Abstract
We investigated whether inhibition of platelet-derived growth factor (PDGF) receptor tyrosine kinase activity would affect pericyte viability, vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor-2 (VEGFR-2) expression and angiogenesis in a model of retinopathy of prematurity (ROP). ROP was induced in Sprague Dawley rats by exposure to 80% oxygen from postnatal (P) days 0 to 11 (with 3 hours/day in room air), and then room air from P12–18 (angiogenesis period). Shams were neonatal rats in room air from P0–18. STI571, a potent inhibitor of PDGF receptor tyrosine kinase, was administered from P12–18 at 50 or 100 mg/kg/day intraperitoneal (i.p.). Electron microscopy revealed that pericytes in the inner retina of both sham and ROP rats appeared normal; however STI571 induced a selective pericyte and vascular smooth muscle degeneration. Immunolabeling for caspase-3 and α-smooth muscle cell actin in consecutive paraffin sections of retinas confirmed that these degenerating cells were apoptotic pericytes. In all groups, VEGF and VEGFR-2 gene expression was located in ganglion cells, the inner nuclear layer, and retinal pigment epithelium. ROP was associated with an increase in both VEGF and VEGFR-2 gene expression and blood vessel profiles in the inner retina compared to sham rats. STI571 at both doses increased VEGF and VEGFR-2 mRNA and exacerbated angiogenesis in ROP rats, and in sham rats at 100 mg/kg/day. In conclusion, PDGF is required for pericyte viability and the subsequent prevention of VEGF/VEGFR-2 overexpression and angiogenesis in ROP.
Retinopathies such as proliferative diabetic retinopathy and retinopathy of prematurity (ROP) are characterized by angiogenesis in the inner retina and pre-retinal space.1–4 In both situations the new blood vessels that form are immature and result in vascular leakage.1–4 It is recognized that pericytes play an important role in the angiogenic process by providing survival signals for endothelial cells and promoting capillary maturation.5,6 A loss in pericytes or pericyte “drop-out” that occurs early in diabetic retinopathy may weaken the vascular wall and initiate endothelial cell death and thereby lead to dilation, microaneurysms, and, ultimately, angiogenesis.7,8
Emerging evidence indicates that platelet-derived growth factor (PDGF) is critical for pericyte viability.9–12 PDGF-B-deficient mice exhibit a loss in brain capillary pericytes which leads to vascular abnormalities including endothelial hyperplasia, capillary dilation and microaneurysms, and, in some instances, angiogenesis.9–12 This may also occur in the retina, since mice with genetic ablation of PDGF-B exhibit increased pericyte loss compared to wild-type mice, a situation that is aggravated by diabetes and ROP.13 Vascular endothelial growth factor (VEGF) is likely to participate with PDGF in regulating both pericyte and endothelial cell survival, as VEGF is known to protect endothelial cells from apoptosis3 and PDGF induces VEGF expression in muscle cells and cultured pericytes.14,15 However, little is known about how PDGF and VEGF interact to influence pericyte loss and angiogenesis in an in vivo model of ischemia-induced retinal angiogenesis.
The action of PDGF isoforms are not restricted to the retinal microvasculature and include the stimulation of cell proliferation in the glomerular mesangium,16 wound repair,17 and an increase in extracellular matrix production.18 Pharmacological blockade of PDGF is therefore a major target for the treatment of a number of diseases including cancer.19 In recent years, PDGF receptor tyrosine kinase (RTK) inhibitors with in vivo activity have been developed.20 These include the signal transduction inhibitor, STI571 (Glivec, Gleevec), a potent and selective inhibitor of PDGF-RTK and v-Abl kinase, with which it shares substantial homology.20 STI571 has been identified as having therapeutic potential for the treatment of disorders characterized by overexpression of PDGF or v-Abl such as glomerular disease and chronic myeloid leukemia.20
The aim of the present study was to determine whether PDGF-RTK inhibition with STI571 would influence pericyte viability and angiogenesis in a rat model of ROP. A potential interaction between PDGF and VEGF/VEGFR-2 was examined by assessment of retinal gene expression.
Materials and Methods
Animals
Pregnant Sprague Dawley rats were provided by The Biological Research Facility at The University of Melbourne, Victoria, Australia. The mothers were randomly divided into 6 experimental groups and 6 to 9 pups investigated from each litter. These groups consisted of: ROP sham, ROP sham treated with STI571 at 50 mg/kg/day intraperitoneal (i.p.), ROP sham treated with STI571 at 100 mg/kg/day i.p., ROP, ROP treated with STI571 at 50 mg/kg/day i.p., and ROP treated with STI571 at 100 mg/kg/day i.p. STI571 was a gift from Dr. E. Buchdunger, Novartis Pharmaceuticals, Basel, Switzerland and was diluted in sterile water before injection.
In the first group, ROP sham, newborn pups and their mother were housed in room air from postnatal days 0 to 18. In groups 2 and 3, ROP shams were housed in room air from birth until postnatal day 18 and treated daily with STI571 between postnatal days 12 and 18. In group 4, ROP was induced by placing newborn pups and their mother in sealed chambers containing 80 ± 5% O2 and 2% CO2 using medical-grade O2 and industrial-grade air. Gas levels in the chamber were monitored twice a day using the ML 205 gas analyzer (AD Instruments Ltd., Castle Hill, NSW, Australia) and chart recorder (Chart version 3.5 program on the MacLab/2E System, AD Instruments). An airflow rate of approximately 2.5L/minute assisted in maintaining adequate levels of metabolically produced CO2 and drops in O2 tension. To promote retinal angiogenesis,4,21 each day pups were exposed to comparatively reduced oxygen levels by housing in room air for 3 hours. Rats remained in the chamber for 11 days (hyperoxic period, postnatal days 0 to 11) and were then housed in room air for a further 5 days (hypoxic-induced angiogenic period, postnatal days 12 to 18). In groups 5 and 6, the ROP protocol was followed and STI571 administered daily during the time in room air (postnatal days 12 to 18).
During the experiment, mothers were provided with water and standard mice chow (GR2, Clark-King and Co., Gladesville, Victoria, Australia) ad libitum and exposed to normal 12 hour light/dark cycles. Pups received nutrition from their mothers. Experimental procedures were consistent with the guidelines set by the Australian National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Scientific Purposes.
Blood Vessel Profiles in the Inner Retina
Following the 18-day experimental period, rats were sacrificed by an intraperitoneal injection of Nembutal (Rhone Merieux, QLD, Australia, 120 mg/kg body weight). One eye from each animal were removed and fixed for 3 hours in 4% paraformaldehyde (Sigma, St. Louis, USA). Eyes were processed in graded alcohol before being embedded in paraffin wax. Eyes were then serially sectioned at 3 μm, 90° to the optic nerve and placed on 1% 3-aminopropyl-triethoxysilane-coated slides (Sigma). Approximately 120 sections/eye were collected and dried overnight at 37°C.
Three to four sections from one eye from each animal were randomly chosen, deparaffinized and stained with Mayer’s hematoxylin (5 minutes) and eosin (5 minutes) (Amber Scientific Laboratories, Belmont, Australia), and cover-slipped. Using an established technique,22 blood vessel profiles (BVPs) were counted in the inner retina which comprised the inner limiting membrane (ILM), ganglion cell layer (GCL), and inner plexiform layer (IPL). BVPs in the vitreous cavity that were adherent to the retina were also counted. A BVP was defined as a blood vessel with a lumen and an endothelial cell. Between four to six fields of retina per section were captured on a Olympus BX51 photomicroscope (Olympus, Tokyo, Japan) at a magnification of ×40 connected to a Spot digital camera and IBM computer (SciTECH Pty. Ltd., Victoria, Australia). Fields of retina were non-overlapping to avoid double sampling of BVPs. The results are expressed as BVPs per high-powered field of inner retina. Quantitation was performed by two investigators masked to the experimental groups.
Transmission Electron Microscopy
Transmission electron microscopy (TEM) was used to evaluate ultrastructural changes to endothelial cells and pericytes in blood vessels in the inner retina of sham rats, ROP rats, and sham and ROP rats treated with 100 mg/kg/day STI571. In brief, eyes were enucleated and placed in 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer for 3 to 7 days. Eyes were then bisected at the equator and the lens and anterior portion of the eye discarded. The optic cup was bisected and each piece placed in fresh 2.5% glutaraldehyde at 4°C for a minimum of 12 hours and post-fixed in 2% osmium tetroxide. Tissues were then processed through graded ethanol and embedded in Spurr’s resin (ProSciTech, Thuringowa, QLd, Australia). Semi-thin sections were prepared, stained with toluidine blue, and retinal orientation evaluated for ultra-thin sectioning. Ultra-thin sections were floated onto 200 mesh copper grids (Agar Scientific Ltd., UK), counter stained with uranyl acetate and lead citrate. Sections were viewed on a Hitachi H-7000 transmission electron microscope.
Immunohistochemistry for Caspase-3 and α-Smooth Muscle Cell Actin
To quantify apoptotic pericytes in the inner retina, consecutive 3-μm paraffin sections of eye were de-waxed and washed for 2 × 5 minutes in 0.1 mol/L phosphate-buffered saline (PBS). For antigen retrieval, sections were immersed in a solution of 1 mol/L sodium citrate and 1 mol/L citric acid and then microwaved for 10 minutes. The sections were then left to cool for 40 minutes, washed for 2 × 5 minutes in 0.1 mol/L PBS and then incubated for 10 minutes in 3% H202 dissolved in 0.1 mol/L PBS. Following washing for 3 × 5 minutes in 0.1 mol/L PBS, the sections were incubated in 0.1 mol/L normal goat serum for 10 minutes and subsequently incubated overnight at 4°C with either a polyclonal antibody to caspase-3 (Promega Corporation, Madison, WI), a marker of cellular apoptosis23 which was diluted 1:400 in 0.1 mol/L PBS or a monoclonal antibody to α-smooth muscle cell actin, a marker of pericytes which was diluted 1:50 in 0.1 mol/L PBS.24 Following thorough washing with 0.1 mol/L PBS, the sections were incubated with for 1 hour with either 1:200 biotinylated goat anti-rabbit immunoglobulin (for caspase-3) or 1:200 biotinylated goat anti-mouse immunoglobulin (for α-smooth muscle cell actin). Sections were then thoroughly washed three times with 0.1 mol/L PBS. In order to visualize protein immunolabeling, the sections were incubated with a pre-formed avidin and biotinylated horseradish peroxidase macromolecular complex (diluted 1:200 with 0.1 mol/L PBS; Vectastain Elite ABC Kit, Vector Laboratories Inc., Burlingame, CA, USA) for 30 minutes, and reacted with 3,3′-diaminobenzidine chromagen solution diluted in imidazole-HCl buffer (containing hydrogen peroxide, pH 7.5). The reaction was stopped by washing in tap water and the sections were then counterstained with 0.5% eosin for 2 minutes and cover-slipped. Positive controls were sections of rat kidney with tumors, while negative controls were sections of eye incubated with 0.1 mol/L PBS instead of the primary antibody. The α-smooth muscle cell antibody, normal goat serum, biotinylated secondary antibodies, and chromagen were purchased from Dako Australia, Botany, NSW, Australia.
For quantitation of apoptotic pericytes, at least three sections were randomly chosen from the entire eye. At least 20 fields of inner retina were quantified in each section from each retina. Six to eight retinas were examined in each experimental group. Sections were viewed at ×100 magnification on an Olympus BX51 microscope (Olympus, Tokyo, Japan) and images captured on a Spot digital camera (SciTech) and projected on a Dell Computer (Dell Inc., USA). Caspase-3-positive cells in the inner retina were identified and confirmed to be pericytes in the consecutive section labeled with α-smooth muscle cell actin.
In Situ Hybridization for VEGF and VEGFR-2
Riboprobes were synthesized from cDNAs encoding mouse VEGF and VEGFR-2 (Dr. S. Stacker, Ludwig Institute, Parkville, Australia).25 The cDNAs were cloned into pGEM 4Z (Promega, Madison, WI, USA) and linearized with HindIII to produce antisense probes using SP6 RNA polymerase (Promega). Three-μm paraffin sections of eye pre-mounted on 1% 3-aminopropyltriethoxysilane coated slides were randomly chosen and de-waxed, re-hydrated in graded ethanol and milliQ water, equilibrated in P buffer (50 mmol/L Tris-HCL (pH 7.5) and 5 mmol/L EDTA), and incubated in 125 μg/ml Pronase E (Bio Scientific, NSW, Australia) in P buffer for 10 minutes at 37°C. Sections were then washed in 0.1 mol/L sodium phosphate buffer (pH 7.2), briefly re-fixed in 4% paraformaldehyde (Crown Scientific Pty. Ltd, Victoria, Australia) for 10 minutes, rinsed in milliQ water, dehydrated in 70% ethanol, and air-dried. Hybridization buffer containing 2 × 104 cpm/ μl riboprobe in 300 mmol/L NaCl, 10 mmol/L Tris-HCL (pH 7.5), 10 mmol/L Na2HPO4, 5 mmol/L EDTA (pH 8.0), 1X Denhardt’s solution, 50% formamide, 17 mg/ml yeast RNA, and 10% w/v dextran sulfate was heated to 85°C for 5 minutes, and 25 μl of this solution was then added to each section. Hybridization was performed overnight at 60°C in 50% formamide-humidified chambers. Sections hybridized with sense-probes for VEGF and VEGFR-2 were used as controls for non-specific binding. After hybridization, slides were washed in 2X SSC containing 50% formamide pre-warmed to 50°C to remove cover-slips. Sections were then washed in the above-described solution for 1 hour at 55°C, rinsed three more times in RNase buffer (10 mmol/L Tris-HCL (pH 7.5), 1 mmol/L EDTA (pH 8.0), 0.5 mol/L NaCl), and incubated with RNase A (150 μg/ml) for 1 hour at 37°C. Sections were later washed in 2X SSC for 45 minutes at 55°C, dehydrated in graded ethanol, air-dried, and exposed to Kodak X-Omat autoradiographic film (Kodak, Rochester, NY, USA) for 5 days. Slides were subsequently dipped in Ilford LM1 emulsion (Ilford, Cheshire, United Kingdom), stored in a light-free box with desiccant at 4°C for 4 weeks, immersed in Kodak D19 developer (Kodak), fixed in Ilford Hypam (Ilford), and stained with hematoxylin and eosin.
Quantitative in Situ Hybridization
Gene expression for VEGF and VEGFR-2 was assessed in the inner retina (ILM, GGL, IPL) and the inner nuclear layer (INL).25 Dark-field images were captured and digitized using a Fujix HC-2000 digital camera (Fuji, Tokyo, Japan). The outline of the ILM, GCL, IPL, and INL was defined by interactive tracing. Gene expression in the inner retina was quantitatively measured to determine the proportion of occupied by autoradiographic grains as previously described,26,27,28 using computerized image analysis (Analytical Imaging Station, Imaging Research). All sections were hybridized to their respective probes in the same experiment and analyzed in duplicate (N = 8 sections per rat, 6 to 9 rats per group).
Animal Numbers and Statistics
Data were analyzed using Statview for Windows (version 5.0.1, SAS Institute Inc., Cary, NC). A two-way AVOVA with Fisher’s post-hoc comparison was applied, with P < 0.05 considered statistically significant.
Results
Body Weight
At the end of the 18-day experimental period there was no significant difference in body weight between all experimental groups (untreated sham, 32 ± 0.7 grams; sham + 50 mg/kg/day STI571, 33 ± 0.6 grams; sham + 100 mg/kg/day STI571, 35 ± 0.8 grams; untreated ROP, 34 ± 1.1 grams; ROP + 50 mg/kg/day STI571, 32 ± 0.9 grams; ROP + 100 mg/kg/day STI51, 34 ± 0.6 grams). Values are mean ± SEM. STI571 at 50 or 100 mg/kg/day had no adverse effects on survival or health of the animals.
Histopathology
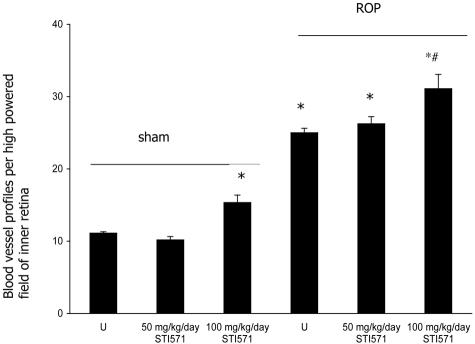
In untreated sham rats, the retinal vasculature appeared normal (Figure 1A). In contrast, in untreated ROP rats, numerous blood vessels were observed in the inner retina with some extending into the vitreous cavity (Figure 1D). In both shams and ROP rats, 50 mg/kg/day of STI571 had no effect on the amount of BVPs in the inner retina (Figure 1, B and E). STI571 at a concentration of 100 mg/kg/day increased BVPs in the inner retina in both sham and ROP rats compared to their respective controls (Figure 1, C and F). Quantitation of BVPs in the inner retina revealed similar results to the qualitative findings (Figure 2). In all groups treated with 100 mg/kg/day STI571 some blood vessels in the inner retina appeared dilated and hemorrhages were observed.
Figure 1-4039.
Three-μm paraffin sections of inner retina from 18-day-old Sprague Dawley rats following retinopathy of prematurity (ROP) and treatment with STI571. A: Untreated sham rats. B: Sham rats treated with 50 mg/kg/day STI571. C: Sham rats treated with 100 mg/kg/day STI571. D: Untreated ROP. E: ROP rats treated with 50 mg/kg/day STI571. F: ROP rats treated with 100 mg/kg/day STI571. Arrows denote blood vessels. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer. Original magnification, ×200.
Figure 2-4039.
Quantitation of blood vessel profiles (BVPs) in the inner retina of 18-day-old Sprague Dawley rats with retinopathy of prematurity (ROP) and treated with STI571. Values are mean ± SEM. N = 6 to 8 rats per group. U, untreated. *, P < 0.001 compared to sham groups. #, P < 0.01 compared to untreated ROP.
Transmission Electron Microscopy
In untreated sham rats, blood vessels and pericytes in the inner retina had a normal morphology (Figure 3A). In sham rats treated with 100 mg/kg/day STI571, some retinal capillaries contained degenerating pericytes as indicated by electron-lucency and the vacuolated appearance of the cytoplasm (Figure 3B). In ROP rats, some capillaries in the inner retina had a closed lumen although pericytes and endothelial cells appeared normal (Figure 3C). In ROP rats treated with 100 mg/kg/day STI571, arteries and arterioles were often damaged as evidenced by electron-lucent and vacuolated pericytes although nuclei appeared normal in some instances (Figure 3D).
Figure 3-4039.
Transmission electron micrographs of blood vessels in the inner retina of 18-day-old rats with retinopathy of prematurity (ROP) and treated with STI571. A: Untreated sham showing a normal capillary with an open lumen (L). Original magnification, ×9000. B: Sham rat treated with 100 mg/kg/day STI571. Retinal capillary contains a pericyte with an electron-lucent appearance and vacuolated cytoplasm. Original magnification, ×5000. C: Untreated ROP showing a closed retinal capillary with apparently a normal pericyte and endothelial cell. Original magnification, ×9000. D: ROP rat treated with 100 mg/kg/day STI571. Disruption of vascular smooth muscle cells in retinal arterioles and venules as shown by necrotic and vacuolated cells, although endothelial cells appear normal in some instances. Original magnification, ×2500.
Immunohistochemistry for Apoptotic Pericytes in the Inner Retina
Retinas from untreated sham rats contained few apoptotic pericytes and this was unchanged with 100 mg/kg/day STI571 (Figure 4, A and B). In ROP rats, apoptotic pericytes in the inner retina were in similar amounts to sham groups (Figure 4C). In ROP rats treated with 100 mg/kg/day STI571, numerous apoptotic pericytes were present in the inner retina and usually associated with branched blood vessels that sometimes penetrated the ILM and were present in the vitreous cavity (Figure 4D). Quantitation of cells immunolabeled for both caspase-3 and α-smooth muscle cell actin confirmed these findings (Figure 4).
Figure 4-4039.
Three-μm paraffin sections of inner retina from 18-day-old Sprague Dawley rats immunolabeled with caspase-3. A: Untreated sham rats. B: Sham rats treated with 100 mg/kg/day STI571. C: Untreated ROP. D: ROP rats treated with 100 mg/kg/day STI571. Original magnification, ×400. N = 6 to 8 rats per group. Caspase-3-positive cells (arrows) were confirmed to be pericytes by examination of α-smooth muscle cell immunolabeling in consecutive sections (not shown). Caspase-3-positive cells are associated with blood vessels (asterisks) and most numerous in ROP rats treated with 100 mg/kg/day STI571 (D). Sections are counterstained with eosin. GCL, ganglion cell layer. Quantitation of apoptotic pericytes is shown graphically. Values are means ± SEM. N = 6 to 8 rats per group. U, untreated. *, P < 0.0001 compared to all groups. **, P < 0.001 compared to sham groups.
Gene Expression for VEGF
VEGF
In untreated shams, VEGF mRNA was distributed over the inner retinal layers (most notably in the INL), the retinal pigment epithelium (RPE), and in blood vessels (Figure 5). In shams treated with 50 mg/kg/day STI571, VEGF gene expression was unchanged compared to controls. In shams treated with 100 mg/kg/day STI571, VEGF mRNA was increased in the inner retina compared with shams (Figure 5). In ROP rats, VEGF gene expression was similar to sham rats treated with 100 mg/kg/day STI571. Compared to untreated ROP rats, VEGF gene expression was increased with 50 mg/kg/day STI571 and further increased with 100 mg/kg/day (Figure 5).
Figure 5-4039.
Paired bright and dark field photomicrographs showing vascular endothelial growth factor (VEGF) gene expression in 3-μm thick paraffin sections of retinas from sham and ROP rats treated with STI571. Sections are counterstained with hematoxylin and eosin. The paired bright and dark field panels on the left show sham groups and the paired bright and dark field panels on the right are ROP groups. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. Original magnification, ×120. In all groups, VEGF mRNA is observed in the GCL, IPL and INL and the RPE. Untreated sham (A and a) and sham + 50 mg/kg/day (B and b) are similar with mRNA most notable over the INL. Sham + 100 mg/kg/day STI571 (C and c) shows an increase in mRNA in the inner retina compared to sham groups. ROP (D and d), shows intense VEGF mRNA in the inner retina and particularly the INL. ROP + 50 mg/kg/day STI571 (E and e) shows increased signal in the inner retina compared to untreated ROP which is further increased in the ROP + 100 mg/kg/day STI571 group (F and f). Results are presented graphically. Values are means ± SEM. N = 6 to 8 rats per group. U, untreated. *, P < 0.0001 compared to all groups except sham + 50 mg/kg/day STI571. *, P < 0.0001 compared to all groups except untreated sham. , P < 0.005 compared to ROP + 50 mg/kg/day STI571. †, P < 0.0001 compared to ROP groups. ‡, P < 0.0001 compared to all groups.
VEGFR-2
Overall, the intensity and distribution of VEGFR-2 gene expression over the entire retina was similar to VEGF in both untreated sham and ROP groups and following treatment with STI571. In untreated shams, VEGFR-2 mRNA was most evident in the inner retina in blood vessels, INL, and RPE (Figure 6). In shams treated with 50 mg/kg/day STI571, VEGF gene expression was unchanged compared to controls. In sham rats treated with 100 mg/kg/day STI571, the intensity of VEGFR-2 mRNA was increased in the GCL, IPL, and INL (Figure 6). In ROP, the intensity of VEGFR-2 mRNA was similar to sham rats treated with 100 mg/kg/day STI571 and increased compared to other sham groups. Compared to untreated ROP rats, VEGF gene expression was increased with 50 mg/kg/day STI571 and further increased with 100 mg/kg/day (Figure 6).
Figure 6-4039.
Paired bright and dark field photomicrographs showing vascular endothelial growth factor 2 (VEGFR-2) gene expression in 3-μm thick paraffin sections of retinas from sham and ROP rats treated with STI571. Sections are counterstained with hematoxylin and eosin. The paired bright and dark field panels on the left show sham groups and the paired bright and dark field panels on the right are ROP groups. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. Original magnification ×120. In all groups, VEGF mRNA is observed in the GCL, IPL and INL and the RPE. Untreated sham (A and a) and sham + 50 mg/kg/day (B and b) are similar with mRNA most notable over the INL. Sham + 100 mg/kg/day STI571 (C and c) shows an increase in mRNA in the inner retina compared to sham groups. ROP (D and d), shows intense VEGF mRNA in the inner retina and particularly the INL. ROP + 50 mg/kg/day STI571 (E and e) shows increased signal in the inner retina compared to untreated ROP which is further increased in the ROP + 100 mg/kg/day STI571 group (F and f). Results are shown graphically. Values are means ± SEM. N = 6 to 8 rats per group. *, P < 0.0001 compared to all groups except sham + 50 mg/kg/day STI571. , P < 0.0001 compared to all groups except untreated sham. †, P < 0.0001 compared to sham groups and ROP + 100 mg/kg/day STI571. ‡, P < 0.05 compared to ROP + 50 mg/kg/day STI571. §, P < 0.001 compared to ROP + 100 mg/kg/day STI571. §§, P < 0.0001 compared to all groups.
Discussion
The major finding is that inhibition of PDGF-RTK following STI571 treatment causes degeneration of pericytes in both the developing retina and rats with ROP. This pathology is accompanied by an increase in VEGF and VEGFR-2 gene expression in discrete sites within the retina, and increased angiogenesis in both sham and ROP animals. These findings are consistent with the view that PDGF plays a critical role in maintaining the integrity of retinal blood vessels, and highlights the importance of PDGF and retinal pericytes in the angiogenic process.
Developmental studies indicate that PDGF is necessary for pericyte viability.9–12 In mice without PDGF-BB or its cognate receptor, capillaries in the brain have few pericytes and develop microaneurysms that rupture late in gestation.10 A similar situation occurs in the retina, with PDGF-deficient mice reported to have fewer pericytes than wild-type mice during the early postnatal phase of the growing retina.13 Similarly, when streptozotocin diabetes is induced in heterozygous PDGF-deficient mice, retinal pericytes are reduced by 50% inducing pathology reminiscent of early diabetic retinopathy.13 Consistent with these findings, we report 100 mg/kg/day STI571 in ROP rats to be associated with the damage and apoptosis of retinal pericytes in the inner retina. Ultrastructural studies indicated that occasionally retinal pericytes in sham rats treated with 100 mg/kg/day STI571 were damaged; however quantitation of apoptosis with caspase-3 and α-smooth muscle cell actin immunohistochemistry revealed this was not significant. Although pericyte morphology and apoptosis was not evaluated in sham and ROP rats treated with the lower dosage of STI571, the dosage of PDGF-B has been linked to the recruitment of retinal pericytes in mice with absence of one PDGF-B allele.29 STI571 is a potent and selective inhibitor of the PDGF α and β receptors and Abl protein tyrosine kinases.20 In cultured vascular smooth muscle cells, STI571 inhibits PDGF tyrosine activity with an IC50 100 times more potent than epidermal growth factor and other tyrosine kinases.20 Recent studies by our group have shown that STI571 specifically blocks PDGF receptor phosphorylation in rat aortic and bovine retinal smooth muscle cells.30 These findings together with evidence that the PDGF-β receptor is found on pericytes,10,31 suggest that STI571 directly compromises pericyte viability. Further evidence supporting this view is provided by our previous study in a rat model of Thy-1.1 glomerulonephritis, where STI571 reduced the proliferation of mesangial cells in the glomerulus,32 cells which are embryologically related to microvascular pericytes.33 Overall, these findings indicate that PDGF is vital for pericyte survival in a number of tissues including the retina.
Physical contact between pericyte and endothelial cells may be necessary to prevent angiogenesis.34 Studies of wound healing have reported that the appearance of pericytes in the wound area correlates with inhibition of endothelial cell proliferation.7,35 In the present study, in both sham and ROP rats treated with 100 mg/kg/day STI571, the appearance of degenerating pericytes in the inner retina occurred together with an increase in blood vessel profiles in the inner retina. The findings of PDGF deficiency exacerbating angiogenesis in ROP is consistent with a study in PDGF-deficient mice subjected to the ROP protocol,13 and a study in mice with endothelial cell-specific PDGF-B ablation where variable pericyte loss was correlated with the extent of vascular abnormalities.29 In both the present and in these previous studies, the increased vascularity associated with PDGF deficiency involved abnormal vessels that were dilated and accompanied by hemorrhages.10,12 Overall, these findings demonstrate the importance of pericyte viability and pericyte-endothelial cell contacts in maintaining the patency of blood vessels.
Tissue hypoxia is a stimulus for VEGF production whose actions include cell proliferation, permeability, and survival.36 In the developing rodent retina, tissue hypoxia stimulates both VEGF and VEGFR-2 expression in blood vessels, astrocytes, ganglion cells, and cells within the inner nuclear layer,3,27,28 findings consistent with those observed in sham retina in the present study. VEGF and VEGFR-2 promote the formation of an endothelial plexus and subsequent coverage by pericytes.3,37 Once a vascular network is complete and tissue hypoxia abated, VEGF and VEGFR-2 are down-regulated, a process that occurs by approximately 3 weeks of age in the developing rodent retina.38 Our findings suggest that PDGF plays an important role in these events. In the developing retinas of sham rats treated with the highest dose of STI571, a deficiency in PDGF leads to some pericyte pathology as viewed by electron microscopy, which although not significant when quantitated for caspase-3 immunohistochemistry, may be sufficient to weaken the vessel wall and promote hypoxia in the surrounding microenvironment. Up-regulation of VEGF and VEGFR-2 gene expression then occurs as observed with in situ hybridization and leads to angiogenesis. A similar situation may have occurred in the ROP model. The introduction of hyperoxia (the first stage of ROP) to the retinas of newborn rats is known to reduce VEGF and VEGFR-2 expression which attenuates retinal angiogenesis and vascular remodeling.3,37 Exposure to the relative hypoxia of room air between postnatal days 12 and 18, results in the up-regulation of VEGF and VEGFR-2 synthesis to such an extent that pathological angiogenesis occurs.28,38 STI571 administered during the angiogenic period in ROP rats not only caused pericyte damage as observed in sham retinas, but marked apoptosis which may have compromised vessel stability, and subsequently increased tissue hypoxia and promoted VEGF-induced angiogenesis. We found that the lower dose of STI571 did not stimulate retinal angiogenesis in either sham rats or ROP; however STI571 was associated with an increase in VEGF and VEGFR-2 mRNA in ROP rats. It is likely that ROP rats, with a more hypoxic retina compared to shams, are more susceptible to the effects of the PDGF-RTK inhibition. This may also occur in diabetes, as reports by our group and others27,39 have shown increased retinal VEGF and VEGFR-2 gene expression in the absence of blood vessel formation.
Despite pericyte “drop-out” being a characteristic early feature of diabetic retinopathy,7 the role of PDGF in this process has not been fully explored. A recent study indicates that retinal PDGF expression is increased after 4 weeks of streptozotocin diabetes in rats and can be down-regulated by protein kinase C-β inhibition.40 However, it is unclear from this study if the rise in retinal PDGF is related to pericyte viability as morphological studies were not performed, and angiogenesis does not develop in this rodent model of diabetes.41,42 It is possible that the rise in PDGF gene expression in the retinas of diabetic rats is a response to hyperglycaemia as cultured bovine retinal pericytes and endothelial cells have been reported to increase PDGF40,43 and PDGF-β receptor44 mRNA in response to high glucose. Studies in humans with the proliferative form of diabetic retinopathy have also reported elevated levels of PDGF-AB in vitreal fluids.45 In disagreement with these findings is a study by Hammes and colleagues13 who reported PDGF-deficient mice with streptozotocin diabetes to exhibit vascular abnormalities. Our findings extend those of Hammes and colleagues39 by showing pericyte degeneration and PDGF deficiency to be associated with increased VEGF expression and angiogenesis. A possible explanation for the discrepancies between these two studies and that of Yokota and colleagues40 is that inhibition of PDGF-RTK with STI571 or a deficiency in PDGF by gene ablation directly reveals the relationship between PDGF, pericytes, VEGF, and angiogenesis.
In summary, our results indicate that PDGF is vital for the maintenance of normal developmental angiogenesis in the retina, and for the prevention of hypoxic-induced angiogenesis in ROP. Inhibition of PDGF-RTK may exacerbate pathological angiogenesis in ROP by stimulating pericyte death and VEGF and VEGFR-2 expression.
Acknowledgments
We thank the Juvenile Diabetes Research Foundation International (JDRFI) and the National Health and Medical Research Council of Australia for their continuing support. Dr. Darren Kelly is a recipient of a JDRFI Career Development Award.
Footnotes
Address reprint requests to Dr. Jennifer Wilkinson-Berka, Department of Physiology, The University of Melbourne, Parkville, Victoria, Australia, 3010. E-mail: jlaberka@unimelb.edu.au.
Supported by Juvenile Diabetes Research Foundation International and The National Health and Medical Research Council of Australia.
References
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;Suppl 2:S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein B, Moss S. Epidemiology of proliferative diabetic retinopathy. Diabetes Care. 1992;15:1875–1891. doi: 10.2337/diacare.15.12.1875. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Peer J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud X, Dorey CK. Extraretinal neovascularization induced by hypoxic episodes in the neonatal rat. Invest Ophthalmol Vis Sci. 1994;35:3169–3177. [PubMed] [Google Scholar]
- Verbeek MM, Otte-Holler I, Wesseling P, Ruiter DJ, de Waal RM. Induction of α-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-β 1. Am J Pathol. 1994;144:372–382. [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β 1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns:IV diabetic retinopathy. Arch Ophthalmol. 1961;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- Buzney SM, Frank RN, Varma SD, Tanishima T, Gabbay KH. Aldose reductase in retinal mural cells. Invest Ophthalmol Vis Sci. 1977;16:392–396. [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kal n M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–554. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes H-P, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, Bucana CD, McMahon G, Gallick GE, Ellis LM. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. EMBO J. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- Affleck DG, Bull DA, Bailey SH, Albanil A, Connors R, Stringham JC, Karwande SV. PDGF(BB) increases myocardial production of VEGF: shift in VEGF mRNA splice variants after direct injection of bFGF, PDGF(BB), and PDGF(AB). J Surg Res. 2002;107:203–209. doi: 10.1006/jsre.2002.6510. [DOI] [PubMed] [Google Scholar]
- Shultz PJ, DiCorleto PE, Silver BJ, Abboud HE. Mesangial cells express PDGF mRNAs and proliferate in response to PDGF. Am J Physiol Heart Circ Physiol. 1988;255:F674–F684. doi: 10.1152/ajprenal.1988.255.4.F674. [DOI] [PubMed] [Google Scholar]
- Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45:319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- Doi T, Vlassara H, Kirstein M, Yamada Y, Striker GE, Striker LJ. Receptor-specific increase in extracellular matrix production in mouse mesangial cells by advanced glycosylation end products is mediated via platelet-derived growth factor. Proc Natl Acad Sci USA. 1992;89:2873–2877. doi: 10.1073/pnas.89.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- Buchdunger E, O’Reilly T, Wood J. Pharmacology of imatinib (STI571) Eur J Cancer. 2002;38 Suppl 5:S28–S36. doi: 10.1016/s0959-8049(02)80600-1. [DOI] [PubMed] [Google Scholar]
- Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci. 1993;34:576–585. [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Alousis NS, Kelly DJ, Gilbert RE. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44:974–999. doi: 10.1167/iovs.02-0392. [DOI] [PubMed] [Google Scholar]
- Vaughan AT, Beth CJ, Villalobos MJ. Surviving apoptosis. Apoptosis. 2002;7:73–177. doi: 10.1023/a:1014374717773. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins at the blood-brain and blood retinal barriers. J Neurocytol. 2001;30:35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Vranes D, Berka JL, Cox A, Kelly DJ, Stacker S, Cooper ME. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab Invest. 1998;78:1017–1027. [PubMed] [Google Scholar]
- Mulder H, Ahren B, Sundler F. Islet amyloid polypeptide and insulin gene expression are regulated in parallel by glucose in vivo in rats. Am J Physiol. 1996;271:E1008–E1014. doi: 10.1152/ajpendo.1996.271.6.E1008. [DOI] [PubMed] [Google Scholar]
- Moravski CJ, Skinner SL, Stubbs AJ, Sarlos S, Kelly DJ, Cooper ME, Gilbert RE, Wilkinson-Berka JL. The renin-angiotensin system influences ocular endothelial cell proliferation in diabetes: transgenic and interventional studies. Am J Pathol. 2003;162:151–160. doi: 10.1016/S0002-9440(10)63806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram J, Shahinfar S, Skinner SL, Wilkinson-Berka JL. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36:1099–1104. doi: 10.1161/01.hyp.36.6.1099. [DOI] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes H-P, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley A, Gilbert RE, Thomas D, Cox A, Price JT, Best J, Jenkins A. STI-571 inhibits in vitro angiogenesis. Biochem Biophys Res Commun. 2003;310:136–143. doi: 10.1016/j.bbrc.2003.08.129. [DOI] [PubMed] [Google Scholar]
- Holmgren L, Glaser A, Pfeifer-Ohlsson S, Ohlsson R. Angiogenesis during human extraembryonic development involves the spatiotemporal control of PDGF ligand and receptor gene expression. Development. 1991;113:749–754. doi: 10.1242/dev.113.3.749. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Kelly DJ, McKay T, Chadban S, Hill PA, Cooper ME, Atkins RC, Nikolic-Patterson DJ. PDGF signal transduction inhibition ameliorates experimental mesangial proliferative glomerulonephritis. Kidney Int. 2001;59:1324–1332. doi: 10.1046/j.1523-1755.2001.0590041324.x. [DOI] [PubMed] [Google Scholar]
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. EMBO J. 1987;1:272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Crocker DJ, Murad TM, Geer JC. Role of the pericyte in wound healing: an ultrastructural study. Exp Mol Pathol. 1970;13:51–63. doi: 10.1016/0014-4800(70)90084-5. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358–C1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Stone J, Chanling T, Peer J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996;37:290–299. [PubMed] [Google Scholar]
- Hammes HP, Lin JH, Bretzel RG, Brownlee M, Breier G. Up-regulation of the vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–406. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- Yokota T, Ma RC, Park JY, Isshiki K, Sotiropoulos KB, Rauniyar RK, Bornfeldt KE, King GL. Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes. 2003;52:8338–8845. doi: 10.2337/diabetes.52.3.838. [DOI] [PubMed] [Google Scholar]
- Su E, Alder V, Yu D, Yu P, Cringle S, Yogesan K. Continued progression of retinopathy despite spontaneous recovery to normoglycemia in a long-term study of streptozotocin-induced diabetes in rats. Graefes Arch Clin Exp Ophthalmol. 2000;238:163–173. doi: 10.1007/s004170050028. [DOI] [PubMed] [Google Scholar]
- Engerman R, Finkelstein D, Aguirre G, Diddie KR, Fox RR, Frank RN, Varma SD. Ocular complications. Diabetes. 1982;31:82–88. doi: 10.2337/diab.31.1.s82. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Adrogue HJ, Nakajima T, Mizutani M, Asano M, Tachi Y, Suzuki S, Yamashita K. Increased production of PDGF by angiotensin and high glucose in human vascular endothelium. Life Sci. 1996;59:1455–1461. doi: 10.1016/0024-3205(96)00473-0. [DOI] [PubMed] [Google Scholar]
- Inaba T, Ishibashi S, Gotoda T, Kawamura M, Morino N, Nojima Y, Kawakami M, Yazaki Y, Yamada N. Enhanced expression of platelet-derived growth factor-β receptor by high glucose: involvement of platelet-derived growth factor in diabetic angiopathy. Diabetes. 1996;45:507–512. doi: 10.2337/diab.45.4.507. [DOI] [PubMed] [Google Scholar]
- Freyberger H, Brocker M, Yakut H, Hammer J, Effert R, Schifferdecker E, Schatz H, Derwahl M. Increased levels of platelet-derived growth factor in vitreous fluid of patients with proliferative diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2000;108:106–109. doi: 10.1055/s-2000-5803. [DOI] [PubMed] [Google Scholar]