Abstract
Moraxella osloensis is a bacterium that is mutualistically associated with Phasmarhabditis hermaphrodita, a nematode that has potential for the biocontrol of mollusk pests, especially the slug Deroceras reticulatum. We discovered that purified M. osloensis lipopolysaccharide (LPS) possesses a lethal toxicity to D. reticulatum when administered by injection but no contact or oral toxicity to this slug. The toxicity of the LPS resides in the lipid A moiety. M. osloensis LPS was semiquantitated at 6 × 107 endotoxin units per mg. The LPS is a rough-type LPS with an estimated molecular weight of 5,300. Coinjection of galactosamine with the LPS increased the LPS's toxicity to the slug two- to four-fold. The galactosamine-induced sensitization of the slug to the LPS was reversed completely by uridine.
Moraxella osloensis is a gram-negative opportunistic human pathogen that has been found to cause several human diseases (1, 2, 6, 14, 15, 23). The bacterium is also associated with Phasmarhabditis hermaphrodita, a parasitic nematode that is lethal to slugs and which has potential for the biocontrol of mollusk pests, especially the gray garden slug Deroceras reticulatum (9, 10, 24). P. hermaphrodita has been found to be associated with different bacterial species (25, 26). M. osloensis was finally selected as the preferred associated bacterium to mass-produce P. hermaphrodita in a monoxenic culture (25). NemaSlug, a commercial product which is based on the nematode-bacterium complex, was launched in England in 1994 (9).
We previously discovered that P. hermaphrodita is a vector that transmits M. osloensis into the shell cavity of D. reticulatum and that the bacterium is the main killing agent (17). We further discovered that M. osloensis produces an endotoxin(s) to kill the slug and indicated that lipopolysaccharide (LPS) from M. osloensis is an active endotoxin (18). The LPS was found to have lethal toxicity against the slug when administered by injection (18). However, for direct application of the endotoxin against slugs, it is important to determine whether the LPS possesses lethal contact or oral toxicity. LPS is usually composed of a lipid A moiety and a polysaccharide moiety (8). The Limulus amebocyte lysate (LAL) assay is a sensitive and powerful test for the semiquantitation of endotoxins (13). We reported recently that M. osloensis LPS is a rough-type LPS (19). However, the toxic moiety, LAL activity, and molecular weight of the LPS are not yet available. Thus, the present study was conducted to further characterize M. osloensis LPS.
Treatment of rabbits, rats, and mice with galactosamine (namely, d-galactosamine-HCl) increased their sensitivity to the lethal effect of an LPS from the bacterium Salmonella enterica serovar Abortusequi several thousandfold. The mechanism of enhanced sensitization was thought to be associated with the liver injury caused by galactosamine, and this enhanced sensitization could be reversed by the administration of uridine (7). Since D. reticulatum is a lower-order animal that does not have a liver (5), we wanted to test whether galactosamine or the galactosamine-uridine combination would have an effect on the susceptibility of D. reticulatum to M. osloensis LPS that is similar to its effect on mammals. We hypothesized that galactosamine induces the susceptibility of D. reticulatum to M. osloensis LPS and that this galactosamine-induced sensitization is blocked by uridine.
Sources of M. osloensis and D. reticulatum as well as the method of M. osloensis LPS purification were the same as those described previously (18). The LPS toxicity against D. reticulatum was determined by using three different methods. The injection toxicity of the LPS was tested by injecting a 50-μl portion of a high concentration of LPS solution (2 mg/ml) into the shell cavity as described previously (16). Slugs injected with distilled water served as controls for the injection toxicity experiment. The contact toxicity of the LPS was determined by applying 5 μl of the LPS solution to the hind dorsal portion of each slug. Slugs receiving 5 μl of distilled water served as controls for the contact toxicity experiment. To test the oral toxicity of the LPS, slugs were placed in a petri dish and fed two pieces of half-dry carrot tubers (2 cm in diameter and 0.5 cm in thickness) that had been treated evenly with the LPS solution around the tops of the tubers (600 μg of LPS per six slugs per dish). The average uptake of the LPS per slug in the oral-toxicity experiment was estimated to be 100 times the percentage of tuber consumption during the experiment, expressed in micrograms. The volume of each tuber was measured before and after the experiment after immersion of the tubers in a 25-ml cylinder containing water, and the increase in volume was recorded. Slugs fed tubers treated with the same amount of distilled water served as controls for the oral-toxicity experiment. Slugs were fed pieces of fresh carrots and cabbage leaves in the injection and contact toxicity experiments. There were three replicate petri dishes, each containing six slugs. All slugs were incubated at 18°C. The numbers of dead slugs were recorded after 4 days.
Lipid A was obtained as a precipitate by hydrolysis of M. osloensis LPS (2 mg/ml) with 1% acetic acid at 100°C for 2 h, followed by centrifugation (16,000 × g, 5 min) and successive washes with distilled water and acetone (12). The supernatant from the LPS hydrolysate containing the polysaccharide moiety was centrifuged (150,000 × g, 3 h, 4°C) to remove lipid A and unhydrolyzed LPS and then lyophilized. A 50-μl portion of the obtained lipid A or polysaccharide (dissolved in the same amount of distilled water as that in the original LPS solution) was injected into the shell cavity. Slugs injected with distilled water served as controls. The rest of the experimental design was as described above for the injection toxicity experiment.
M. osloensis LPS was diluted serially with endotoxin-free water (Sigma Chemical Co., St. Louis, Mo.). Equal volumes (0.1 ml) of each LPS dose and LAL (Sigma Chemical Co.) were mixed and incubated at 37°C for 1 h. The gelation of LAL at each LPS dose was determined by inverting the mixture. A firm gel was considered a positive reaction (11). Escherichia coli O55:B5 LPS (Sigma Chemical Co.) was purchased as a standard endotoxin to semiquantitate M. osloensis LPS. The endotoxin activity in the lipid A or polysaccharide moiety was also analyzed by the LAL assay. The sensitivity of the LAL assay is 0.05 to 0.1 endotoxin unit (EU) per ml.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described previously (19). LPSs were then visualized by the classic silver staining method (21). Commercially available rough-type LPSs from E. coli J5 (Mr = 4,200) and EH100 (Mr = 5,500) (22) were used as LPS controls and Mr markers (Sigma Chemical Co.).
In preliminary experiments, we determined the lethal and sublethal doses of galactosamine and uridine for slugs. A dose of 0.3 mg of galactosamine per slug or 1 mg of uridine per slug did not cause any slug mortality, whereas 1 mg of galactosamine per slug and 3 mg of uridine per slug caused about 30 and 80% mortality, respectively, at day 4 after injection into the shell cavity. The weight of each adult slug used in the experiments was in the range of 0.6 to 0.8 g. Furthermore, a mixture of 3 mg of uridine and 1 mg of galactosamine per slug had no effect on slug mortality. Thus, we used the following treatments: (i) 0.3 mg of galactosamine plus 0.01 mg of LPS with or without 1 mg of uridine per slug and (ii) 1 mg of galactosamine plus 0.03 mg of LPS with or without 3 mg of uridine per slug. Each mixture (in a 50-μl solution) was injected into the shell cavity. Slugs injected with endotoxin-free water (as a solvent in the experiment), galactosamine alone (0.3 or 1 mg per slug), or LPS alone (0.01 or 0.03 mg per slug) served as controls. The rest of the experimental design was as described above for the injection toxicity experiment.
All data presented in percentage values were arcsine transformed and subjected to one-way analysis of variance by using the statistical software STATISTICA Kernel release 5.5 (StatSoft Inc., Tulsa, Okla.). Significant differences among treatment results were determined by using Tukey's honestly significant difference tests at a P value of 0.05.
Compared to the results with the controls, M. osloensis LPS caused significant slug mortality (P < 0.05) only when injected into the shell cavity (Table 1). In the oral-toxicity experiment, the slugs were found to eat the top halves of the tubers (data not shown); thus, the average uptake of the LPS was estimated to be 50 μg per slug. Therefore, M. osloensis LPS possesses a lethal toxicity when administered by injection but no contact or oral toxicity against D. reticulatum, which implies that it is not feasible to directly apply the LPS for slug control. It is suggested that P. hermaphrodita is a necessary natural vector of M. osloensis for the biological control of slugs. The lipid A moiety resulted in 61.1% ± 5.6% (mean ± standard error; n = 3 petri dishes) slug mortality, which differed significantly (P < 0.05) from the results for the controls, but the polysaccharide moiety did not (data not shown). Therefore, the toxicity of M. osloensis LPS resides in the lipid A moiety but not in the polysaccharide moiety. In addition, the slugs killed by the LPS or lipid A were found to lie on their sides, and their bodies and tentacles were contracted. Their skin surfaces were sticky because they secreted a lot of slime before death. Their mouths occasionally were prolapsed.
TABLE 1.
Percentage mortality of D. reticulatum slugs following injection, contact, or oral administration of purified LPS from 3-day M. osloensis culturesa
| Treatment group | % Mortality (mean ± SE)b
|
||
|---|---|---|---|
| Injection | Contact | Oral | |
| Control | 5.6 ± 5.6A | 5.6 ± 5.6A | 5.6 ± 5.6A |
| Treated | 77.8 ± 5.5B | 5.6 ± 5.6A | 11.1 ± 5.6A |
n = 3.
Petri dishes differed significantly at a P value of <0.05 as indicated by different letters for each type of LPS administration.
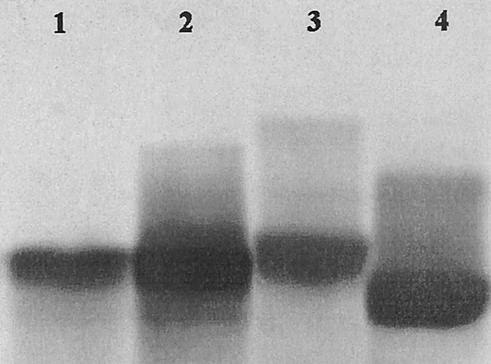
A 0.1-pg amount of M. osloensis LPS (in 0.1 ml of the LPS dilution) induced the gelation of LAL, but 0.05 pg of LPS did not. Thus, the minimal dose of M. osloensis LPS required for the gelation was estimated to be 1 pg per ml. Moreover, 0.06 EU per ml was the minimal dose for the standard endotoxin (E. coli O55:B5 LPS) to cause a positive reaction. Therefore, M. osloensis LPS was semiquantitated to be 6 × 107 EU/mg by the LAL assay. Furthermore, the lipid A moiety also caused a positive reaction, but the polysaccharide moiety did not. As with the results with the rough-type LPS controls, only one main band was detected in the gel for M. osloensis LPS. The LPS Mr was about 5,300, as estimated from a comparison of the electrophoretic mobility of the band representing M. osloensis LPS with that of bands representing the two Mr markers (Fig. 1).
FIG. 1.
Detection of LPS by SDS-PAGE following silver staining. One-microgram samples were analyzed by SDS-PAGE except as noted otherwise. Lanes 1 and 2, M. osloensis at 1 and 5 μg, respectively; lane 3, E. coli EH100; lane 4, E. coli J5. The figure was created with Photoshop 5.5 software (Adobe Systems Inc.).
The lethal (1-mg/slug) or sublethal (0.3-mg/slug) dose of galactosamine enhanced the susceptibility of D. reticulatum to M. osloensis LPS (0.03 or 0.01 mg/slug, respectively), but uridine (3 or 1 mg/slug, respectively) inhibited the sensitization effect completely (Table 2). A mixture of galactosamine and LPS caused slug mortality that differed significantly (P < 0.05) from the mortality caused by treatment with galactosamine alone or LPS alone. However, coinjection of uridine with the galactosamine-LPS mixture caused slug mortality similar only to that caused by the treatment with LPS alone.
TABLE 2.
Mortality of D. reticulatum slugs following injection into the shell cavity of galactosamine alone, M. osloensis LPS alone, galactosamine and M. osloensis LPS, or a mixture of galactosamine, M. osloensis LPS, and uridinea
| Substance injected | % Mortality (mean ± SE) with indicated level of treatmentb
|
|
|---|---|---|
| Lethalc | Sublethald | |
| Endotoxin-free water | 0A | 0A |
| Galactosamine | 33.3 ± 9.6B | 0A |
| LPS | 27.8 ± 11.1B | 5.6 ± 5.6A |
| Galactosamine + LPS | 77.8 ± 5.5C | 27.8 ± 5.5B |
| Galactosamine + LPS + uridine | 27.8 ± 5.5B | 0A |
n = 3 petri dishes.
Values in the same row differed significantly at a P value of <0.05 as indicated by different letters.
Lethal treatments were the following: galactosamine (1 mg/slug) alone, M. osloensis LPS (0.03 mg/slug) alone, 1 mg of galactosamine plus 0.03 mg of LPS per slug, and 1 mg of galactosamine plus 0.03 mg of LPS plus 3 mg of uridine per slug.
Sublethal treatments were the following: galactosamine (0.3 mg/slug) alone, M. osloensis LPS (0.01 mg/slug) alone, 0.3 mg of galactosamine plus 0.01 mg of LPS per slug, and 0.3 mg of galactosamine plus 0.01 mg of LPS plus 1 mg of uridine per slug.
The present study demonstrated that galactosamine enhances the susceptibility of D. reticulatum to M. osloensis LPS and that the induced sensitization can be blocked completely by uridine. Galanos et al. (7) reported that the administration of galactosamine in experimental mammals increases their susceptibility to the LPS from Salmonella serovar Abortusequi several thousandfold and that the sensitization effect is inhibited completely by uridine. They suggested that the mechanisms leading to the sensitization effect are associated with the early metabolic alterations in the liver that are elicited by galactosamine (7). Galactosamine causes a high level of accumulation of UDP-galactosamine derivatives and leads to a depletion of hepatic UTP in the liver, thus inhibiting the biosynthesis of macromolecules and eventually resulting in the damage and death of hepatocytes (4). However, the hepatic lesions elicited by galactosamine can be blocked completely by uridine (3).
It is not fully clear why the galactosamine-induced sensitization also exists in D. reticulatum organisms, which do not have livers. Compared with mammals such as rabbits, rats, and mice, slugs are lower-order animals. However, the slug hepatopancreas has been found to have some primitive liver-like functions (e.g., production of digestive enzymes and detoxification of dietary metals) (5, 20). Thus, the hepatopancreas may be the potential target of galactosamine in the slug. Compared with the several-thousandfold increase in the sensitization of experimental mammals to LPSs induced by galactosamine, the galactosamine-induced increase in the sensitization of D. reticulatum to M. osloensis LPS is only two- to fourfold. Since the liver is an important organ and the main one for clearance of endotoxins in mammals (7), it is understandable that liver damage caused by galactosamine dramatically increases the susceptibility of mammals to LPSs. Although galactosamine was thought to be a specific hepatotoxic agent (4), the involvement of other target organs cannot be excluded. Since important organs, including the kidney, lung, and heart, are located in the mantle region of D. reticulatum (24), it is also possible that galactosamine damages another important organ(s) in the slug, leading to the induced sensitization of D. reticulatum to M. osloensis LPS. This appears to be the first report of galactosamine-induced sensitization to an LPS in an animal without a liver.
Acknowledgments
This work was supported by a graduate research competitive grant from the Ohio Agricultural Research and Development Center and by a presidential fellowship from Ohio State University to L. Tan.
REFERENCES
- 1.Berrocal, A. M., I. U. Scott, D. Miller, and H. W. Flynn. 2002. Endophthalmitis caused by Moraxella osloensis. Graefe's Arch. Clin. Exp. Ophthalmol. 240:329-330. [DOI] [PubMed] [Google Scholar]
- 2.Buchman, A. L., M. J. Pickett, L. Mann, and M. E. Ament. 1993. Central venous catheter infection caused by Moraxella osloensis in a patient receiving home parenteral nutrition. Diagn. Microbiol. Infect. Dis. 17:163-166. [DOI] [PubMed] [Google Scholar]
- 3.Decker, K., and D. Keppler. 1972. Galactosamine-induced liver injury, p. 183-199. In H. Popper and F. Schaffner (ed.), Progress in liver diseases, vol. 4. Grune & Stratton, New York, N.Y. [PubMed]
- 4.Decker, K., and D. Keppler. 1974. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev. Physiol. Biochem. Pharmacol. 71:77-103. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriadis, V. K. 2001. Structure and function of the digestive system in Stylommatophora, p. 237-257. In G. M. Barker (ed.), The biology of terrestrial mollusks. CABI Publishing, New York, N.Y.
- 6.Fijen, C. A. P., E. J. Kuijper, H. G. Tjia, M. R. Daha, and J. Dankert. 1994. Complement deficiency predisposes for meningitis due to nongroupable meningococci and Neisseria-related bacteria. Clin. Infect. Dis. 18:780-784. [DOI] [PubMed] [Google Scholar]
- 7.Galanos, C., M. A. Freudenberg, and W. Reutter. 1979. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 76:5935-5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galanos, C., O. Luderitz, E. T. Rietschel, and O. Westphal. 1977. Newer aspects of the chemistry and biology of bacterial lipopolysaccharides with special reference to their lipid A component, p. 239-335. In T. W. Goodwin (ed.), Biochemistry of lipids II. International review of biochemistry, vol. 14. University Park Press, Baltimore, Md.
- 9.Glen, D. M., and M. J. Wilson. 1997. Slug-parasitic nematodes as biocontrol agents for slugs. Agro Food Ind. Hi-Tech 8:23-27. [Google Scholar]
- 10.Grewal, P. S., S. K. Grewal, R. A. J. Taylor, and R. B. Hammond. 2001. Application of molluscicidal nematodes to slug shelters: a novel approach to economic biological control of slugs. Biol. Control 22:72-80. [Google Scholar]
- 11.Hochstein, H. D., R. J. Elfin, J. F. Cooper, and S. M. Wolff. 1973. Further development of Limulus amebocyte lysate test. Bull. Parenter. Drug Assoc. 27:139-148. [PubMed] [Google Scholar]
- 12.Masoud, H., M. B. Perry, and J. C. Richards. 1994. Characterization of the lipopolysaccharide of Moraxella catarrhalis: structural analysis of the lipid A from M. catarrhalis serotype A lipopolysaccharide. Eur. J. Biochem. 220:209-216. [DOI] [PubMed] [Google Scholar]
- 13.Roth, R. I., J. Levin, and S. Behr. 1989. A modified Limulus amebocyte lysate test with increased sensitivity for detection of bacterial entotoxin. J. Lab. Clin. Med. 114:306-311. [PubMed] [Google Scholar]
- 14.Stryker, T. D., W. J. Stone, and A. M. Savage. 1982. Renal-failure secondary to Moraxella osloensis endocarditis. Johns Hopkins Med. J. 151:217-219. [PubMed] [Google Scholar]
- 15.Sugarman, B., and J. Clarridge. 1982. Osteomyelitis caused by Moraxella osloensis. J. Clin. Microbiol. 15:1148-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan, L., and P. S. Grewal. 2001. Infection behavior of the rhabditid nematode Phasmarhabditis hermaphrodita to the grey garden slug Deroceras reticulatum. J. Parasitol. 87:1349-1354. [DOI] [PubMed] [Google Scholar]
- 17.Tan, L., and P. S. Grewal. 2001. Pathogenicity of Moraxella osloensis, a bacterium associated with the nematode Phasmarhabditis hermaphrodita, to the slug Deroceras reticulatum. Appl. Environ. Microbiol. 67:5010-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan, L., and P. S. Grewal. 2002. Endotoxin activity of Moraxella osloensis against the grey garden slug, Deroceras reticulatum. Appl. Environ. Microbiol. 68:3943-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan, L., and P. S. Grewal. 2002. Comparison of two silver staining techniques for detecting lipopolysaccharides in polyacrylamide gels. J. Clin. Microbiol. 40:4372-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triebskorn, R., and H. R. Kohler. 1996. The impact of heavy metals on the grey garden slug, Deroceras reticulatum (Muller): metal storage, cellular effects and semi-quantitative evaluation of metal toxicity. Environ. Pollut. 93:327-343. [DOI] [PubMed] [Google Scholar]
- 21.Tsai, C., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 22.Vaneechoutte, M., G. Verschraegen, G. Claeys, and A.-P. V. D. Abeele. 1990. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J. Clin. Microbiol. 28:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuori-Holopainen, E., E. Salo, H. Saxen, M. Vaara, E. Tarkka, and H. Peltola. 2001. Clinical “pneumococcal pneumonia” due to Moraxella osloensis: case report and a review. Scand. J. Infect. Dis. 33:625-627. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, M. J., D. M. Glen, and S. K. George. 1993. The rhabditid nematode Phasmarhabditis hermaphrodita as a potential biological control agent for slugs. Biocontrol Sci. Technol. 3:503-511. [Google Scholar]
- 25.Wilson, M. J., D. M. Glen, S. K. George, and J. D. Pearce. 1995. Selection of a bacterium for the mass production of Pharmarhabditis hermaphrodita (Nematoda: Rhabditidae) as a biocontrol agent for slugs. Fundam. Appl. Nematol. 18:419-425. [Google Scholar]
- 26.Wilson, M. J., D. M. Glen, J. D. Pearce, and P. B. Rodgers. 1995. Monoxenic culture of the slug parasite Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) with different bacteria in liquid and solid phase. Fundam. Appl. Nematol. 18:159-166. [Google Scholar]