Abstract
The susceptibility of the egg, pupa, and adult of Haematobia irritans (L.) (Diptera: Muscidae) to isolates of the fungi Metarhizium anisopliae (Metsch.) Sor., Beauveria bassiana (Bals.) Vuill., and Paecilomyces fumosoroseus (Wize) Brown and Smith, was evaluated under laboratory conditions. Groups of 20 eggs than 4 h old, pupae less than 48h old and adults were sprayed with a conidial suspension of each isolate. Eggs, pupae and adults of horn fly were susceptible to these entomopathogenic fungi. For treated eggs, the isolates Ma3, Ma 15, Ma25, Pfr1, and Pfr8 reduced adult emergence to 3.8% to 6.3% in comparison with the control (72%). The mortality of pupae infected by the isolates Ma2, Ma25, and Pfr10 ranged between 50% and 71.3%. Mortality of adults after treatment with the isolates Ma6, Ma 10, Ma 14, Ma 15, Pfr 1, Pfr 9, Pfr 10, Pfr 11, and Pfr12 were higher than 90%. The isolate Ma6 produced the lowest LC50 against adult horn flies (8.08 × 102conidia/ml). These findings supported the hypotheses that isolates of M. anisopliae, and P. fumosoroseus are pathogenic against the different biological stages of horn flies by reducing adult emergence when applied on groups of eggs and pupae, and producing mortality when applied to adults.
Keywords: Metarhizium anisopliae, Beauveria bassiana, Paecilomyces fumosoroseus, horn fly, biological control Descriptores: Metarhizium anisopliae, Beauveria bassiana, Paecilomyces fumosoroseus, moscas del cuerno, control biológico
Resumen
La susceptibilidad de los estados biológicos de larva, pupa y adulto de Haematobia irritans (L.) (Diptera: Muscidae) a aislados de los hongos Metarhizium anisopliae (Metsch.) Sor., Beauveria bassiana (Bals.) Vuill., y Paecilomyces fumosoroseus (Wize) Brown y Smith, fue evaluada bajo condiciones de laboratorio. Grupos de 20 huevos de 4 h de edad fueron colocados sobre 50 g de excremento de vaca fresco en una proporción de 10:1 de excremento de vaca: harina de pescado deshidratada y ellos se asperjaron con una suspensión a una concentración de 1 × 106 conidias/ml. Otros grupos de 20 pupas de menos de 48 h de edad fueron asperjados con una suspensión conteniendo 1 × 108 conidias/ml, y cuatro grupos de 20 adultos fueron asperjadas con una suspensión de cada aislado. Los huevos, pupas y adultos de la mosca de los cuernos fueron susceptibles a los hongos entomopatógenos. Los aislados Ma2, Ma3, Ma 15, Ma25, Pfr1, y Pfr8 causaron una reducción en la emergencia de los adultos desde 3.8 a 6.3% en comparación con el testigo (72%). Las pupas fueron micosados por los aislados Ma2, Ma25 y Pfr10 en un rango entre 50 y 71.3%; los porcentajes de mortalidad de adultos mayor que 90% con los aislados Ma6, Ma 10, Ma 14, Pfr 9, Pfr 10, Pfr 11 y Pfr12. La CL50 más baja (8.08 ×102conidias/ml) en contra de adultos de moscas de los cuernos, la produjo el aislado Ma6. Los resultados apoyan la hipótesis de que los aislados de M. anisopliae y P. fumosoroseus son patógenos de diferentes estados biológico de las moscas del cuerno y que estos hongos reducen la emergencia de adultos cuando son aplicados a grupos de huevos y pupas y producen mortalidad cuando son aplicados a adultos de esta plaga.
Introduction
The horn fly, Haematobia irritans (L.) (Diptera: Muscidae) lay eggs on fresh cow manure. Maggots hatch in a few days, finish development in 8-10 days and leave the manure to pupate. Adults feed on cattle blood. H. irritans is widely distributed in North and Latin America and is considered to be a major bloodsucking pest of pastured cattle due to significant losses in cattle production (Guglielmone et al., 1997). This pest has also been recently reported attacking confined dairy cattle in Central Mexico (Cruz-Vázquez et al., 1999). The economic impact on livestock production by H. irritans has been estimated to approach $1 billion annually in the North America (Cupp et al., 1998). A horn fly population of about 700-1000 adults per animal caused weight losses between 40-90 g per day in beef cattle during a 4-month period (Kunz et al., 1984). In 1992, the horn fly was the most important and widespread cattle pest in the US, with an annual economic impact on cattle production estimated at $730.3 million (Byford et al., 1992), but the stable fly has taken its place in most of the country. Occasionally, H. irritans feeds on other species such as goats, horses, mules, dogs, but only rarely attacks humans (Greer and Butler, 1973; Torres and Prieto, 1993).
Chemical insecticides have bee used to control this pest, but widespread resistance has occurred throughout the United States (Sheppard and Joyce, 1992). Capsules of insecticides fed orally to cattle are used to control dung-inhabiting flies but also adversely affect the abundance of biological control agents in dung (Wall and Strong, 1987). Horn fly control is becoming increasingly difficult due to development of resistance to chlorinated hydrocarbons, carbamates, pyrethroids, and organophosphates from Canada (Mwangala and Galloway, 1993) to Argentina (Guglielmone et al., 2001). Therefore, alternative means of control that are not potentially harmful to humans, other animals and the environment have been sought. A number of resistance management strategies for the horn fly have been proposed (Sparks et al., 1985) to delay or prevent insecticide resistance (Byford et al., 1987).
Biological control is an alternative being investigated for control of livestock ectoparasites (Hogsette, 1999). The horn fly is susceptible to entomopathogenic fungi (Steenberg et al., 2001a). Beauveria bassiana (Bals.) Vuill, Lecanicillium (=Verticillium) lecanii (Zimmermann) Viegas, V. fusisporum (W. Gams) and Furia americana (Thaxter) Humber have been isolated from this insect pest (Steenberg et al., 2001a). The species, B. bassiana and P. farinosus killed 100%, and 90% of adults 4 days postreatment, respectively, at a concentration of 1.7x109 spores under laboratory conditions (Steenberg et al., 2001b).
Watson et al. (1995) evaluated the larvicidal activity of B. bassiana against the house fly, Musca domestica with the aim of controlling larvae in the bedding of confined cattle.
There are no reports of the effects of Metarhizium anisopliae (Metsh.), P. fumosoroseus, and B. bassiana against the eggs, and pupae of H. irritans. The objective of this study was to evaluate the susceptibility of eggs, pupae, and adults of H. irritans to these entomopathogenic fungi.
Materials and Methods
Stock colonies
Horn flies were provided by the Centro Nacional de Parasitología Animal (CENAPA-SAGARPA) (National Center for Animal Parasitology) in Jiutepec, Morelos, Mexico. The colony was maintained under laboratory conditions following the methodology and techniques of Schmidt et al. (1967). Constituents of the larval medium included fresh cow manure, and sardine meal Mazarina (Maz Industrial SA de CV, Estero de Urias, Mazatlán, Sinaloa, México) at a 10:1 ratio. Larvae were maintained at 30° C, and 60-70% RH. Pupae were collected by flotation, air dried, and placed in plastic containers with a capacity of 500 ml. Adults were fed ad libitum with bovine defibrined blood on saturated cellucotton pads covered with gauze, and maintained at 30° C, and 70-80% RH, in total darkness (Mendes and Linhares, 1999).
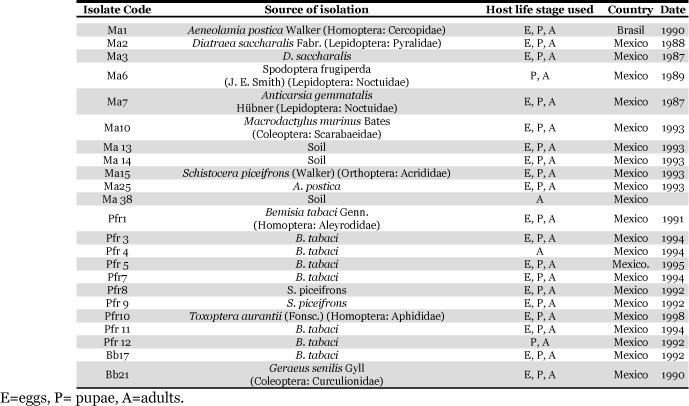
Table 1 shows the origin of the fungal isolates used. For egg inoculation, 9 isolates of M. anisopliae, 8 of P. fumosoroseus, and 2 of B. bassiana were used. For evaluating the susceptibility of pupae, 10 isolates of M. anisopliae, 9 of P. fumosoroseus, and 2 of B. bassiana were used. To evaluate the susceptibility of adults, 11 isolates of M. anisopliae, 10 of P. fumosoroseus, and 2 of B. bassiana were used. All the fungal isolates are maintained in the Entomopathogenic Fungi Collection of the Facultad de Ciencias Biológicas y Agropecuarias, of the Universidad de Colima. The isolates of P. fumosoroseus were provided by the Centro Nacional de Referencia de Control Biológico-Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (CNRCBSAGARPA), located in Tecomán, Colima, México, and the Ma 1 strain of M. anisopliae was graciously provided by Dr. A. Mendoça (BIOTEC, Alagaos, Brasil).
Table 1.
Origin of M. anisopliae (Ma), P. fumosoroseus (Pfr) and B. bassiana (Bb) fungal isolates used against different host life stages of the horn fly, Haematobia irritans.
Fungi were mass produced on Sabouraud dextrose Agar medium with 1% yeast extract and chloramphenicol at 500 ppm (Moorhouse et al., 1993). They were incubated at 25 ± 1° C, and 70% RH for three weeks. Conidia of each 21-day-old isolate were harvested by scraping, then suspending them in sterile distilled water containing 0.1% Tween 80. The suspension was poured into a sterile glass tube and mixed using a vortex mixer. Conidial concentrations were measured with an improved Neubauer hemocytometer (Reichert Scientific Instruments, Buffalo, New York) and adjusted by dilution with 0.1% Tween 80 for each life stage of horn fly as described below.
Susceptibility of eggs to entomopathogenic fungi
Fresh cow manure (50 g), mixed with fish meal in a ratio 10:1 (wt:wt), was placed in clear plastic containers (6 cm diameter × 6 cm height). Twenty eggs of H. irritans less than 4 h old were placed on the cow manure: fish meal mixture in each container using a camel hair brush. Four groups of 20 eggs each were inoculated with 4 ml sprays of a conidial suspension of each isolate of the entomopathogenic fungi at a concentration of 1 × 106 conidia/ml (1.1x105 conidia/cm2 of manure surface). The conidial suspensions were sprayed using a hand sprayer. Four control groups of eggs were sprayed with sterile distilled water with 0.1% Tween 80. After the inoculation, the eggs were incubated at 30o C, and 58% RH, in total darkness. The emergence of adults was observed and recorded at 48 h-intervals until adult emergence was complete. Dead horn flies were incubated in sterile Petri dishes on filter paper moistened with sterile distilled water at 25° C and ≈100% RH for 4 days to allow growth of surface mycelia of the fungi.
Susceptibility of pupae to entomopathogenic fungi
Groups of 20 pupae less than 48 h old were placed in Petri dishes (60 × 15 mm) lined with two moistened layers of Whatman No. 1 filter paper. Pupae were inoculated with 4 ml of conidial suspension using a hand sprayer. The suspension was adjusted to a concentration of 1x108conidia/ml to achieve 3.5x106 conidia/cm2. Untreated controls were sprayed with sterile distilled water containing 0.1% Tween 80 to similar groups of 20 pupae. The Petri dishes were incubated at 25 ± 1° C and 70% RH, in total darkness. Mycosis in the pupae was recorded during 24 h intervals until adult emergence. Pupae showing symptoms or signs of mycosis were placed in Petri dishes (60 × 15 mm) with a moistened double-lined filter paper to allow for the growth of surface mycelia.
Susceptibility of adults to entomopathogenic fungi
A suspension of conidia of each 21 day-old isolate (1 × 108conidia/ml) was used to inoculate with hand sprayer adults less than 48 h old to determine their susceptibility to entomopathogenic fungi (Watson et al., 1995). Four groups of 20 adults were counted out (Cantagui et al., 1995) and immobilized by exposure to cold temperature (4 ± 1° C) for 2 minutes (Crosby et al., 1991). Adults were placed on a moistened double-lined filter paper in plastic 500-ml containers (10 cm height × 10 cm diameter) and, spayed with a 1 × 108 conidia/ml suspension of each isolate. Untreated controls consisted of four groups of 20 flies, sprayed with sterile distilled water containing Tween 80 (Poprawsky et al., 1985). After inoculation, a 10-cm lid was placed on each container. The interior of the lid was replaced with a 2 mm mesh metallic mosquito screen to allow for air circulation and feeding the flies. Adults were fed at libitum with bovine defibrined blood in saturated cellucotton pads covered with gauze (Schmidt et al., 1967). All containers were placed in insulated boxes with a 2-cm layer of water and maintained at 27 ± 1° C.
Twenty four treatments with four replications each were evaluated using a completely randomized design. Emergence data, the percent of pupae, and adults exhibiting mycosis were transformed using arcsine transformation and subjected to ANOVA. Beginning 48 hours after inoculation, dead adults were recorded at 24-h intervals. Dead adults were placed in Petri dishes (60 × 15 mm) with a moistened double-lined filter paper to allow the growth of surface mycelia (Arthurs and Thomas, 2001). Means were separated by Tukey test (P < 0.05) (SAS Institute, 1989).
Mean lethal concentration of the most pathogenic fungi against horn fly adults
The most pathogenic isolates of M. anisopliae, and P. fumosoroseus from the adult susceptibility studies were selected to determine the mean lethal concentrations (LC50). Conidia of 20 d-old incubations of each isolate were used and the concentrations were adjusted to 1 × 102, 1 × 103, 1 × 104, 1 × 105, 1 × 106, and 1 × 107 conidia/ml. Groups of 20 adults 24-48 d-old were counted out (Butt et al. 1994) and immobilized as described above. Adults were placed on a moistened double-lined filter paper in plastic 500-ml containers (10 cm height × 10 cm diameter) and, each group was sprayed with the correspondent concentration of the suspension of each isolate. Untreated controls consisted of four groups of 20 flies, sprayed with sterile distilled water with 0.1% Tween 80 (Poprawsky et al., 1985). After inoculation, adults were handled as described above.
Seven treatments with four replications each for each selected fungal isolate were evaluated using a completely randomized design. Beginning 48 h after inoculation, dead adults were recorded at 24-h intervals. Dead adults were placed in Petri dishes (60 × 15 mm) with a moistened double-lined filter paper to allow for the growth of surface mycelia (Butt et al. 1994; Smith et al. 2000; Arthurs and Thomas, 2001). Data of mortality percentage were normalized by arcsine transformation prior to the analysis. Mean lethal concentrations and associated statistics were estimated by Probit analysis (SAS Institute, 1989).
Results
Effects of application of entomopathogenic fungi on eggs
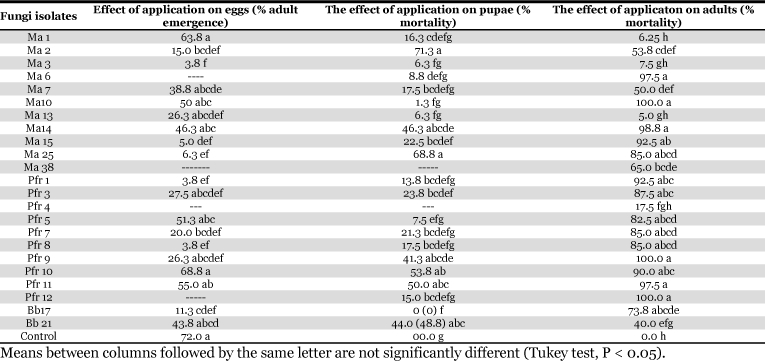
Application of the entomopathogenic fungi M. anisopliae, P. fumosoroseus, and B. bassiana, to eggs of H. irritans caused reduced adult emergence. The most effective isolates were, Ma 3, Ma 25, Ma 15, Pfr 1, Pfr 8, and Bb 17 (Table 2). Significant differences between some treatments compared to controls were demonstrated (F = 8.65, P < F=0.0001, df = 19) by the following fungal strains: Ma3, Ma15, Ma25, Pfr1, and Pfr8, all with adult horn fly emergence values lower than 7%.
Table 2.
Percentage horn fly adult emergence and mortality of pupae and adults, respectively, as response to M. anisopliae (Ma), P. fumosoroseus (Pfr), and B. bassiana (Bb) at doses of 1 × 106 conidia/ml on eggs and 1 × 108 conidia/ml on pupae and adults.
Effects of application of entomopathogenic fungi on pupae
The pupae of H. irritans were also susceptible to isolates of the entomopathogenic fungi. The highest mortality was obtained with the isolates Ma2, Ma25, Pfr9, Pfr10, Pfr11 and Bb 24 (Table 2). The ANOVA showed differences between treatments (F = 11.7, F < P = 0.0001, df = 21). The Tukey test indicated no significant differences between isolates Ma2, Ma14, Ma25, Bb21, Pfr9, Pfr10, and Pfr11 and these treatments produced the highest levels of mortality (Table 2).
Susceptibility of adults to entomopathogenic fungi
Application of entomopathogenic fungal isolates to adults caused a range of mortality between 5, and 100%. The most effective isolates were Ma6, Ma10, Ma14, Ma15, and Ma25, Bb21, Bb17, Pfr3, Pfr7, Pfr8, Pfr9, Pfr10, Pfr11, and Pfr12 (Table 2). The ANOVA showed differences between treatments (F = 28.66; F < P = 0.0001; df = 23). Tukey test showed no significant differences (P<0.05) between the isolates Pfr9, Pfr12, Ma10, Ma14; Ma6, Pfr11, Ma15, Pfr3, Pfr10, Pfr1, Pfr7, Pfr8, Pfr5, Ma25, and Bb17 all of which produced the highest mortality (Table 2).
Mean lethal concentration of the most pathogenic fungi against horn fly adults
The LC50s for the M. anisopliae isolates Ma6, Ma10, and Ma14 ranged from 8.08 × 102 to 9.28 × 106conidia/ml (Table 3) with the most virulent isolate being Ma 6. The LC50s for the P. fumosoroseus isolates were similar to the less virulent M. anisopliae strains (Table 3).
Table 3.
LC50 for selected isolates of Metarhizium anisopliae (Ma) and Paecilomyces fumosoroseus (Pfr) against horn fly adults.

Discussion
The hypothesis of this research was that egg, pupa and adult of Haematobia irritans are susceptible to the entomopathogenic fungi M. anisopliae, P. fumosoroseus, and B. bassiana, by reducing adult emergence when applied on groups of eggs and pupae and producing mortality when applied to adults. The results showed that these stages are susceptible to the different isolates of the evaluated fungi. However, differences were detected in the susceptibility between and among isolates of these three fungal species.
Treatments with the fungi M. anisopliae, and P. fumosoroseus, on horn fly eggs reduced adult emergence. When the entomopathogenic fungi attacked the eggs, the eggs did not hatch and we recovered the fungi from cadavers. Healthy eggs hatched in about 36 hours, and pupae produced healthy horn flies. We did not observe saprophytic contamination in controls. The isolates Ma 2, Ma 3, Ma 15, Ma 25, Pfr 1 and Pfr 8 reduced emergence to less than 7%, in comparison with the emergence of about 72.5% in the control treatments. These results corroborate the need to evaluate fungal isolates of different species, origins, and strains for selecting the most effective isolate or species with the object of regulating insect pest populations. These findings support the previous reports of Lezama-Gutiérrez et al. (1996), who reported that the eggs of fall armyworm, Spodoptera frugiperda (J. E. Smith) are susceptible to entomopathogenic fungi, resulting in control before the pest can harm the crop.
The use of entomopathogenic fungi as a strategy for larval control of Musca domestica has been suggested by Watson et al. (1995), particularly if the larvae remain in confined areas. They reported mortality of about 56% after applying a concentration of 1 × 1010 conidia/cm3 to manure. In our research, the results suggest that the use of entomopathogenic fungi could reduce the horn fly populations although it would be necessary to evaluate the fungal persistence in the inoculated manure. Because of the effects of manure pH, and microbial flora on the fungal persistence in naturally dried manure remain unknown, as well as the impact of fungi on parasitoids, it will be necessary to evaluate their performance under field conditions (Hu and Frank, 1996).
Horn fly pupae were also susceptible to entomopathogenic fungi. The isolates Ma2, and Ma25 of M. anisopliae caused high levels of mycosis in pupae. The remaining isolates caused less than 50% of infection in pupae. Similar results have been reported by Ekesi et al. (2002) using entomopathogenic fungi against Ceratitis capitata (Wiedeman) pupae with mortality of 94%.
There was considerable variability in the pathogencity of the fungal isolates. Three isolates of M. anisopliae (Ma6, Ma10, and Ma14), and four of P. fumosoroseus (Pfr9, Pfr10, Pfr11, and Pfr12) exhibited the highest pathogenicity causing adult mortality rates higher than 90%. The isolates of B. bassiana caused adult mortality rates between 40% and 73.8%. Steenberg et al. (Steenberg et al., 2001a) reported that B. bassiana caused 100% adult mortality in H. irritans seven days post inoculation. Their mortality values are higher than ours, but it could be due differences in the concentrations used. Steenberg et al. (Steenberg et al., 2001a) also reported susceptibility of horn fly adults to Paecilomyces farinosus with mortality rates of 90% after 7 days of inoculation. They also mentioned that other fungi such as M anisopliae and P. fumosoroseus showed high pathogenicity against horn flies.
Differences in virulence (LC50) between selected isolates of M. anisopliae, and P. fomosoroseus were determined against horn fly adults and showed that Ma6 had the lowest LC50 (8.08 × 102conidia/ml). Several studies on other Diptera mentioned that high mortalities were produced with isolates of M. anisopliae (Lezama-Gutiérrez et al., 2000), B. bassiana (Watson et al., 1995, Meadow et al., 1996), and P. fumosoroseus (Steenberg et al., 2001b) but we highlight the low concentration needed to diminish horn fly populations to 50% with the isolates Ma6. Our findings corroborate their results and the hypothesis that selected isolates of M. anisopliae, and P. fumosoroseus can be considered as microbial control agents for use in an IPM program against the horn flies.
Overall, our results support the selection of the isolates Ma2, Ma 3 and Ma25, as candidates for biological control of different biological stages of H. irritans. The isolate Ma25 reduced the adult emergence to 6.3%, caused mycosis on 69% of the horn fly pupae bioassayed, and reduced adult emergence when applied to eggs.
Acknowledgments
The authors acknowledge the support of this work by the grant SAGARPA-2002-CO1-0853/A-1obtained from the Fondo Sectorial de Investigación en Materia Agrícola, Pecuaria, Acuacultura, Agrobiotecnología y Recursos Fitogenéticos of the CONACYT-Mexico, and to CONACYT for granting to the first author with the grant No. 174412. We also thank Dr. John J. Hamm (USDA-ARS Crop Protection and Management Research Unit, Tifton, GA) for review of the manuscript. This paper is a contribution of the Universidad de Colima-Facultad de Ciencias Biológicas y Agropecuarias, Tecomán, Colima, México, and the University of Nebraska Agricultural Research Division, Lincoln, NE 68583. Journal Series No 14797, Department of Entomology, University of Nebraska Lincoln.
References
- Arthurs S, Thomas MB. Effect of dose pre-mortem host incubation temperature and thermal behaviour on host mortality, mycosis and sporulation of Metarhizium anisopliae var. acridum in Schistocerca gregaria. Biocontrol Science and Technology. 2001;11:411–420. [Google Scholar]
- Byford RL, Lockwood JA, Smith SM, Sparks TC, Luther DG. Insecticide mixtures as an approach to the management of pyrethroid-resistant horn flies (Diptera: Muscidae) Journal of Economic Entomology. 1987;80:111–116. doi: 10.1093/jee/80.1.111. [DOI] [PubMed] [Google Scholar]
- Byford RL, Craig ME, Crosby BL. A review of ectoparasites and their effects on cattle production. Journal of Animal Science. 1992;70:597–602. doi: 10.2527/1992.702597x. [DOI] [PubMed] [Google Scholar]
- Cantagui MA, Campbell JB, Thomas GD, Boxler DJ. Average daily gains of Brahman-Crossbred term exposure to stable flies (Diptera: Muscidae) Journal of Economic Entomology. 1995;88:1349–1352. doi: 10.1093/jee/86.4.1144. [DOI] [PubMed] [Google Scholar]
- Crosby BL, Byford RL, Kinzer HG. Insecticide resistance in the horn fly, Haematobia irritans (L.), in New Mexico:survey and control. Southwestern Entomologist. 1991;16:301–309. [Google Scholar]
- Cruz-Vázquez C, Vitela-Mendoza I, Ramos-Parra M, Quintero-Martínez MT, García-Vázquez M. Presencia de Haematobia irritans (L.) (Diptera: Muscidae) en ganado lechero estabulado de Aguascalientes, México: Informe preliminar. Veterinaria Mexico. 1999;30(2):205–208. [Google Scholar]
- Cupp EW, Cupp MS, Ribeiro JM, Kunz SE. Blood-feeding strategy of Haematobia irritans (Diptera: Muscidae) Journal of Medical Entomology. 1998;35(4):591–595. doi: 10.1093/jmedent/35.4.591. [DOI] [PubMed] [Google Scholar]
- Ekesi S, Maniania NK, Lux SA. Mortality in three African tephritid fruit fly puparia and caused by the entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Science and Technology. 2002;12:7–17. [Google Scholar]
- Finney DJ. 1971 Probit analysis. Cambridge University Press, England. [Google Scholar]
- Greer NI, Butler DF. Comparisons of horn fly development in manure of five animal species. Florida Entomologist. 1973;56(3):197–199. [Google Scholar]
- Guglielmone AA, Anziani OS, Mangold AJ, Lorgi RE, Volpogni MM, Flores SG. Seasonal variation of Haematobia irritans (Diptera: Muscidae) in a recently infested region of Central Argentina. Bulletin of Entomological Research. 1997;87:55–59. [Google Scholar]
- Guglielmone AA, Castelli ME, Volpogni MM, Medus PD, Martins RJ, Duarez VH, Anziani OS, Mangold AJ. Toxicity of cypermethrin and diazinon to Haematobia irritans (Diptera: Muscidae) in its American Southern range. Veterinary Parasitology. 2001;101:67–73. doi: 10.1016/s0304-4017(01)00490-3. [DOI] [PubMed] [Google Scholar]
- Hogsette JA. Management of ectoparasites with biological control organisms. International Journal of Parasitology. 1999;29:147–151. doi: 10.1016/s0020-7519(98)00190-8. [DOI] [PubMed] [Google Scholar]
- Hu GY, Frank JH. Effect of the red imported fire ant (Hymenoptera: Formicidae) on dung-inhabiting arthropods in Florida. Environmental Entomology. 1996;25:1290–1296. [Google Scholar]
- Kunz SE, Miller AJ, Simms PL. Economics of controlling horn flies (Diptera: Muscidae) in range cattle management. Journal of Economic Entomology. 1984;77:657–660. [Google Scholar]
- Lezama-Gutiérrez R, Alatorre-Rosas R, Bojalil-Jaber LF, Molina-Ochoa J, Arenas-Vargas M, González-Ramírez M, Rebolledo-Domínguez O. Virulence of five entomopathogenic fungi (Hyphomycetes) against, Spodoptera frugiperda (Lepidoptera: Noctuidae) eggs and neonate larvae. Vedalia. 1996;3:35–39. [Google Scholar]
- Lezama-Gutiérrez R, Trujillo de la Luz A, Molina-Ochoa J, Rebolledo-Domínguez O, Pescador-Rubio A, López-Edwards M, Aluja M. Virulence of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) on Anastrepha ludens (Diptera: Tephritidae): Laboaratory and field trials. Journal of Economic Entomology. 2000;93(4):1080–1084. doi: 10.1603/0022-0493-93.4.1080. [DOI] [PubMed] [Google Scholar]
- Meadow R, Vandenberg JD, Shelton AM, Watson DW. Microbial control of cabbage maggot-Preliminary screenings of fungal isolates. Arthropod Management Tests. 1996;21:443. [Google Scholar]
- Mendes J, Linhares AX. Diapause, pupation, sites and parasitism of the hornfly, Haematobia irritans, in South-eastern Brazil. Medical and Veterinary Entomology. 1999;13:1–6. doi: 10.1046/j.1365-2915.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- Moorhouse ER, Gillespie AT, Charnley AK. Selection of virulent and persistent Metarhizium anisopliae isolates to control black vine weevil (Otiorhynchus sulcatus) larvae on glasshouse begonia. Journal of Invertebrate Pathology. 1993;62:67–52. [Google Scholar]
- Mwangala FS, Galloway TD. Susceptibility of horn flies, Haematobia irritans L.(Diptera: Muscidae), to pyrethroids in Manitoba. Canadian Entomologist. 1993;125:47–53. [Google Scholar]
- Poprawsky TJ, Robert RH, Majchrowicz L, Boivin G. Susceptibility of Delia antiqua (Diptera:Anthomyiidae) to eleven isolates of entomopathogenic hyphomycetes. Environmental Entomology. 1985;14:557–561. [Google Scholar]
- SAS Institute. 1989 SAS/STAT users guide, version 6, 4th ed. SAS Institute, Cary, NC. [Google Scholar]
- Schmidt CD, Harris RL, Hoffman RA. Mass rearing of the horn fly, Haematobia irritans (Diptera: Muscidae), in the laboratory. Annals of the Entomological Society of America. 1967;60:508–510. [Google Scholar]
- Sheppard DC, Joyce JA. High levels of pyrethroid resistance in horn flies (Diptera: Muscidae) selected with cyalothrin. Journal of Economic Entomology. 1992;85:1587–1593. doi: 10.1093/jee/85.5.1587. [DOI] [PubMed] [Google Scholar]
- Sparks TC, Quisenberry SS, Lockwood JA, Byford RL, Roush RT. Insecticide resistance in the horn fly, Haematobia irritans. Journal of Agricultural Entomology. 1985;2:217–233. [Google Scholar]
- Steenberg T, Jensen KMV, Jespersen JB. Microbial control of flies on pastured cattle. DJF-rapport NR. 2001a;49:87–90. [Google Scholar]
- Steenberg T, Jespersen JB, Jensen KMV, Nielsen BO, Humbert RA. Entomopathogenic fungi in flies associated with pasture cattle in Denmark. Journal of Invertebrate Pathology. 2001b;77:186–197. doi: 10.1006/jipa.2001.5021. [DOI] [PubMed] [Google Scholar]
- Torres P, Prieto O. 1993 La mosca de los cuernos Haematobia irritans. pp. 357–457.In Enfermedades parasitarias de importancia económica en bovinos, bases epidemiológicas para su prevención y control. Nari, A. y Fiel, C. (Eds). Editorial Hemisferio Sur. Montevideo, Uruguay. [Google Scholar]
- Wall R, Strong L. Environmental consequences of treating cattle with the antiparasitic drug ivermectin. Nature. 1987;327:418–421. doi: 10.1038/327418a0. [DOI] [PubMed] [Google Scholar]
- Watson DW, Geden CJ, Rutz DA. Efficacy of Beauveria bassiana for controlling the house fly and stable fly (Diptera: Muscidae) Biological Control. 1995;5:405–411. [Google Scholar]


