Abstract
Advanced glycation end products (AGEs) have been shown to play a role in tubular epithelial-myofibroblast transdifferentiation (TEMT) in diabetic nephropathy, but the intracellular signaling pathway remains unknown. We report here that AGEs signal through the receptor for AGEs (RAGE) to induce TEMT, as determined by de novo expression of a mesenchymal marker (α-smooth muscle actin, α-SMA) and loss of epithelial marker (E-cadherin), directly through the MEK1-ERK1/2 MAP kinase pathway, which is TGF-β independent. This is supported by the following findings: AGEs induced de novo α-SMA mRNA expression as early as 2 hours followed by a loss of E-cadherin before TGF-β mRNA expression at 24 hours and occurred in the absence of TGF-β and AGE-induced activation of ERK1/2 MAP kinase at 15 minutes and TEMT at 24 hours were completely blocked by a neutralizing RAGE antibody, a soluble RAGE receptor, an ERK1/2 MAP kinase inhibitor (PD98059), and DN-MEK1, but not by a neutralizing TGF-β antibody. Thus, this study demonstrates that AGEs activate the RAGE-ERK1/2 MAP kinase pathway to mediate the early TEMT process. The findings from this study suggest that targeting the RAGE or the ERK MAP kinase pathway may provide new therapeutic strategies for diabetic nephropathy and shed new light on the pathogenesis of diabetic nephropathy.
Reducing sugars react non-enzymatically with amino groups of proteins to form Amadori products, which undergo further complex reactions to form advanced glycation end products (AGEs).1,2 AGEs have been implicated in many of the diabetic complications, including diabetic nephropathy.3,4 Tubulointerstitial fibrosis, a prominent phenomenon in diabetic nephropathy, is characterized by an increasing number of interstitial myofibroblasts that mediate excessive deposition of interstitial matrix components leading to tubulointerstitial fibrosis.5–7 Indeed, myofibroblast formation is a key step in the development of tubulointerstitial fibrosis and has been shown to be a major determinant of disease progression in glomerulonephritis including diabetic nephropathy.8–12 We and other investigators have previously shown that interstitial myofibroblasts may derive from tubular epithelial cells (TECs) through tubular epithelial-myofibroblast transdifferentiation (TEMT) in both experimental and human chronic kidney diseases.13–17 This is determined by de novo expression of the mesenchymal marker, α-SMA, and the loss of an epithelial marker, E-cadherin. The importance of TEMT in tubulointerstitial fibrosis is further demonstrated in a recent study in a transgenic mouse model of ureteral obstructive kidney disease in which large numbers of interstitial fibroblasts are derived from TECs as demonstrated by expression of fibroblast specific protein-1 (FSP-1) reporter gene.18 In addition, disruption of tissue-type plasminogen activator also blocks tubular epithelial-to-myofibroblast transition and tubulointerstitial fibrosis in obstructive nephropathy in mice.19 All these demonstrate a critical role for TEMT in tubulointerstitial fibrosis.20 There are numbers of mediators including TGF-β, fibroblast growth factor-2, epithelial growth factor, and IL-1 that play a role in TEMT in vitro and in vivo.21–22 In contrast, overexpression of hepatocyte growth factor is able to inhibit TGF-β-induced TEMT.23 These observations indicate that regulation of TEMT may involve multiple signaling pathways.
Oldfield et al24 has reported that TEMT is also an important event in both experimental and human diabetic nephropathy and is mediated by engagement of AGEs to one of its receptor for AGEs, RAGE. This finding provides evidence that the TEMT process may play a critical role in the pathogenesis of diabetic nephropathy. However, the intracellular mechanism(s) whereby AGEs regulate TEMT remains completely unknown. It has been shown that AGEs interact with RAGE leading to activation of multiple signaling pathways including NF.κB, p38 MAP kinase, Ras-extracellular signal-regulated kinase1/2 (ERK1/2), and stress-activated protein kinase/c-Jun-NH2-terminal kinase (SAPK/JNK) signaling pathways.25–28 The MAP kinase cascades have been shown to be important in cell proliferation, differentiation including EMT, and apoptosis.29–31 Activation of RAGE has been shown to induce ERK1/2 MAP kinase phosphorylation and is capable of driving uncommitted embryonic stem cells to differentiate toward a neuronal phenotype.32 Taken together, we hypothesize that AGEs may signal through RAGE to activate ERK1/2 MAP kinase to induce TEMT. This has been tested in the present study.
Materials and Methods
Cell Culture
A normal rat kidney tubular epithelial cell (TEC) line (NRK52E) was used in this study. Cells were grown in Dulbecco’s modified Eagle’s medium/F12 (Gibco, BRL, Gaithersburg, MD) containing 10% fetal bovine serum (BSA) in 6-well plastic plates or 8-chamber glass slides (Nunc, Naperville, CT) at 37°C. Cells were stimulated with AGE-BSA or control BSA at concentrations of 0, 10, 30, and 100 μg/ml for periods of 5, 15, 30, and 60 minutes, and 2, 4, and 6 hours for ERK1/2 signaling detection and periods of 2, 6, 12, and 24 hours for examination of α-SMA and E-cadherin mRNA and protein expression. The preparation and degree of modification of AGE-BSA and control BSA were described previously.24 All AGE-BSA preparations and dilutions were performed under endotoxin-free conditions and were passed over an endotoxin-binding affinity polymyxin column (Detoxi-gel, Pierce, Rockford, IL). Before in vitro study, reagents were tested for endotoxin levels by the Limulus amebocyte assay (E-Toxate, Sigma, St. Louis, MO) and were found to contain less than 0.2 ng/ml of endotoxin.
To block the binding of AGE to RAGE, a rabbit neutralizing anti-RAGE antibody (10 μg/ml)24 or a soluble and truncated receptor for AGE (sRAGE, 100 μg/ml) was used.25 To block the TGF-β activity induced by AGEs, a rabbit neutralizing TGF-β antibody (10 μg/ml, R&D System, Minneapolis, MN) or an isotype control normal rabbit IgG (10 μg/ml, R&D), and a dominant-negative TGF-β receptor II (DN-TβRII, an original gift from Rik Derynck, University of California San Francisco) or a control plasmid (pcDAN3) were used. To inhibit AGE-induced ERK1/2 MAP kinase activities, MAPK inhibitor PD98059 (10 μmol/L, Calbiochem, La Jolla, CA), a dominant-negative (DN) or wild-type (WT) MEK1 (DN-MEK1, WT-MEK1, a gift from Dr. Michael J. Weber, University of Virginia), and an empty vector control (pcDNA3) plasmids were used. Each experiment was repeated at least three times throughout the study. The transfection was conducted using Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. After a 24-hour transfection, cells were rested with serum-free medium for 24 hours and then were stimulated with AGE-BSA or BSA as described above.
RNA Extraction and Real-Time Reverse Transcriptase Polymerase Chain Reaction
Total RNA from cultured TEC was isolated and real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed using the method described previously.33 Real-time, one-step RT-PCR was performed with SYBR Green PCR Reagents (Sigma), the Thermoscript RT-PCR system (Invitrogen) and the Opticon DNA Engine (MJ Research Inc.), according to manufacturer’s instructions. One hundred of total RNA was reverse transcribed before PCR as follows: 94°C for 2 minutes followed by 40 cycles at 94°C for 15 seconds, 58°C for 30 seconds, 72°C for 30 seconds with final extension at 72°C for 10 minutes. Primers used in this study were: α-SMA, fwd 5′-CTCTGGTGTGTGACAATGGTCC-3′, rev 5′-CGAAGCTCGTTATAGAAGGAGTG-3′; E-cadherin, fwd 5′-CCTGATGCCAGACCGGAAGTGATTCG-3′, rev 5′-GATCTGACTCAGAGTTCAG-3′, TGF-β1, fwd 5′-CCTGGATACCAACTACTGCTTC-3′, rev 5′-CGATCATGTTGGACAACTGCTC-3′, GAPDH, fwd 5′-ACCCCCAATGTATCCGTTGT-3′, rev 5′-TACTCCTTGGAGGCCATGTA-3′. Amplicon sizes were 265 bp (α-SMA), 247 bp (E-cadherin), 314 bp (TGF-β1), and 299 bp (GAPDH). Reaction specificity was confirmed by electrophoresis analysis of products before real-time PCR and bands of expected size were detected. Ratios for α-SMA/GAPDH, E-cadherin/GAPDH, and TGF-β1/GAPDH mRNA were calculated for each sample and expressed as the mean ± SD.
Western Blot Analyses
NRK52E TEC grown in six-well plates with or without different stimuli at various time points were analyzed by Western blotting as described previously.33 Briefly, after cells were lysed in 1 ml of 1% Nonidet P-40, 25 mmol/L Tris-HCl, 150 mmol/L NaCl, 10 mmol/L EDTA, pH 8.0, containing a 1:50 dilution of a protease inhibitor cocktail (P2714; Sigma), samples were centrifuged at 14,000 × g for 5 minutes to pellet cell debris. Samples (20 μg) were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled for 5 minutes, electrophoresed on a 10% SDS polyacrylamide gel, and electroblotted onto Hybond-ECL nitrocellulose membrane (Amersham International, Buckinghamshire, UK). The membrane was blocked in PBS containing 5% BSA and 0.02% Tween 20. To detect phosphorylated ERK1/2, total ERK1/2, α-SMA, and E-cadherin, the membrane was incubated for 1 hour with mouse monoclonal (mAb) to phosphorylated ERK1/2, rabbit polyclonal antibodies (Ab) to ERK1/2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), and mAbs to α-SMA (1A4, Sigma) and E-cadherin (Transduction Laboratories, Lexington, KY). After washing, the membrane was incubated with a 1:20,000 dilution of peroxidase-conjugated rabbit anti-mouse IgG or swine anti-rabbit IgG in PBS containing 1% normal goat serum and 1% fetal calf serum. The blot was then developed using the ECL detection kit (Amersham International) to produce a chemiluminescence signal, which was captured on X-ray film.
Two-Color Immunocytochemistry
NRK52E TEC were cultured in eight-chamber glass slides with or without different stimuli at various time points as described above. Cells were analyzed for TEMT using microwave-based two-color immunostaining.33,34 Briefly, cells were fixed in 2% paraformaldehyde and preincubated with 10% fetal calf serum and 10% normal sheep serum to block non-specific binding. Cells were then incubated with the anti-α-SMA mAb or an irrelevant isotype control IgG (the mAb 73.5 that recognizes human CD45R antigen) at 4°C overnight. After inactivation of endogenous peroxidase, cells were incubated with peroxidase-conjugated rabbit anti-mouse IgG and then by the mouse peroxidase anti-peroxidase complexes. After being washed, slides were developed with diaminobenzidine to produce a brown product. After color developing, cells were treated with 10 minutes of microwave oven heating in 10 mmol/L sodium citrate (pH 6.0) at 2450 MHz and 800 watts of power to block antibody cross-reactivity and to facilitate antigen retrieval. Cells were then incubated with the anti-E-cadherin mAb or control mAb (73.5) for 60 minutes and then with alkaline phosphatase-conjugated goat anti-mouse IgG and mouse alkaline phosphatase anti-alkaline phosphatase complexes. Finally, sections were developed with Fast Blue BB Base (Sigma), counterstained with hematoxylin, and cover-slipped in an aqueous mounting medium. All procedures were performed at room temperature.
ELISA Analysis for TGF-β1 Protein
After TEC were cultured in 24-well plates (5 × 104 cells/well) with or without different stimuli at various time points as described above, the supernatants were collected and measured for TGF-β levels by ELISA (R&D) according to the manufacturer’s specifications. Experiments were performed in triplicates and results were expressed as the mean ± SD.
Quantitative Analyses
AGE-induced de novo expression of α-SMA and lose of E-cadherin by TECs were quantitatively analyzed as described previously.33 Briefly, the percentage of positive cells in eight-chamber slides stained for mAbs to α-SMA and E-cadherin as described above was determined by counting at least 1000 cells under high power (×400) in each well. Analysis was performed blind on coded slides.
Statistical Analyses
Data obtained from this study are expressed as the mean ± SD. Statistical analyses were performed using GraphPad Prism 3.0 (GraphPad Software, Inc. San Diego, CA). Differences in p-ERK1/2, α-SMA, E-cadherin, and TGF-β1 expression were assessed by one-way analysis of variance or by t-test.
Results
AGEs Induce TEMT Directly and Independently of TGF-β
It is noted that de novo expression of α-SMA and loss of E-cadherin have been used as the markers to determine TEMT in both in vivo and in vitro.15–17,19,20,22–24, 33 In the present study, we first examined the kinetic changes of these two distinct markers during AGE-induced TEMT and tested the hypothesis that AGEs induce TEMT directly and independently of TGF-β. As shown in Figure 1, AGEs, but not control BSA, were able to induce TEMT at 24 hours as demonstrated by de novo α-SMA expression and partial loss of E-cadherin by TECs (Figure 1, A and B). This was further demonstrated by real time PCR and Western blot analyses as shown in Figures 2 and 3. Indeed, AGEs induced significant α-SMA mRNA expression in both a time- and dose-dependent manner, being significant at 2 hours, and significantly stimulated α-SMA protein expression at 6 hours (Figure 2), which preceded the significant reduction of E-cadherin mRNA and protein expression first detected at 24 hours (Figure 3).
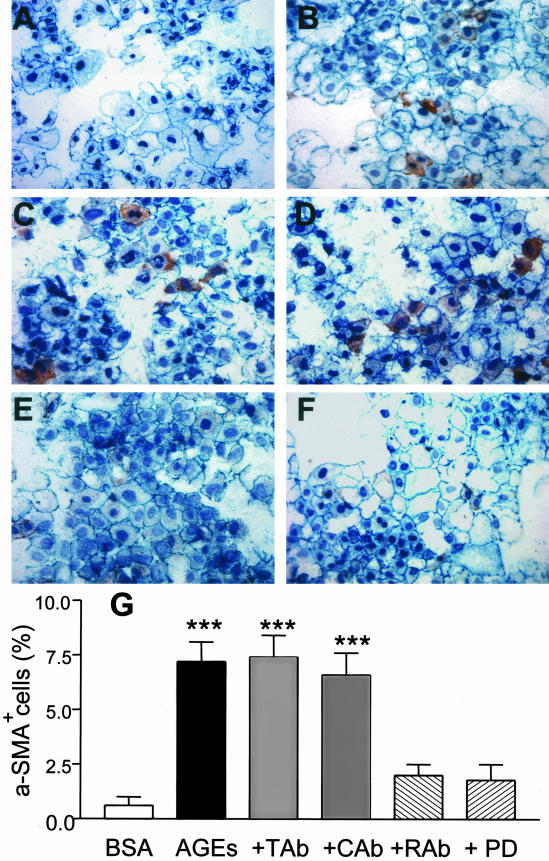
Figure 1.
Double immunocytochemistry demonstrates that AGEs induce TEMT as determined by de novo expression of α-SMA and a partial loss of E-cadherin in NRK52E cells. NRK52E cells were stimulated with control BSA (30 μg/ml, A) or AGE-BSA (30 μg/ml, B–F) for 24 hours in the presence of an isotype control normal rabbit IgG (10 μg/ml, C), a neutralizing TGF-β antibody (10 μg/ml, D), a neutralizing RAGE antibody (10 μg/ml, E), or a specific ERK1/2 MAP kinase inhibitor (PD 98059, 10 μmol/L, F). Cells were stained with mAbs to α-SMA (myofibroblasts, red) and E-cadherin (epithelial cells, blue). The percentage of transformed cells (α-SMA+) is shown in (G). Results show that AGE-induced α-SMA expression in TECs is blocked by a neutralizing RAGE and an ERK1/2 MAP kinase inhibitor, but not by a neutralizing TGF-β antibody. Tab, anti-TGF-β antibody; Cab, control antibody; Rab, anti-RAGE antibody; PD, PD 98059. Data represent the mean ± SD for five experiments. Nuclei are stained with hematoxylin. Magnification, ×250. ***, P < 0.001 compared with BSA, anti-RAGE antibody, and ERK1/2 inhibitor.
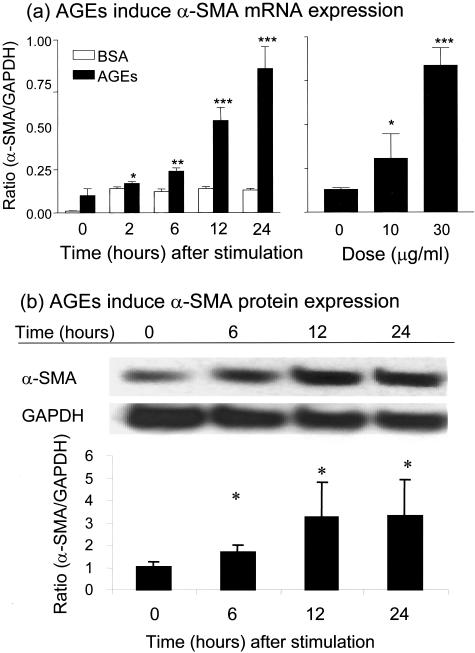
Figure 2.
Real-time PCR and Western blot analysis demonstrate that AGEs induce de novo expression of α-SMA by NRK52E cells. a: Real-time PCR shows that AGE-BSA (30 μg/ml), but not BSA (30 μg/ml), induce α-SMA mRNA expression in a time-dependent (left panel) and a dose-dependent manner (right panel, at 24 hours after AGE stimulation). b: Western blotting shows that AGEs induce α-SMA protein expression, being significant at 6 hours after AGE-BSA (30 μg/ml) stimulation. Each bar represents the mean ± SD for at least three independent experiments. *, P < 0.05, **, P < 0.01, ***, P < 0.001 compared to either the BSA control or with time (or dose) 0.
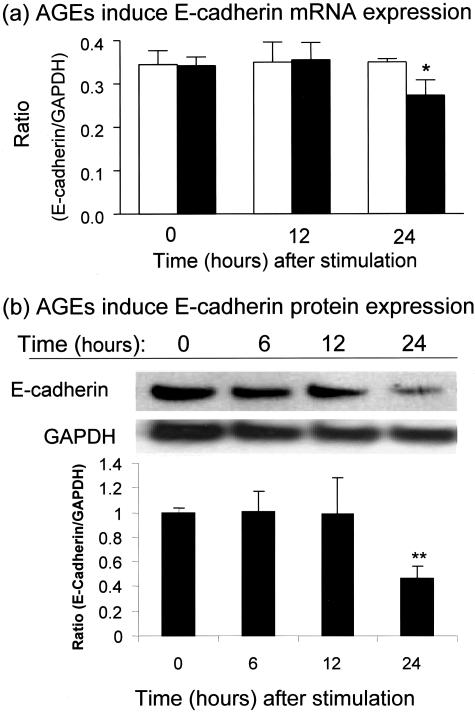
Figure 3.
Real-time PCR and Western blot analysis demonstrate that AGEs induce loss of E-cadherin by NRK52E cells. Results show that AGE-BSA (30 μg/ml, closed bars), but not BSA (30 μg/ml, open bars), significantly reduce E-cadherin mRNA and protein expression at 24 hours as demonstrated by real-time PCR (a) and Western blotting (b). Each bar represents the mean ± SD for three independent experiments. *, P < 0.05, **, P < 0.01 compared to either the BSA control or with time 0.
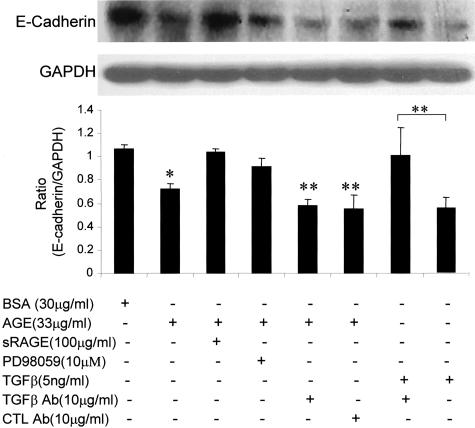
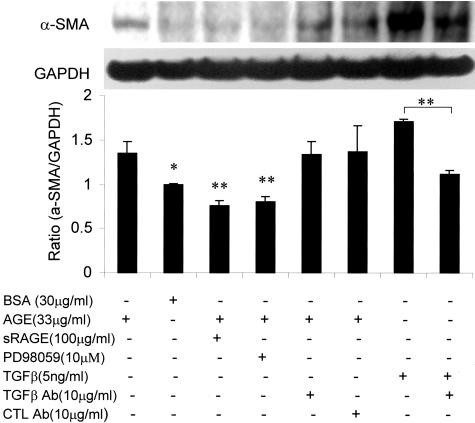
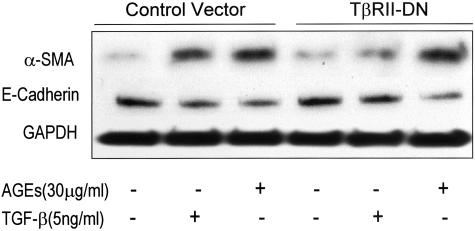
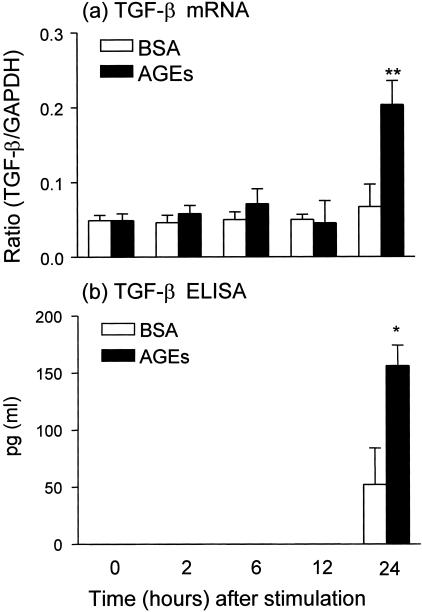
We next examined the mechanism by which AGEs induce TEMT. Because TGF-β has been shown to be a key mediator for TEMT,16,20–23 we tested whether early AGE-induced TEMT is TGF-β dependent. As shown in Figures 1 and 4–6, addition of a neutralizing TGF-β antibody did not inhibit AGE-induced TEMT in terms of de novo α-SMA expression and loss of E-cadherin as demonstrated by two-color immunocytochemistry (Figure 1, C and D), real time PCR (Figure 4), and Western blot analysis (Figures 5 and 6). This was further demonstrated by the finding that overexpression of DN-TβRII in TEC did not block AGE-induced TEMT in terms of α-SMA expression and a loss of E-cadherin, although TGF-β-induced TEMT was completely inhibited (Figure 7). In addition, there was no TGF-β in the medium as measured by ELISA and no significant TGF-β mRNA expression was detected by real time PCR until 24 hours after AGE-stimulation (Figure 8).
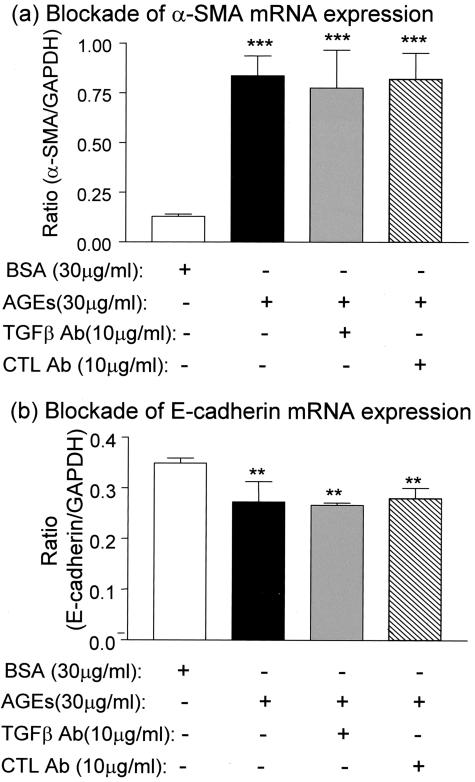
Figure 4.
Real-time PCR demonstrates that AGE-induced α-SMA mRNA and reduced E-cadherin mRNA expression are TGF-β-independent. Results show that AGE-induced α-SMA (a) and reduced E-cadherin (b) mRNA expression by NRK52E cells at 24 hours are not inhibited by a neutralizing TGF-β antibody. CTL Ab, control antibody. Each bar represents the mean ± SD for three independent experiments. **, P < 0.01, ***, P < 0.001 compared to the BSA control.
Figure 5.
Western blot analysis demonstrates that AGE-induced loss of E-cadherin protein expression is TGF-β-independent and RAGE-ERK-dependent. Results show that AGE-induced loss of E-cadherin expression by NRK52E cells at 24 hours is not inhibited by a neutralizing TGF-β antibody (A), but is prevented by addition of sRAGE and an ERK1/2 inhibitor, PD98059. The specificity of a neutralizing anti-TGF-β antibody to block TGF-β-induced loss of E-cadherin is demonstrated in the last two lanes. CTL Ab, control rabbit antibody (IgG). Each bar represents the mean ± SD for four independent experiments. *, P < 0.05, **, P < 0.01, compared to the BSA control, sRAGE, and PD98059, or as indicated.
Figure 6.
Western blot analysis demonstrates that AGE-induced α-SMA protein expression is TGF-β-independent, but RAGE-ERK-dependent. Results show that AGE-induced α-SMA expression by NRK52E cells at 24 hours is not inhibited by a neutralizing TGF-β antibody, but is completely blocked by sRAGE and an ERK1/2 inhibitor, PD98059. The specificity of a neutralizing anti-TGF-β antibody to block TGF-β-induced α-SMA expression is shown in the last two lanes. CTL Ab, control rabbit antibody (IgG). Each bar represents the mean ± SD for three independent experiments. *, P < 0.05, **, P < 0.01, compared to control BSA, sRAGE, and PD98059 or as indicated.
Figure 7.
Western blot analysis demonstrates that overexpression of DN-TβRII cannot block AGE-induced TEMT. Results show that the AGE-increased α-SMA and reduced E-cadherin expression by NRK52E cells at 24 hours is not inhibited by overexpression of DN-TβRII. In contrast, overexpression of DN-TβRII prevents TGF-β-induced TEMT in terms of increased α-SMA and decreased E-cadherin expression. Each sample represents the results from at least three independent experiments.
Figure 8.
Real-time and ELISA demonstrate that AGEs induce de novo TGF-β mRNA and protein expression at 24 hours. Results shown that AGE-BSA (30 μg/ml), but not BSA (30 μg/ml), induce TGF-β mRNA expression at 24 hours as demonstrated by real-time PCR (a). This is associated with the TGF-β protein secretion in the medium as demonstrated by ELISA (b). Each bar represents the mean ± SD for three independent experiments at triplicates. *, P < 0.05, **, P < 0.01 compared to the BSA control.
AGEs Induce TEMT via the RAGE and ERK1/2 MAP Kinase Signaling Pathway
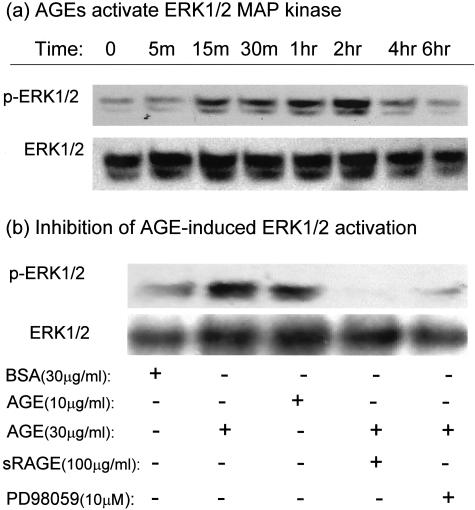
We further explored the signaling pathway where by AGEs induce TEMT via a TGF-β-independent mechanism. It has been shown that engagement of RAGE by AGE activates the ERK1/2 MAP kinase pathway, leading to embryonic stem cell differentiation.32 We tested whether the ERK1/2 MAP kinase pathway plays a role in the pathogenesis of AGE-induced TEMT. As shown in Figure 9, treatment of NRK52E cells with AGEs, but not BSA, resulted in the activation of ERK1/2 in both a time- and a dose-dependent manner, being significant at 15 minutes and declining to baseline levels at 4 hours (Figure 9a). This is confirmed by the finding that an ERK1/2 MAP kinase inhibitor (PD98059) was able to completely block AGE-induced ERK1/2 activation at 30 minutes (Figure 9b). In addition, the ability of sRAGE to completely inhibit AGE-induced ERK1/2 activation at the peak of 30 minutes (Figure 9b) indicated AGEs activating ERK1/2 signaling through RAGE.
Figure 9.
Western blot analysis demonstrates that AGEs signal through RAGE to activate the ERK1/2 signaling pathway. a: A representative Western blot shows that AGE-BSA (30 μg/ml) induces ERK1/2 phosphorylation in NRK52E in a time-dependent manner, being significant at 15 minutes. b: AGEs, but not BSA, induce ERK1/2 phosphorylation (at 30 minutes) in a dose-dependent manner and this is blocked by both sRAGE and a specific ERK1/2 inhibitor, PD 98059. Each sample represents the results from three independent experiments.
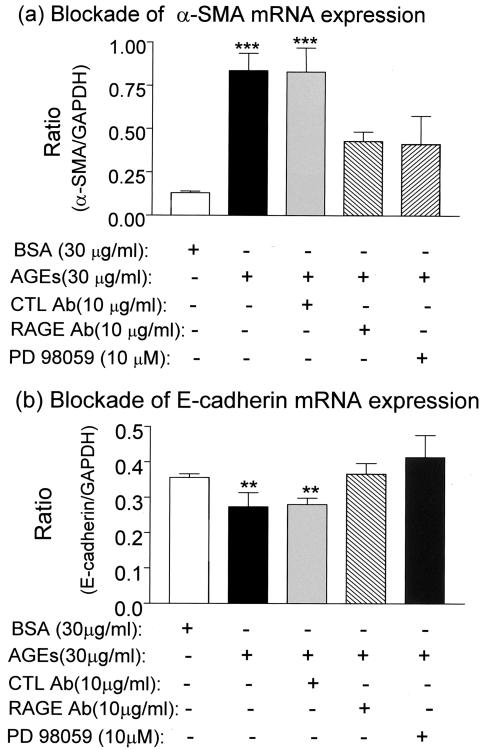
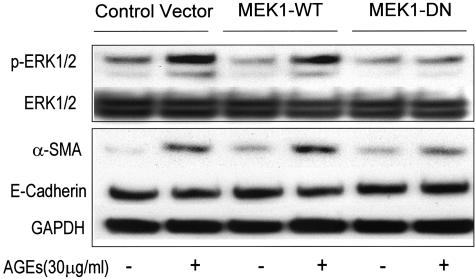
We further examined the potential role of the RAGE-ERK MAP kinase cascade in AGE-induced TEMT. As shown in Figure 1, E to G, two-color immunohistochemistry showed that both neutralizing anti-AGE antibody and ERK1/2 inhibitor (PD98059) were able to completely block AGE-induced TEMT in terms of increased α-SMA and decreased E-cadherin expression at 24 hours. Similar results were also found by real-time PCR (Figure 10) and Western blot analyses (Figures 5 and 6). AGE-induced up-regulation of α-SMA and decrease in E-cadherin expression were substantially inhibited by the neutralizing anti-RAGE antibody (Figure 10), sRAGE (Figures 5 and 6), and the ERK1/2 inhibitor (Figures 5, 6, and 10). The critical role of MEK1-ERK signaling pathway in AGE-induced TEMT was further demonstrated in TEC that were transfected with the WT or DN-MEK1 plasmid. As shown in Figure 11, overexpression of DN-MEK1 resulted in substantial inhibition of AGE-induced p-ERK1/2 activation, which was associated with the inhibition of α-SMA expression and prevention of the loss of E-cadherin. However, overexpression of WT-MEK1 caused no significant changes in p-ERK1/2 and expression of α-SMA and E-cadherin.
Figure 10.
Real-time PCR demonstrates that AGE-induced de novo α-SMA and loss of E-cadherin mRNA expression are regulated by RAGE through the ERK1/2 MAP kinase pathway. Real-time PCR demonstrates that AGE-induced α-SMA (a) and reduced E-cadherin (b) mRNA expression by NRK52E cells at 24 hours are completely blocked by a neutralizing RAGE antibody (RAGE Ab) and a specific ERK1/2 MAP kinase inhibitor, PD 98059. CTL Ab, control antibody (rabbit IgG). Each bar represents the mean ± SD for three independent experiments. **, P < 0.01, ***, P < 0.001 compared to the BSA control and treatments with the neutralizing RAGE antibody and PD 98059.
Figure 11.
Western blot analysis shows that AGE-induced p-ERK1/2, α-SMA expression, and loss of E-cadherin are regulated by the ERK1/2 MAP kinase pathway. Results show that AGE-induced p-ERK1/2, up-regulation of α-SMA and decrease in E-cadherin expression by NRK52E cells at 24 hours are completely blocked by overexpression of DN-MEK1. However, cells with overexpression of WT-MEK1 show no significant increase in p-ERK1/2 and α-SMA or decrease in E-cadherin expression when compared to empty control vector. Each sample represents the results from at least three independent experiments.
Discussion
There is increasing evidence that AGEs are an important mediator of diabetic nephropathy including tubulointerstitial fibrosis.1–5 Interstitial myofibroblasts are a major cell type and play a critical role in the development of tubulointerstitial fibrosis in both diabetic and non-diabetic kidney diseases.6–12 Myofibroblasts within the diseased kidney may derive from the transformed tubular epithelial cells under pathological conditions.13–19 TGF-β is a key mediator responsible for this transdifferentiation in vivo and in vitro.16,17,20,22,23 Indeed, in a recent study, we have demonstrated that blockade of the TGF-β signaling pathway by overexpression of Smad7 results in a complete inhibition of TGF-β-induced TEMT in vitro.33 Importantly, it has been also demonstrated that TEMT occurs in both experimental and human diabetic nephropathy and that AGEs are able to induce TEMT, which is blocked by both a neutralizing RAGE and an anti-TGF-β antibody.24 However, it remains completely unknown as to the specific signaling pathway(s) by which AGEs induce TEMT.
A new and significant finding in the present study is that AGEs signal through RAGE to activate the ERK1/2 MAP kinase pathway to induce TEMT at 24 hours and that this process is TGF-β independent. The identification of a TGF-β-independent signaling pathway involved the AGE-induced early process of TEMT is demonstrated by a number of findings in the present study. Firstly, AGEs are able to induce de novo α-SMA expression as early as 2 hours, which precedes the synthesis of TGF-β that occurs after 24 hours of exposure to AGEs. Secondly, the early TEMT induced by AGEs occurred when there was no TGF-β detectable within the medium. Finally, blockade of TGF-β signaling using a neutralizing anti-TGF-β antibody and overexpression of a DN-TβRII could not block AGE-induced TEMT at 24 hours as assessed by de novo α-SMA expression or a loss of E-cadherin.
Interestingly, in contrast to the TEMT process induced by TGF-β in which loss of E-cadherin is a critical step in this process,16 we found that de novo expression of α-SMA mRNA by tubular epithelial cells is the first evidence for TEMT induced by AGEs as demonstrated by quantitative real-time PCR. Indeed, AGEs induce significant α-SMA expression at 2 hours, which precedes the reduction of E-cadherin that occurs at 24 hours. This indicates that the activation of the mesenchymal gene may be a key process in TEMT induced by AGEs. This discrepancy between these two studies may also relate to the difference in stimuli, cells, and methods.
The mechanism by which AGEs induce TEMT directly and independently of TGF-β was shown to be regulated by RAGE and the ERK1/2 MAP kinase-dependent pathway. Specifically, AGEs are able to activate ERK1/2 within 15 minutes and this is blocked by a soluble truncated receptor for AGE, sRAGE, and an inhibitor to ERK1/2, PD98059. Furthermore, AGE-induced TEMT is completely inhibited by addition of a neutralizing anti-RAGE antibody, a soluble RAGE, an ERK1/2 MAP kinase inhibitor, PD98059, and specifically, by overexpression of DN-MEK1. Thus, the present study provides the first evidence that AGEs induce TEMT directly and independently of TGF-β via the RAGE-ERK1/2 MAP kinase pathway.
Engagement of RAGE by AGEs is critical for the pathogenesis of diabetic complications. Overexpression of RAGE results in the development of diabetic nephropathy and blockade of RAGE prevents a range of diabetic vascular complications, including atherosclerosis.35–37 In addition, AGEs may also play an important role in a non-diabetic context of transdifferentiation. For example, up-regulation of RAGE is associated with myofibroblast transdifferentiation in rat hepatic stellate cells.38 The present study provides further information that blockade of the AGE-RAGE interaction and RAGE-activated ERK1/2 MAP signaling results in complete inhibition of TEMT, a critical process in renal tubulointerstitial fibrosis. This suggests that targeting RAGE and/or the ERK1/2 MAP kinase pathway may provide new therapeutic strategies for the prevention and treatment of diabetic nephropathy.
While RAGE-ERK MAP kinase signaling appears to be important in the early process of TEMT, it should be noted that TEMT is a complex process and may involve multiple signaling pathways. It is possible that the TGF-β signaling pathway may also play a role in AGE-induced TEMT. This is supported by the previous finding that AGEs are able to stimulate TGF-β production and the addition of a neutralizing TGF-β antibody is able to partially inhibit TEMT and collagen matrix production after AGE stimulation for 6 days.24,39–41 Indeed, the present study also found that AGEs could stimulate tubular TGF-β expression at 24 hours, although this does not appear to contribute to the early process of TEMT. This finding is consistent with TGF-β-dependent signaling pathways participating and enhancing further the later process of TEMT induced by AGEs.24 Interestingly, we also found that AGE-induced TGF-β expression is also RAGE-ERK1/2 dependent since the addition of a neutralizing anti-RAGE antibody, sRAGE, or an ERK MAP kinase inhibitor were able to completely block AGE-induced TGF-β mRNA expression.41 Thus, it is likely that AGEs mediate TEMT directly through the RAGE-ERK MAP kinase pathway and indirectly via the TGF-β signaling pathway. The latter phenomenon may involve multiple intracellular signaling processes,42,43 which requires further investigation.
Footnotes
Address reprint requests to Dr. Hui Y. Lan, M.D., Ph.D., Department of Medicine-Nephrology, Baylor College of Medicine, One Baylor Plaza, Alkek N520, Houston, TX 77030. E-mail: hlan@bcm.tmc.edu.
Supported in part by grants from the Juvenile Diabetes Research Foundation (JDRF 1–2001-596 and 4–2003-30) and NIH George M. O’Brien Kidney Research Center (1P50 DK064233–01).
J.H.L. and W.W. made equal contributions to this study.
References
- Bucala R, Cerami A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, Friedman EA, Cerami A, Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325:836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001;44:1957–1972. doi: 10.1007/s001250100000. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- Essawy M, Soylemzoglu O, Muchaneta-Kubara EC, Shortland J, Brown CB, El Nahas AM. Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant. 1997;12:43–50. doi: 10.1093/ndt/12.1.43. [DOI] [PubMed] [Google Scholar]
- Fogo AB. Diabetic nephropathy: it’s in the numbers. Kidney Int. 2002;61:2274–2275. doi: 10.1046/j.1523-1755.2002.00389.x. [DOI] [PubMed] [Google Scholar]
- Fogo AB. Pathology of progressive nephropathies. Curr Opin Nephrol Hypertens. 2000;9:241–246. doi: 10.1097/00041552-200005000-00006. [DOI] [PubMed] [Google Scholar]
- Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- Badid C, Mounier N, Costa AM, Desmouliere A. Role of myofibroblasts during normal tissue repair and excessive scarring: interest of their assessment in nephropathies. Histol Histopathol. 2000;15:269–280. doi: 10.14670/HH-15.269. [DOI] [PubMed] [Google Scholar]
- Hewitson TD, Becker GJ. Interstitial myofibroblasts in IgA glomerulonephritis. Am J Nephrol. 1995;15:111–117. doi: 10.1159/000168813. [DOI] [PubMed] [Google Scholar]
- Yang N, Wu LL, Nikolic-Paterson DJ, Ng YY, Yang WC, Mu W, Gilbert RE, Cooper ME, Atkins RC, Lan HY. Local macrophage and myofibroblast proliferation in progressive renal injury in the rat remnant kidney. Nephrol Dial Transplant. 1998;13:1967–1974. doi: 10.1093/ndt/13.8.1967. [DOI] [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: fSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- Ng YY, Huang TP, Yang WC, Chen ZP, Yang AH, Nikolic-Paterson DJ, Atkins RC, Lan HY. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998;53:864–876. doi: 10.1046/j.1523-1755.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinde K, Nikolic-Paterson DJ, Huang XR, Sakai H, Kurokawa K, Atkins RC, Lan HY. Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis. 2001;38:761–769. doi: 10.1053/ajkd.2001.27693. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110:1525–1538. doi: 10.1172/JCI16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-β regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-β1-dependent mechanism in vitro. Am J Kidney Dis. 2001;37:820–831. doi: 10.1016/s0272-6386(01)80132-3. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002;13:96–107. doi: 10.1681/ASN.V13196. [DOI] [PubMed] [Google Scholar]
- Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcos M, Sayed AA, Bierhaus A, Yard B, Waldherr R, Merz W, Kloeting I, Schleicher E, Mentz S, Abd el Baki RF, Tritschler H, Kasper M, Schwenger V, Hamann A, Dugi KA, Schmidt AM, Stern D, Ziegler R, Haering HU, Andrassy M, van der Woude F, Nawroth PP. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51:3532–3544. doi: 10.2337/diabetes.51.12.3532. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Sturgis L, Haidacher J, Zhang XN, Sherwood SJ, Bjercke RJ, Juhasz O, Crow MT, Tilton RG, Denner L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-κB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495–1504. doi: 10.2337/diabetes.50.6.1495. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21ras-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- Ellenrieder V, Hendler SF, Boeck W, Seufferlein T, Menke A, Ruhland C, Adler G, Gress TM. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61:4222–4228. [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Rauvala H. Receptor for advanced glycation end products (RAGE) signaling induces CREB-dependent chromogranin expression during neuronal differentiation. J Biol Chem. 2002;277:38635–38644. doi: 10.1074/jbc.M202515200. [DOI] [PubMed] [Google Scholar]
- Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-β on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol. 2002;13:1464–1472. doi: 10.1097/01.asn.0000014252.37680.e4. [DOI] [PubMed] [Google Scholar]
- Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC. A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem. 1995;43:97–102. doi: 10.1177/43.1.7822770. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, Okamoto H, Yamamoto H. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–268. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Stern D. M:rAGE: a new target for the prevention and treatment of the vascular and inflammatory complications of diabetes. Trends Endocrinol Metab. 2000;11:368–375. doi: 10.1016/s1043-2760(00)00311-8. [DOI] [PubMed] [Google Scholar]
- Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor β response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31:1094–1106. doi: 10.1053/he.2000.6126. [DOI] [PubMed] [Google Scholar]
- Pugliese G, Pricci F, Romeo G, Pugliese F, Mene P, Giannini S, Cresci B, Galli G, Rotella CM, Vlassara H, Di Mario U. Up-regulation of mesangial growth factor and extracellular matrix synthesis by advanced glycation end products via a receptor-mediated mechanism. Diabetes. 1997;46:1881–1887. doi: 10.2337/diab.46.11.1881. [DOI] [PubMed] [Google Scholar]
- Throckmorton DC, Brogden AP, Min B, Rasmussen H, Kashgarian M. PDGF and TGF-β mediate collagen production by mesangial cells exposed to advanced glycosylation end products. Kidney Int. 1995;48:111–117. doi: 10.1038/ki.1995.274. [DOI] [PubMed] [Google Scholar]
- Li JH, Huang XR, Zhu HJ, Oldfield M, Cooper M, Truong LD, Johnson RJ, Lan HY. Advanced glycation end products activate Smad signaling via TGF-β-dependent and -independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 2004;18:176–178. doi: 10.1096/fj.02-1117fje. [Epub ahead of print April 22, 2003] [DOI] [PubMed] [Google Scholar]
- Bottinger EP, Bitzer M. TGF-β signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- Lan HY. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003;12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]