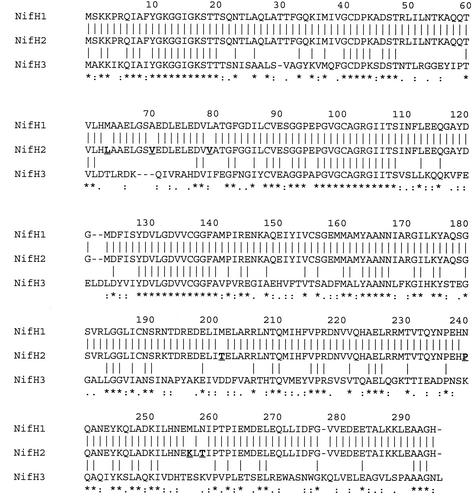
Figure 2 shows an alignment of the deduced amino acid sequences of the P. azotofixans NifH proteins. They are more divergent in their C termini. Stretches of 10 or more conserved amino acids were observed for residues 10 to 21, 97 to 109, and 129 to 141. When the amino acid residues of NifH1 and NifH2 were compared, seven were found to differ; this constitutes 97% identity. Comparing either NifH1 or NifH2 with NifH3 yielded a comparatively low 43% identity. A high (97%) identity was also observed when the partial reading frames of nifB genes were translated to their respective amino acids. At the nucleotide level, alignment of the two nifBH gene clusters also revealed a high degree of identity (94%), with no significant changes until 57 bases downstream of the presumptive termination codon of the nifH ORF (data not shown). These data led us to postulate that nifBH gene clusters of P. azotofixans had undergone a gene duplication process, resulting in the nifB1H1 and nifB2H2 gene organizations.
FIG. 2.
ClustalX (9) alignment of deduced amino acids sequences of the three NifH proteins of P. azotofixans. Amino acids are designated with one-letter abbreviations. Gaps (indicated by hyphens) were introduced for maximal matching. The conserved amino acids are indicated by stars below the alignment; periods and colons denote similar amino acids and acceptable substitutions, respectively, at the indicated amino acid positions. NifH2 amino acid residues which differ from those in the corresponding positions of NifH1 are in bold and underlined.
Phylogenetic analysis.
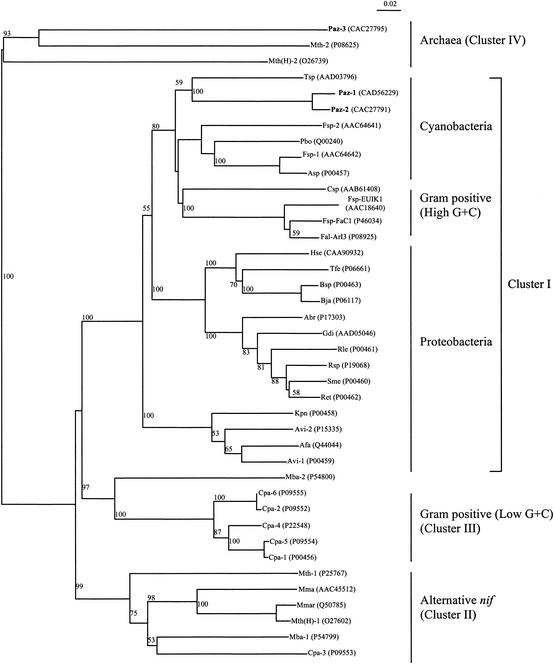
The nifH phylogenetic tree had been well established (20, 26, 32, 35, 36) and is largely consistent with the 16S rRNA gene phylogeny (34). Our data (Fig. 3) are in agreement with the division of the NifH topology into four major clusters, as described by Chien and Zinder (5, 6). When the complete nifH coding sequences were used, the clustering of P. azotofixans NifH1 and NifH2 yielded several interesting observations. Earlier nifH-based phylogenetic analyses of P. azotofixans involved partial sequences of nifH fragments derived by PCR amplification (1, 26, 36). Discrepancies between our study and those of other investigators (1, 26, 36) in the placement of P. azotofixans NifH proteins were probably due to their use of short-length nifH fragments, which reduced the resolving power of the analyses. When phylogeny was based on partial nifH gene sequences, determined by Zehr et al. (36), P. azotofixans NifH did not cluster with NifH proteins of any group of bacteria. Further observations and the branching order of the NifH phylogeny seemed to suggest that P. azotofixans NifH lies within the cyanobacterial clade (1, 36).
FIG. 3.
Tree showing phylogeny of NifH polypeptide sequences, constructed by the neighbor-joining method (27). Graphic representation of the tree was made using NJPlot software (23). The database accession numbers are indicated after the abbreviations. Cluster I to IV assignments are described elsewhere (5, 6). The data was analyzed with 100 bootstrap values. The values presented above the nodes are the bootstrap values generated. Bootstrap values below 50% are not shown. The scale bar represents 0.02 substitution per site. Abbreviations: Abr, Azospirillum brasilense; Afa, Alcaligenes faecalis; Asp, Nostoc sp. strain PCC7120; Avi, Azotobacter vinelandii; Bsp, Bradyrhizobium sp. strain ANU289; Bja, Bradyrhizobium japonicum; Cpa, Clostridium pasteurianum; Csp, Cyanothece sp. strain ATCC 51142; Fal-ArI3, Frankia alni strain ArI3; Fsp-EUIK1, Frankia sp. strain EUIK1; Fsp-FaC1, Frankia sp. strain FaC1; Fsp, Fischerella sp. strain UTEX1931; Gdi, Gluconacetobacter diazotrophicus; Hse, Herbaspirillum seropedicae; Kpn, Klebsiella pneumoniae; Mba, Methanosarcina barkeri; Mma, Methanococcus maripaludis; Mth(H), Methanothermobacter thermoautotrophicus (ΔH); Mmar, Methanothermobacter marburgensis strain Marburg; Mth, Methanothermococcus thermolithotrophicus; Paz, Paenibacillus azotofixans; Pbo, Plectonema boryanum; Ret, Rhizobium etli; Rle, Rhizobium leguminosarum; Rsp, Rhizobium sp. strain NGR234; Sme, Sinorhizobium meliloti; Tfe, Acidithiobacillus ferrooxidans; Tsp, Trichodesmium sp. strain IMS101.
Use of the complete DNA sequences of the three nifH genes in a reanalysis of NifH phylogeny demonstrated clustering of P. azotofixans NifH1 and NifH2 within the Cyanobacteriaceae grouping (Fig. 3). The NifH protein from a filamentous, nonheterocystous marine cyanobacterium, Trichodesmium sp. strain IMS101, showed the highest degrees of identity with P. azotofixans NifH1 (80%) and NifH2 (79%), respectively. Interestingly, neither NifH1 nor NifH2 clustered with the NifH proteins of other gram-positive diazotrophs, such as Frankia spp. (a high-G+C firmicute) and Clostridium pasteurianum (a low-G+C firmicute).
The third putative nifH gene product of P. azotofixans (NifH3) clustered with NifH proteins of members of the Archaea domain, Methanothermococcus thermolithotrophicus and Methanothermobacter thermoautotrophicus. Again, this putative NifH did not cluster with those of the other phyletically related gram-positive microorganisms, such as Frankia spp. or C. pasteurianum. This is the first report of a gram-positive diazotroph having a NifH protein that clusters with Nif proteins of confirmed methanogenic diazotrophs. Based on the NifH phylogenetic analysis (Fig. 3), P. azotofixans NifH3 also did not fall within the anf nitrogenase clade. Rosado et al. (26) reported a nifH phylogenetic tree in which the proteins of three Paenibacillus strains, P. azotofixans P3E20 and RBN4 and P. durum DSMZ1735, formed a cluster with the alternative (anf) nitrogenases. It is not known at this point whether the putative anf nitrogenase reported by Rosado et al. (26) exists in this P. azotofixans type strain as well. It has not yet been determined whether this nifH3 gene product is a functional nitrogenase. It has been postulated that genes from this cluster are related to genes involved in bacteriochlorophyll synthesis and probably have a function unrelated to nitrogen fixation (5, 12).
The question of horizontal transfer of the nifH gene has been debated among evolutionists for the last 3 decades (10, 17, 18). The strongest evidence yet for horizontal nifH gene transfer came from the pioneering phylogeny studies of nifH genes (17, 18). Verification of a horizontal-transfer event is difficult, especially with the limited genetic data from Paenibacillus strains. Nevertheless, our data on NifH phylogeny revealed some unanticipated features that brought us to postulate that the gene transfer phenomenon exists. The most striking evidence for the occurrence of a gene transfer event was the unusual placement of NifH3 among the highly divergent members of the Archaea. Smith et al. (31) described a phylogenetic congruency test based on the assumption that a NifH tree corresponds to conventional NifH phylogenies (20, 26, 32, 35, 36); if there was any odd placement, a horizontal-gene-transfer event may have occurred. Furthermore, the low level of identity (43%) between P. azotofixans NifH3 and the other two NifH proteins likely indicates that there are two different groupings of orthologous gene products. The vast differences in the sequences among NifH3 proteins compared to NifH1 and NifH2 seemed to suggest that a duplication event was unlikely; otherwise, like the five C. pasteurianum NifH proteins (33), all three P. azotofixans NifH proteins would be grouped in the same cluster.
It is not presently known whether all three nif homologue clusters are located in the genome or on plasmids (if any even exist), as in some diazotrophic systems (19, 24). It will also be of interest to determine whether the phylogenies of complete nifH genes of other Paenibacillus strains conform to the conventional nifH phylogenetic topology.
Nucleotide sequence accession numbers.
The sequencing data obtained in this study have been deposited in the EMBL database under the following accession numbers: AJ299453, AJ299454, and AJ515294.
Acknowledgments
This work was supported by research grants from the Malaysian Ministry of Science, Technology and Environment (MOSTE); a Universiti Sains Malaysia short-term grant; and the Malaysian Toray Science Foundation (MTSF).
REFERENCES
- 1.Achouak, W., P. Normand, and T. Heulin. 1999. Comparative phylogeny of rrs and nifH genes in the Bacillaceae. Int. J. Syst. Bacteriol. 49:961-967. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Morales, A., M. Betancourt-Alvarez, K. Kaluza, and H. Hennecke. 1986. Activation of the Bradyrhizobium japonicum nifH and nifDK operons is dependent on promoter-upstream DNA sequences. Nucleic Acids Res. 14:4207-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ash, C., J. A. E. Farrow, S. Wallbanks, and M. D. Collins. 1991. Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small-subunit-ribosomal RNA sequences. Lett. Appl. Microbiol. 13:202-206. [Google Scholar]
- 4.Brigle, K. E., W. E. Newton, and D. R. Dean. 1985. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene 37:37-44. [DOI] [PubMed] [Google Scholar]
- 5.Chien, Y.-T., and S. H. Zinder. 1994. Cloning, DNA sequencing, and characterization of a nifD-homologous gene from the archaeon Methanosarcina barkeri 227 which resembles nifD1 from the eubacterium Clostridium pasteurianum. J. Bacteriol. 176:6590-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien, Y.-T., and S. H. Zinder. 1996. Cloning, functional organization, transcript studies, and phylogenetic analysis of the complete nitrogenase structural genes (nifHDK2) and associated genes in the archaeon Methanosarcina barkeri 227. J. Bacteriol. 178:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean, D. R., and M. R. Jacobson. 1992. Biochemical genetics of nitrogenase, p. 763-834. In G. Stacey, R. H. Burris, and H. J. Evans (ed.), Biological nitrogen fixation. Chapman and Hall, New York, N.Y.
- 8.De Lima, B. D., and E. G. C. Penido. 1989. Inoculation in vitro of wheat seedlings and wheat straw cultures with Bacillus azotofixans. Plant Soil 113:133-136. [Google Scholar]
- 9.Higgins, D. G., and P. M. Sharp. 1988. Clustal: a package for performing multiple alignment on a computer. Gene 73:237-244. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch, A. M., H. I. McKhann, A. Reddy, J. Liao, Y. Fang, and C. R. Marshall. 1995. Assessing horizontal transfer of nifHDK genes in eubacteria: nucleotide sequence of nifK from Frankia strain HFPCcI3. Mol. Biol. Evol. 12:16-27. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson, M. R., K. E. Brigle, L. T. Bennett, R. A. Setterquist, M. S. Wilson, V. L. Cash, J. Beynon, W. E. Newton, and D. R. Dean. 1989. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J. Bacteriol. 171:1017-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler, P. S., J. McLarnan, and J. A. Leigh. 1997. Nitrogenase phylogeny and the molybdenum dependence of nitrogen fixation in Methanococcus maripaludis. J. Bacteriol. 179:541-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler, P. S., C. Blank, and J. A. Leigh. 1998. The nif gene operon of the methanogenic archaeon Methanococcus maripaludis. J. Bacteriol. 180:1504-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligon, J. M., and J. P. Nakas. 1987. Isolation and characterization of Frankia sp. strain FaC1 genes involved in nitrogen fixation. Appl. Environ. Microbiol. 53:2321-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons, E. M., and T. Thiel. 1995. Characterization of nifB, nifS, and nifU genes in the cyanobacterium Anabaena variabilis: NifB is required for the vanadium-dependent nitrogenase. J. Bacteriol. 177:1570-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norel, F., and C. Elmerich. 1987. Nucleotide sequence and functional analysis of the two nifH copies of Rhizobium ORS571. J. Gen. Microbiol. 133:1563-1576. [Google Scholar]
- 17.Normand, P., and J. Bousquet. 1989. Phylogeny of nitrogenase sequences in Frankia and other nitrogen-fixing microorganisms. J. Mol. Evol. 29:436-447. [DOI] [PubMed] [Google Scholar]
- 18.Normand, P., M. Gouy, B. Cournoyer, and P. Simonet. 1992. Nucleotide sequence of nifD from Frankia alni strain ArI3: phylogenetic inferences. Mol. Biol. Evol. 9:495-506. [DOI] [PubMed] [Google Scholar]
- 19.Nuti, M. P., A. A. Lepidi, R. K. Prakash, R. A. Schilperoort, and F. C. Cannon. 1979. Evidence for nitrogen fixation (nif) genes on indigenous Rhizobium plasmids. Nature 282:533-535. [Google Scholar]
- 20.Ohkuma, M., S. Noda, R. Usami, K. Horikoshi, and T. Kudo. 1996. Diversity of nitrogen fixation genes in the symbiotic intestinal microflora of the termite Reticulitermes speratus. Appl. Environ. Microbiol. 62:2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira, S. S., L. Seldin, and M. C. F. Bastos. 1993. Identification of structural nitrogen-fixation (nif) genes in Bacillus polymyxa and Bacillus macerans. World J. Microbiol. Biotechnol. 9:387-389. [DOI] [PubMed] [Google Scholar]
- 22.Oppenheim, D. S., and C. Yanofsky. 1980. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics 95:785-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrière, G., and M. Gouy. 1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 24.Quinto, C., H. de la Vega, M. Flores, J. Leemans, M. A. Cevallos, M. A. Pardo, R. Azpiroz, M. L. Girard, E. Calva, and R. Palacios. 1985. Nitrogenase reductase: a functional multigene family in Rhizobium phaseoli. Proc. Natl. Acad. Sci. USA 82:1170-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosado, A. S., F. S. de Azevedo, D. W. da Cruz, J. D. van Elsas, and L. Seldin. 1998. Phenotypic and genetic diversity of Paenibacillus azotofixans strains isolated from the rhizoplane or rhizosphere soil of different grasses. J. Appl. Microbiol. 84:216-226. [Google Scholar]
- 26.Rosado, A. S., G. F. Duarte, L. Seldin, and J. D. Van Elsas. 1998. Genetic diversity of nifH gene sequences in Paenibacillus azotofixans strains and soil samples analyzed by denaturing gradient gel electrophoresis of PCR-amplified gene fragments. Appl. Environ. Microbiol. 64:2770-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitou, N., and M. Nei. 1987. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Seldin, L., J. D. Van Elsas, and E. G. C. Penido. 1984. Bacillus azotofixans sp. nov., a nitrogen-fixing species from Brazilian soils and grass roots. Int. J. Syst. Bacteriol. 34:451-456. [Google Scholar]
- 30.Shine, J., and L. Dalgarno. 1974. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementary to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 71:1342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, M. W., D.-F. Feng, and R. F. Doolittle. 1992. Evolution by acquisition: the case for horizontal gene transfer. Trends Biochem. Sci. 17:489-493. [DOI] [PubMed] [Google Scholar]
- 32.Ueda, T., Y. Suga, N. Yahiro, and T. Matsuguchi. 1995. Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nifH gene sequences. J. Bacteriol. 177:1414-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, S.-Z., J.-S. Chen, and J. Johnson. 1988. The presence of five nifH-like sequences in Clostridium pasteurianum: sequence divergence and transcription properties. Nucleic Acids Res. 16:439-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young, J. P. W. 1992. Phylogenetic classification of nitrogen-fixing organisms. p. 43-86. In G. Stacey, R. H. Burris, and H. J. Evans (ed.), Biological nitrogen fixation. Chapman and Hall, New York, N.Y.
- 36.Zehr, J. P., M. Mellon, S. Braun, W. Litaker, T. Steppe, and H. W. Paerl. 1995. Diversity of heterotrophic nitrogen fixation genes in a marine cyanobacterial mat. Appl. Environ. Microbiol. 61:2527-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]