Abstract
Wasting and renal diseases are frequent complications of HIV (human immunodeficiency virus) infection and are associated with accelerated disease progression and increased mortality. Transgenic mice expressing HIV1 under control of the CD4 promoter develop an AIDS-like disease and were used in the present work to study HIV1-induced wasting and kidney pathology. In this study, we reported that disease evolution paralleled increases in serum urea and creatinine levels, indicating an early and progressive deterioration of kidney function; meanwhile the wasting syndrome characterized by up-regulation of the ubiquitine-proteasome pathway and increased level of serum 3-methyl-histidine levels occurred at later stages just prior to death. Further examination of kidney and muscle pathologies revealed a progressive accumulation of CD45+ cells, first affecting the kidneys. In addition, the onset of disease is accompanied by elevated levels of circulating “regulated on activation, normal and secreted T cell expressed and secreted” (RANTES). These results prompted us to assess the effects of AS602868, a specific small molecule inhibitor of IkappaB kinase 2 (IKK2) on disease progression. Inhibition of the NF-κB pathway indeed resulted in increased lifespan, kidney and lean body mass preservation. These beneficial results were associated with a reduction of CD45+ cells infiltrating the kidneys, amelioration of the renal architecture, and reduced level of circulating RANTES. Together our data provide evidence that IKK2 inhibitors have therapeutic relevance in the treatment of HIV1-associated disorders.
Human immunodeficiency virus 1 (HIV1)-infected patients develop a multi-organ disease, of which wasting and HIV-associated nephropathy (HIVAN) are frequent complication associated with accelerated disease progression1–3 and increased mortality.4–6 The etiology of the cachexia is likely to be multi-factorial, resulting from interactions between decreased caloric intake, malabsorption and altered energy utilization or expenditure secondary to hormonal and/or metabolic abnormalities.3 There is also extensive evidence implicating cytokines in the development of acquired immunodeficiency syndrome (AIDS)-associated wasting.7 Because AIDS involves a variety of pathologies ranging from opportunistic infections to kidney and cardiac failure, it is relevant, in the quest to find new treatments, to determine whether any of these pathologies can mediate the cachexia process.
In most clinical situations, limited access to human tissue leads to the dependence for experimentation using relevant animal models of disease. The transgenic (Tg) mouse line expressing the entire HIV1 coding sequence under the control of the regulatory sequences of the human CD4 gene8 spontaneously develop many pathologies that closely resemble those observed in HIV-infected patients, such as the severe atrophy of the lymphoid organs, wasting, and pathology of the kidney, lung, and heart, leading to premature death.8,9 As in humans, these pathologies have been widely associated with mononuclear cell infiltration within diseased organs. This model therefore offers a unique opportunity to follow the development of associated pathologies of an AIDS-like syndrome in vivo as well as to test potential therapeutic strategies.
For this purpose, we first characterized the course of disease progression by following the evolution of metabolic parameters in the serum of these transgenic animals. Our results clearly demonstrated that kidneys are affected as early events of the disease. Disease onset was also associated with increased circulating levels of the protein, “regulated on activation, normal and secreted T cell expressed and secreted” (RANTES). At later stages of the disease, these Tg animals also suffered from severe cachexia. In addition, a skeletal muscle wasting syndrome was observed sharing similarities with other well-described models of wasting such as cancer cachexia.10
The kidney and muscle pathologies were both associated with accumulation of leukocytes within diseased organs. Interestingly, infiltration of the kidneys with mononuclear cells preceded infiltration of the skeletal muscles, providing further evidence that kidney failure developed before the initiation of weight loss. As an approach to modulate disease progression, we tested the effect of the compound, AS602868, a specific chemical inhibitor of IkappaB kinase 2 (IKK2).11 Indeed, nuclear factor kappa B (NF-κB) activation is associated with the inflammatory states12–14 and is involved in the regulation of RANTES expression.15 IKK2 is the main upstream kinase responsible for NF-κB-dependent gene expression, and thus represents an attractive therapeutic target in this model.
The therapeutic potential of AS602868 was first evaluated via the ex vivo treatment of cultured kidney cells. After 48 hours of culture, this IKK2 inhibitor dose-dependently inhibited the production of endogenous chemokines and pro-inflammatory cytokines. The effect was observed both in the absence or presence of exogenous TNF-α. Following oral administration, AS602868 treatment significantly increased survival times, alleviated kidney pathology, and preserved muscle and lean body mass. These beneficial effects were accompanied by a reduction of circulating RANTES. Our data further define the wasting and kidney syndromes developed by the CD4C/HIV1 transgenic mouse line and demonstrate the potential therapeutic value of IKK2 inhibitors in the treatment of HIV1-associated inflammatory disorders.
Materials and Methods
Animals
The generation and characterization of the CD4C/HIVMutA Tg mice have been previously described.8,9 Heterozygote transgenic mice were obtained by crossing CD4C/HIV transgenic males from Dr. P. Jolicoeur’s Laboratory with wild-type C3H females (IFFA-CREDO, France). Animals were housed in specific pathogen-free facilities and had free access to water and a standard diet chow. Except for the first survival study, only females were enrolled in subsequent experiments.
Categorization of Disease Severity
Three disease stages were defined as a function of serum urea levels. Twelve to 40 mmol/L were observed in young healthy Tg animals and designated as stage Tg1, 40 to 80 mmol/L as an intermediate (stage Tg2) disease stage, and >80 mmol/L in the most severely diseased animals (stage Tg3).
Serum Analyses
Mouse serum was collected in BD microtainer SST tubes (BD, Franklin Lakes, NJ) and centrifuged at 10,000 rpm for 10 minutes. Sera were analyzed for different biological parameters (namely cholesterol, triglycerides, aspartate amino-transferase (ASAT), alanine aminotransferase (ALAT), creatinine, albumin, glucose, urea, and lipase) immediately after collection using a Cobas Mira Plus automated analyzer (Roche Diagnostics).
Serum 3-methylhistidine levels were determined by HPLC. Proteins were precipitated by adding 1 ml of chilled ethanol (−20°C) to 100 μl of serum diluted 1:2 with 0.9% NaCl. After overnight incubation at −20°C, the solution was centrifuged at 14,000 rpm for 10 minutes. Nine-hundred μl of supernatant were dried under vacuum and re-suspended in 25 μl of water. Amino acids were quantified using a Waters AccQ.Tag chemistry and a Hewlett Packard HP1090 liquid chromatograph.
The level of circulating RANTES was determined in serum by ELISA accordingly to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). To assess inflammation status, we measured the levels of several pro-inflammatory cytokines (IL-10, IL-12p70, IL-6, TNF-α, and IFN-γ and MCP-1) in mouse serum using cytometric bead array (CBA) kit (BD Pharmingen) and according to the manufacturer’s instructions.
Histopathology
Mice were anesthetized with pentobarbital (Sanofi, France), and then perfused intracardially with 4% paraformaldehyde. Kidneys and soleus muscles were immediately collected and immersion-fixed overnight in 4% paraformaldehyde in PBS. Organs were embedded in paraffin and 5-μm sections were stained with hematoxylin-eosin (Merck) and then subjected to histological analysis. The presence of leukocytes was assayed by immunostaining on paraffin sections with a biotinylated monoclonal antibody to mouse CD45 (30-F11, Pharmingen). Sections were first deparaffinized, treated with PBS containing 0.03% H202 to block endogenous peroxidase activity, followed by microwave treatment in 10 mmol/L sodium citrate buffer. Sections were then incubated with the antibody (1/100) overnight at 4°C. Biotinylated antibody was developed with the StepABComplex kit (DAKO) and peroxidase activity was detected using FAST DAB (Sigma Aldrich, St. Louis, MO). Samples were analyzed in a blinded manner by two examiners (A.H. and Y.S.).
Single-Cell Suspension
Single-cell suspensions were prepared from the spleen, kidneys, and gastrocnemius/soleus muscles. Briefly, mice were anesthetized with pentobarbital, perfused intracardially with ice-cold PBS, and the organs were removed and weighed. Cells were obtained by sequential enzymatic digestion in Iscove’s medium, containing Collagenase IV (8 mg/ml) (Worthington Biochemical Corp, Lakewood, NJ) and DNase (1% in PBS, Sigma), 3 × 20 minutes, at 37°C with gentle shaking. Cells were harvested and transferred into 4 ml of Iscove’s medium/1 ml FCS (GIBCO) on ice. Cells were then washed twice with fresh medium and filtered through a 70-μm cell strainer (BD Falcon) before proceeding with further analysis.
Analysis of Inflammatory Infiltrates
All flow-cytometric analysis for surface antigens was performed using a phycoerythrin (PE)-coupled monoclonal antibody directed against mouse CD45 (30-F11) obtained from BD Pharmingen (San Diego, California). Single-cell suspensions were pre-incubated with the 2.4G2 monoclonal antibodies (BD Pharmingen) to block FcγR-binding, and then incubated with the relevant monoclonal antibody for 30 minutes on ice. Subsequently, cells were washed twice with fresh medium and analyzed with a FACScalibur (BD Biosciences, Mountain View, CA) with CellQuest software.
Northern Blot
Total RNA was extracted from the gastrocnemius muscle using the Trizol (Gibco BRL) procedure. Twenty-five μg samples of total RNA were electrophoresed on 1.2% agarose gels and then transferred and covalently bound to nylon HYBOND N+ membrane. Pre-hybridization for 2 hours and hybridization overnight were performed at 68°C in ExpressHyb hybridization solution (BD Biosciences), followed by successive washes in 2X SSC/1% SDS and 0.5X SSC/0.05% SDS. Membranes were hybridized with 32P cDNA fragments labeled by random priming (High Prime, Boehringer Mannheim). Probes encoding ubiquitin or the C2 and C9 subunits of the proteasome were used. After stripping, the membranes were re-probed with a cDNA fragment encoding GAPDH.
Treatment with an IKK2 Inhibitor
A potent, reversible and ATP-competitive inhibitor of IKK2 with an IC50 = 62 nmol/L (Ki = 20 nmol/L) has been identified and designated AS602868. This compound is able to prevent TNF-induced IκB-α phosphorylation and NF-κB activation in Jurkat leukemic T cells as well as the TNF-induced-disruption of mitochondrial potential, -decrease in anti-apoptotic gene transcription, -caspase activation, and -DNA fragmentation.11 On oral administration, AS602868 suppressed TNF-α production in response to LPS challenge in mice (ED50 4 mg/kg).
Ex Vivo Effects of AS602868
To assess the effects of AS602868 treatment on chemokine and cytokine expression, kidney single-cell suspensions, prepared as described previously, were cultured at 5 105 cells per well in 96-well plates and pre-incubated for 30 minutes in the absence or presence of increasing concentrations of AS602868. The following culture medium was used: RPMI 1640 with 10% fetal calf serum (FCS), streptomycin, penicillin, and L-glutamine. Cells were either stimulated with TNF-α (600U/well) or not. Fifty μl out of 150 μl total volume of supernatants were harvested after 48 hours and assayed for IL-10, IL-6, IL12, TNF-α, IFN-γ, and MCP-1 using the mouse inflammation cytometric bead array kit (BD Pharmingen) and for RANTES using ELISA (R&D). All kits were used according to the manufacturer’s instructions.
In Vivo Effects of AS602868
To assess the effects of AS602868 on disease progression, age-matched CD4C/HIV Tg females were chosen according to their serum urea level (stage Tg1, ie, between 12 and 20 mmol/L) at the beginning of the experiment (n = 6/group). Six times per week the mice received an oral administration of 150 μl of the IKK2 inhibitor (45 mg/kg) or vehicle (0.5% carboxymethylcellulose/Tween 0.25% (Sigma)). Disease progression was evaluated at weekly intervals by monitoring body weight and serum parameters. To study the effects of a short-term treatment, mice were sacrificed after 8 weeks of treatment. Organs of interest such as kidneys, gastrocnemius muscles, and spleen were removed and weighed. One kidney and both muscles were enzyme digested and subsequently analyzed by flow cytometry for the quantification of CD45+ cells. The second kidney was used for histology. Results of two independent experiments are shown. For the survival study, mice were enrolled in the experiment when their serum urea level reached between 12 to 20 mmol/L and were then treated six times per week with either 15 or 45 mg/kg of the IKK2 inhibitor or the vehicle alone. Animals were sacrificed when the serum urea level was above 100 mmol/L. Both serum biological parameters and body weight were monitored weekly.
Statistical Analysis
The statistical significance was determined by performing the two-tailed Student’s t-test. Survival was analyzed by the Kaplan-Meier method, and the significance of differences was determined by the log-rank test.
Results
Disease Progression in the CD4C/HIV1 Transgenic Model
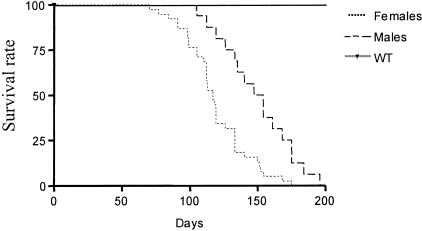
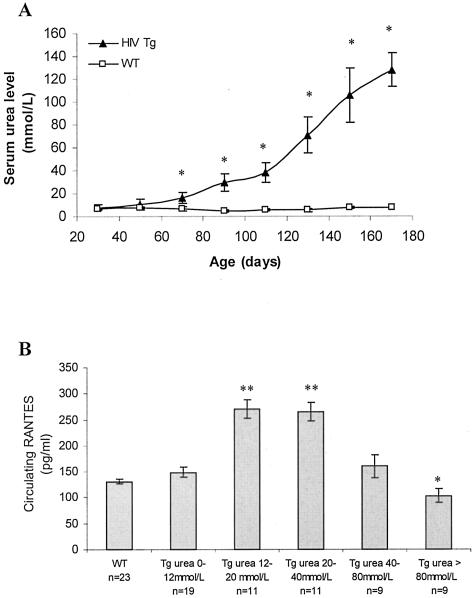
Premature death and multiple organ involvement are observed in this murine CD4C/HIV transgenic model.8 Indeed, the lifespan of these transgenic animals is limited with that of females being shorter than males (117 days versus 150, respectively, Figure 1). The external manifestations of the disease include hypoactivity, weakness, failure to thrive, and ruffled coat appearance that usually appear during the fourth month of age and are predictive of near death. However, the course of events leading to their dramatically reduced survival time still remains to be determined. To address this issue, the levels of several serum parameters were evaluated in CD4C/HIV Tg animals at different ages, before and after the appearance of the previously described externally visible symptoms. During the early stage of disease, altered serum markers were indicative of kidney dysfunction, such as urea, creatinine, and albumin levels, as well as a lipid metabolic disturbance reflected by cholesterol and lipase activity (Table 1). In contrast, at later stages, many more parameters were altered including markers of wasting such as creatine kinase (Table 1), providing evidence that the process of wasting commences after the initiation of kidney impairment. However, the most striking change observed occurred with serum urea levels, increasing up to 17-fold. The serum urea level increased progressively, reaching a statistically significant difference at 70 days of age, whereas no changes with age occurred in WT animals (Figure 2A). As CD4C/HIV Tg mice have a heterogeneous disease onset, there was a need to have an appropriate marker to follow disease progression. Based on the above results, the serum urea level was chosen as the marker to define disease stages, with 12 to 40 mmol/L as the early-stage range (Tg1), 40 to 80 mmol/L as the intermediate (Tg2), and >80 mmol/L for the most severely diseased animals (Tg3).
Figure 1.
Effect of HIV1 transgene expression on mice survival. The survival of CD4C/HIV1 transgenic mice is markedly reduced compared to non-transgenic C3H controls. In addition CD4C/HIV1 transgenic males (n = 15) survive longer than females (n = 45) (P = 0.0005, log-rank test).
Table 1.
Evolution with Age of Serum Biological Parameters in WT and Tg Animals
| WT | HIV-Tg (0–50 days) | HIV-Tg (50–100 days) | HIV-Tg (>100 days) | |
|---|---|---|---|---|
| Cholesterol (mmol/L) | 2.98 ± 0.14 | 5.95 ± 0.50** | 9.09 ± 0.50*** | 8.80 ± 0.73*** |
| Creatinine (μmol/L) | 42.51 ± 5.12 | 48.49 ± 4.14 | 111.52 ± 7.29*** | 140.08 ± 10.95*** |
| Glucose (mmol/L) | 8.41 ± 0.24 | 8.59 ± 0.31 | 7.53 ± 0.17 | 5.65 ± 0.80*** |
| Triglycerides (mmol/L) | 2.28 ± 0.19 | 2.48 ± 0.37 | 2.15 ± 0.22 | 2.29 ± 0.41 |
| Urea (mmol/L) | 7.57 ± 0.55 | 13.04 ± 1.01* | 52.62 ± 1.88*** | 127.06 ± 6.14*** |
| Albumin (g/L) | 44.91 ± 1.42 | 33.73 ± 1.93*** | 29.94 ± 1.54*** | 21.49 ± 1.01*** |
| ASAT (U/L) | 192.92 ± 22.28 | 152.13 ± 21.59 | 157.28 ± 8.21 | 480.62 ± 135.3 |
| ALAT (U/L) | 102.43 ± 17.21 | 77.67 ± 12.38 | 90.47 ± 11.88 | 117.20 ± 14.52* |
| CK (U/L) | 1506.0 ± 302.3 | 1278.3 ± 383.7 | 1081.3 ± 227.5 | 4686.6 ± 1122.4* |
| LDH (U/L) | 883.6 ± 119.2 | 976.0 ± 88.0 | 713.5 ± 104.8 | 816.0 ± 722.3 |
| Lipase (U/L) | 45.33 ± 6.88 | 57.07 ± 3.71 | 82.62 ± 4.00*** | 55.29 ± 8.84 |
Serum biological parameters were evaluated in WT (n = 20) and CD4C/HIV transgenic animals at different ages (n = 20/group). No significant variations with age were observed in WT animals. Data are means ± SD.
, P < 0.05;
, P < 0.01;
, P < 0.005.
Figure 2.
A: Evolution with time of serum urea level. Serum urea level was determined in WT and CD4C/HIV Tg animals at different ages. Data are means ± SD. *, P < 0.01. As such, serum urea level was thereafter used as a marker of disease progression. B: Evolution of circulating RANTES level in WT and CD4C/HIV1 Tg animals, grouped according to their serum urea level. Data are means ± SD. *, P < 0.05; **, P < 0.001
In many clinical situations including AIDS, pro-inflammatory cytokines and chemokines are involved in disease pathogenesis. As such, the levels of these immune mediators were determined in the serum of age-matched WT and transgenic animals at different disease stages. The levels of several pro-inflammatory cytokines (IL-6, IL-10, IL-12p70, and IFN-γ) were not altered in the serum of Tg animals (Table 2). TNF-α and MCP-1 levels were slightly increased in transgenic animals, independently of disease severity (data not shown). In contrast, a significant increase in circulating RANTES was observed. In addition, a correlation between elevated levels of RANTES and disease onset and progression (Figure 2B) was observed, suggesting that RANTES may be involved in disease pathogenesis.
Table 2.
Comparison of Pro-Inflammatory Cytokines and MCP-1 Levels in WT and CD4C/HIV1
| IL-12 (pg/ml) | TNF-α (pg/ml) | IFN-γ (pg/ml) | MCP-1 (pg/ml) | IL-10 (pg/ml) | IL-6 (pg/ml) | |
|---|---|---|---|---|---|---|
| WT (n = 15) | 0.00 | 1.48 ± 2.56 | 0.00 | 35.25 ± 11.25 | 0.00 | 0.66 ± 1.48 |
| CD4C/HIV1 Tg (n = 19) | 0.00 | 30.81 ± 14.43 | 0.09 ± 0.39 | 51.43 ± 26.8 | 2.72 ± 11.54 | 1.24 ± 1.76 |
| P value | <0.001 | ns | <0.05 | ns | ns |
Serum pro-inflammatory cytokines and MCP-1 were determined using CBA kit.
Values are expressed as mean ± SD; ns, not significant.
Kidneys and Skeletal Muscles Are Severely Affected by the HIV1 Transgene Expression
As suggested by the analysis of serum parameters, kidney and muscle function appeared to be severely affected by the expression of the HIV1 proteins. Autopsy revealed severe atrophy of the kidneys, the specific loss of the epidydimal fat, and decreased skeletal muscle weights (soleus, gastrocnemius, tibialis) (Table 3). A significant total body weight loss was also observed. However, as some mice develop edema, comparison of the carcass weight provided a more relevant representation of the muscle and lean body mass wasting than the wet weight (49% decrease versus 22%, respectively, Table 3). However, macroscopic examination revealed that not all organs appeared atrophied in late-stage disease, as indicated by unaltered liver, heart, and brain weights.
Table 3.
Decreased Fat, Kidney and Skeletal Muscle Individual Weight in CD4C/HIV1 Diseased Animals (Stage Tg3)
| WT (n = 18) | HIV-Tg (n = 31) | % Decline | |
|---|---|---|---|
| Body weight (g) | 26.44 ± 3.54 | 20.57 ± 2.89 | 22*** |
| Carcass (g) | 5.98 ± 1.43 | 3.04 ± 1.83 | 49*** |
| Epididymal fat (mg) | 282.2 ± 79.4 | 53.7 ± 4.3 | 81*** |
| Soleus (mg) | 7.65 ± 2.6 | 5.9 ± 0.4 | 23* |
| Gastrocnemius (mg) | 131.2 ± 29.7 | 92.6 ± 3.4 | 29*** |
| Tibialis (mg) | 55.0 ± 8.7 | 37.7 ± 3.6 | 31** |
| Kidneys (mg) | 193.5 ± 51.6 | 103.8 ± 8.5 | 44*** |
| Spleen (mg) | 90.5 ± 19.3 | 80.3 ± 11.1 | 7 |
| Brain (mg) | 462.6 ± 6.6 | 453.0 ± 5.5 | 2 |
| Liver (g) | 1.33 ± 0.17 | 1.1 ± 0.08 | 12* |
| Heart (mg) | 131.1 ± 17.8 | 142.8 ± 8.5 | −7 |
Data are means ± SD.
, P < 0.05;
, P < 0.01;
, P < 0.005.
Skeletal muscle weight loss along with the specific depletion of fat reserves are indicative of an HIV-1-associated wasting disease process also encountered in a relatively high percentage of AIDS patients.3,16,17 To provide information on the molecular mechanisms leading to a muscle wasting process in this model, the expression of the ubiquitin-proteasome pathway related proteins, a proteolytic pathway responsible for the accelerated proteolysis seen in a variety of wasting conditions18–20 were studied. By Northern blot analysis, increased levels of ubiquitin and the 20S proteasome C2 and C9 α-subunit proteins in the gastrocnemius muscle of diseased animals were observed (Figure 3A). In addition, the serum level of 3-methyl-histidine, a well-known and widely accepted specific marker of myosin breakdown,21,22 was significantly elevated (fivefold increase) in the CD4C/HIV Tg group compared to WT mice (Figure 3B). These data are consistent of a wasting process linked to increased protein degradation.
Figure 3.
Documentation of a muscle wasting process in CD4C/HIV1 transgenic diseased animals. A: Up-regulation of the ubiquitin-proteasome pathways in CD4C/HIV transgenic gastrocnemius muscles. Northern blot analysis of the expression of ubiquitin, the C2, and C9 proteasome core proteins. GADPH serves as an internal control. B: Increased 3-methylhistidine level in the serum of CD4C/HIV Tg animals (WT, n = 4; CD4C/HIV Tg, disease stage Tg3, n = 4). *, P < 0.005.
Kidney Damage Precedes Muscle Structure Disorganization
We next investigated the extent of the kidney and skeletal muscle pathology. H&E-stained paraffin sections of kidney and soleus muscle from WT and CD4C/HIV Tg animals from all disease stages were prepared and analyzed. Renal damage was detected early in disease progression (stage Tg1; Figure 4D), consistent with the early increase in serum urea levels. The renal tubules presented microcystic tubular and tubulointerstitial pathology characterized by tubular degeneration and mononuclear cell infiltration (Figure 4, D and F). In contrast, the skeletal muscle histopathology of age-matched WT and early-stage transgenic animals (stage Tg1) presented similar anatomy (Figure 4, A and C); only sections from CD4C/HIV Tg mice at stage Tg3 (Figure 4E) were altered from controls. Analysis of stage Tg3 skeletal muscle specimens revealed the presence of numerous infiltrating cells, abnormally small rounded muscle fibers with highly variable diameters, and consistently paler coloration than that of age-matched WT controls (Figure 4, F and G). Infiltration of leukocytes into the kidneys and skeletal muscles of transgenic mice was confirmed by immunostaining with an anti-CD45 (ie, pan leukocyte) antibody (Figure 4H). These data confirmed that during disease development, alterations to kidney structure and function precedes skeletal muscle pathology.
Figure 4.
Muscle and kidney histopathology in WT and in CD4C/HIV transgenic mice at disease stage Tg1 and Tg3. A, C, and E: H&E staining of 5-μm soleus transversal sections from WT, Tg1, and Tg3 CD4C/HIV animals, respectively. Mononuclear cell infiltrate is only observed in diseased animals (Tg3) whereas muscle sections are similar in WT and CD4C/HIV Tg1 animals. Representative pictures are shown (×40). B, D, and F: H&E staining of WT, Tg1, and Tg3 CD4C/HIV kidney sections. Severe kidney damage is already detected in young transgenic animals (Tg1) (×20). G: Higher magnification of Tg3 soleus muscle section revealing the presence of numerous infiltrating cells (×60) and (H), immunostaining with an anti-CD45 antibody of Tg3 kidney sections revealing the presence of infiltrating leukocytes (×20). Samples were analyzed in a blinded manner by two examiners (A.H. and Y.S.)
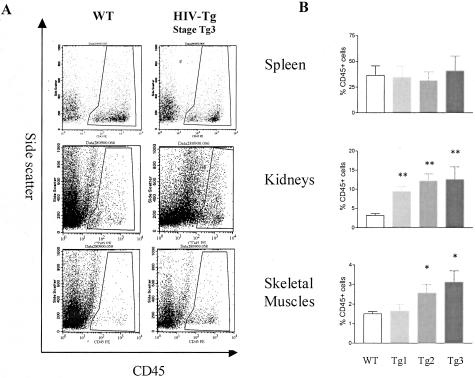
To further document whether the leukocyte infiltration observed by histology was an early event in HIV disease progression, the cellular infiltration was analyzed by flow cytometry. Single-cell suspensions obtained after sequential enzymatic digestion of the spleen, kidneys, and gastrocnemius muscles were labeled with the anti-CD45 antibody and the proportion of hematopoietic cells present within the organ of WT and CD4C/HIV Tg mice quantified (Figure 5A). While FACS analysis of the spleen showed no dramatic change with disease progression, the proportion of CD45+ cells increased in both the kidneys as well as skeletal muscles of the CD4C/HIV Tg animals (Figure 5B). In the kidneys, a higher proportion of CD45+ cells was already observed in the Tg1 stage and increased progressively to reach 12% of the total population of kidney cells at stage Tg3. In contrast, the infiltration observed in the skeletal muscles appeared as a later event only when animals were classified in the Tg2 stage.
Figure 5.
Cellular infiltrates quantification by flow cytometry. (A and B) Spleen (top), kidney (middle) and skeletal muscle (bottom) single-cell suspensions were obtained by sequential enzymatic digestion and then processed for flow cytometric analysis. A: FACS analysis reveals an increased proportion of CD45+ cells in the kidneys and the gastrocnemius muscles from CD4C/HIV transgenic diseased mouse (urea >80 mmol/L, stage Tg3; right) compared to age-matched WT (left). Representative profiles are shown; results from multiple experiments are summarized in B. B: The proportion of CD45+ cells has been evaluated in the spleen, kidneys, and gastrocnemius muscles from WT (n = 21), and CD4C/HIV transgenic mice at different disease stages Tg1, Tg2, and Tg3 (Tg 1: urea 12 to 40 mmol/L, n = 17; Tg2: urea 40 to 80 mmol/L, n = 11; Tg3: urea >80 mmol/L, n = 8). Data are means ± SEM (* P < 0.05; ** P < 0.001).
An IKK2 Inhibitor Reduces Chemokine and Pro-Inflammatory Cytokines Production in Ex Vivo Kidney Cell Preparation
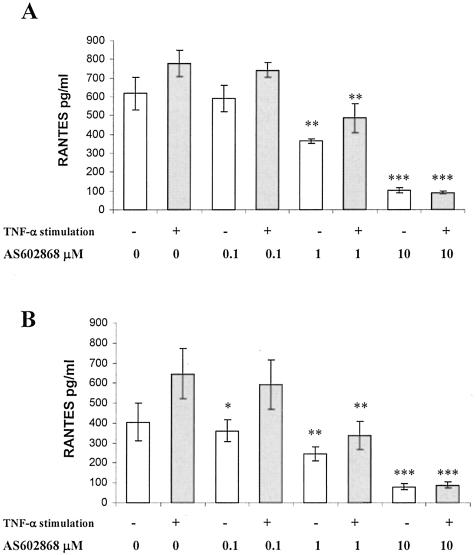
As the NF-κB pathway is responsible for key signaling events leading to cellular infiltration and elevated RANTES levels,15,23 we questioned whether the disease progression observed in our study could be altered using a small molecular weight inhibitor of NF-κB. In a first series of experiments, the effect of AS602868, a specific inhibitor via IKK2 and the downstream expression of genes transcriptionally regulated by NF-κB, was tested in an ex vivo assay. Single-cell suspensions of kidney cells of age-matched WT and CD4C/HIV1-Tg mice were obtained by enzymatic digestion. AS602868 dose-dependently prevented RANTES production by WT or Tg kidney cell suspensions, with or without the presence of TNF-α (Figure 6). Similar results were obtained while studying the effects of AS602868 on pro-inflammatory cytokines expression by cultured kidney cells (data not shown). AS602868 is thus able to reduce chemokine and cytokine expression in kidney cell ex vivo culture.
Figure 6.
AS602868 dose-dependently inhibits RANTES expression in kidney cell cultures. Single-cell suspensions obtained after enzymatic digestion of age-matched WT kidney (A) and Tg2 transgenic mice (B) were incubated with increasing doses of AS602868 and 30 minutes later, TNF-α (600U/well) or medium alone was added, the cell supernatants then harvested after 48 hours and analyzed for RANTES expression by ELISA. Each condition was analyzed in triplicate. Data are means ± SD. Experiment was repeated twice (WT, n = 4; CD4C/HIV1 Tg stage Tg2, n = 4), (*, P < 0.05; **, P < 0.01; ***, P < 0.005 versus control without inhibitor).
Treatment with an IKK2 Inhibitor Delays Renal Pathology and Preserves Muscle Mass
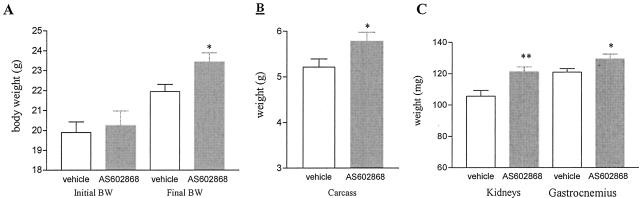
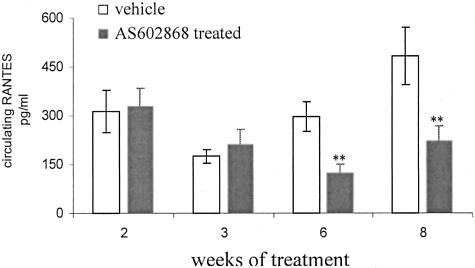
As AS602868 inhibited NF-κB regulated-genes in the CD4C/HIV1 transgenic kidney cell culture assay, we next investigated its in vivo effect on disease progression. First, the effects of an 8-week treatment were evaluated. Seventy- to 90-day-old mice with a urea level between 12 to 20 mmol/L were treated six times per week during 8 weeks with an oral dose of either the IKK2 inhibitor (45 mg/kg) or the vehicle alone. Disease progression was evaluated weekly by monitoring body weight and serum parameters. As shown in Figure 7A, mice treated with the IKK2 inhibitor gained more weight than the vehicle-treated animals (body weight at the end of the treatment period: 23.43 grams for AS602868 versus 21.95 grams for vehicle-treated animals). At the end of the treatment period, animals were sacrificed and the organs of interest analyzed. The total body weight gain, previously observed in IKK2 inhibitor-treated mice, was confirmed when the dried carcass mass was measured (5.78 grams for AS602868 versus 5.21 grams for vehicle-treated animals) (Figure 7B). The observed reduction of kidney and gastrocnemius muscle weights was less pronounced in the group receiving AS602868 (kidney weight: 121+/−3.2 mg versus 105+/−3.6 mg for vehicle-treated animals, age-matched controls, 142+/−22.4 mg; gastrocnemius: 131+/−3.0 mg, versus 120+/−2.1 mg, age-matched controls 129+/−12.4 mg) (Figure 7C). In addition, IKK2 inhibition resulted in an amelioration of the kidney pathology as indicated by the reduced proportion of CD45+ cells and the preserved renal architecture (Figure 8, A and B). The tubular atrophy was less pronounced in the AS602868-treated group. These beneficial effects were accompanied by reduced level of circulating RANTES (Figure 9). Due to the young age of mice enrolled in this study, no difference in the proportion of CD45+ cells infiltrating the muscles was detected (data not shown).
Figure 7.
Effect of an IKK2 inhibitor (AS602868, 45 mg/kg, six times per week) during 8 weeks of short-term treatment on total body weight, carcass, and kidneys and gastrocnemius individual weights. A: Total body weight at the beginning and at the end of the 8-week treatment period. Note that young mice (around 70 days) were enrolled in this study and therefore are still gaining weight. B: Carcass weight (age-matched non-transgenic control mice, 5.8 ± 0.7 grams). C: Kidneys and gastrocnemius muscle weights are partially preserved at the end of the treatment period (kidney and muscle weights of age-matched non-transgenic controls, 142 ± 22.4 mg and 129 ± 12.4 mg, respectively). *, P < 0.05; ** P < 0.005.
Figure 8.
Amelioration of kidney pathology with an IKK2 inhibitor (AS602868) after 8 weeks of treatment. A: The proportion of CD45+ cells infiltrating the kidneys is significantly reduced in AS602868-treated (n = 8) compared to vehicle-treated animals (n = 8). **, P < 0.01. Horizontal bar indicates the basal level of CD45+ cells in non-transgenic controls. B: H&E kidney histology (×40); vehicle-treated animals (top) and AS602868-treated animals (bottom) showing that kidney structure was less damaged in AS602868-treated group, in particular tubular atrophy is less pronounced. Eight animals per group analyzed, at least three sections/animals were examined. Samples were analyzed in a blinded manner by two examiners (A.H. and Y.S.) Three representative pictures are shown for each group.
Figure 9.
IKK2 inhibitor (AS602868) reduces the level of circulating RANTES. Serum levels of circulating RANTES were determined by ELISA in the serum of vehicle-treated (n = 8) and AS602868-treated animals (n = 8) after 2, 3, 6, and 8 weeks of treatment. Results are expressed as means ± SD. **, P < 0.001
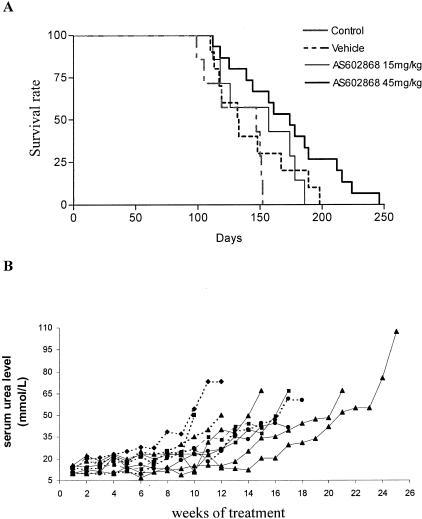
As the 8-week course of treatment provided significant improvement in disease symptoms and progression, we next evaluated the effects of a long-term treatment. In this study, two doses of the IKK2 inhibitor, ie, 15 and 45 mg/kg were included. No significant effect on survival was achieved with the lower dose, while the lifespan of CD4C/HIV Tg animals treated with 45 mg/kg of the IKK2 inhibitor was significantly increased (P = 0.0357) and in some animals exceeded 200 days (Figure 10A). Although AS602868 treatment was not sufficient to block disease totally, the increase in serum urea level was delayed in the IKK2 inhibitor-treated group (45 mg/kg) as compared to vehicle-treated animals (Figure 10B). These data confirm that the beneficial effect observed after 8 weeks of treatment could lead to an increased survival when the treatment is prolonged. These data support the notion that IKK2 and more generally the NF-κB system plays a significant role in the development of kidney pathology and decreased lean body mass observed in the spontaneous disease of CD4C/HIV Tg mice.
Figure 10.
AS602868 long-term treatment delays disease progression and dose-dependently increases survival rates. A: Long-term administration of AS602868 increases survival rates at 45 mg/kg (P = 0.0357, log-rank test). Control non-treated, n = 9; vehicle-, n = 11; AS602868-treated 45 mg/kg, n = 16; 15 mg/kg, n = 8. B: Serum urea level was monitored at weekly intervals in vehicle-treated (dashed line, n = 4) and AS602868-treated mice (solid line, n = 5). Representative profiles of three experiments are shown.
Discussion
In the present study, we have documented the evolution of the AIDS-like disease which develops in the CD4C/HIV1 transgenic mouse line. Analysis of serum parameters provides further insight into one of the predominate features, ie, kidney destruction and eventual failure. Indeed, progressive deterioration of renal structure and function participated in disease evolution. At late-stage disease, the CD4C/HIV1 Tg animals also suffer from a severe wasting process that is likely due to an increased protein breakdown as shown by the up-regulation of the ubiquitin-proteasome pathway and the elevated level of serum 3-methylhistidine. This result is in accordance with previous studies reporting that among the different proteolytic pathways responsible for protein catabolism, the ubiquitin-proteasome is considered to be the most important in a range of catabolic states such as cancer24 and chronic renal failure.25 Currently, process wasting associated with AIDS is poorly understood. However, Llovera and colleagues26 have also observed an up-regulation of the ubiquitin-proteasome pathway in the skeletal muscles of AIDS patients.
In contrast to these similarities, certain features differentiate the wasting syndrome manifested in CD4C/HIV1 Tg mice versus human AIDS.27 Diarrhea, gastrointestinal disturbances, or hypertriglyceridemia are not observed in this mouse line. Furthermore, in HIV-infected patients, rapid weight loss has been associated with acute infections,28 which the mice were not subjected to being house in SPF conditions. Therefore our data indicated that the wasting syndrome in this Tg model can develops in the absence of secondary opportunistic infections and in the absence of viral particles. No classical inflammatory cytokines detected in the serum of the CD4C/HIV Tg mice except TNF-α whose level remained marginal compared to those observed in the serum of cancer cachectic mice.29 Furthermore, using a genetic approach, these transgenic mice can develop the AIDS-like disease in the absence of IFNγ and IL-6.30 Therefore, these pro-cachectic factors are not essential for developing HIV-associated wasting in this animal model of disease which could have therapeutic implications.
In contrast, the increased levels of RANTES in the serum of the CD4C/HIV transgenic animals and the progressive accumulation of leukocytes in the kidneys and muscles could be of major importance to disease progression. Mononuclear cell infiltration in skeletal muscle has been reported in HIV-infected patients31 and the elevation of serum RANTES has been shown to correlate with rapid progression of the disease.32 Therefore, mononuclear cell recruitment may play a pivotal role not only in the kidney but also in the muscle pathologies associated with HIV1 protein expression. Interestingly, we have shown that in the CD4C/HIV Tg mice, appearance of inflammatory cells initially occurred in the kidneys, later in the skeletal muscles, and paralleled the increase in serum RANTES, suggesting that this chemokine is involved in their recruitment to diseased organs. This is consistent with previous data from Hanna et al8 reporting positive immunoreactivty against RANTES in damaged tissues. Kidney dysfunction was confirmed by an increase in serum urea and creatinine levels and by a severe atrophy and a dramatic destruction of kidney structures. Whether kidney failure participates in CD45+ cells infiltration in muscles and wasting is an unsolved question, and requires further investigation. However, there is precedent for this concept as chronic renal failure is associated with loss of lean body mass,33 likely due to specific stimulation of the ATP-dependent proteasome pathway in the muscle.25
Time-course characterization of disease progression allowed us to use this model to test therapeutic approach. As such, we tested the effect of oral administration of a chemical small molecule inhibitor of IKK2 activity, AS602868. We first showed that this compound was able to dose-dependently reduce the expression of RANTES in kidney cell culture. We next tested the in vivo effect of AS602868 on disease progression. Treatment with the AS602868 compound slows down CD45+ cell infiltration in kidney and also reduced muscle weight loss and favored body weight gain. However, in this experiment, the effects of AS602868 on proteolysis (transcription of the ubiquitine-proteasome pathway and serum 3-methyl-histidine levels) were not assayed. A possible explanation would be that IKK2 inhibitors interact with the activation of NF-κB pathway previously reported in some muscle wasting models.34 However, gel-shift experiments did not reveal evidence of NF-κB activation in the muscles of cachectic CD4C/HIV Tg mice (data not shown) and therefore suggest that IKK2 inhibitor is not directly acting on muscle. There is now increasing evidence that the NF-κB pathway plays a fundamental role in regulating acute inflammation and inhibition of this pathway with natural products, such as gliotoxin or parthenolide has already been successful in treating immune renal disease.35 More recently, Chen et al,36 demonstrated that genetic ablation of IKKβ in enterocytes prevented the systemic inflammatory response normally triggered by gut ischemia. However, IKKβ removal also resulted in severe apoptotic damage after reperfusion.
To our knowledge, this is the first evidence that inhibiting IKK2 function could have significant effect on HIV1-mediated disease progression. The exact mechanisms are still unknown. However, our experiments on ex vivo kidney cultured cells as well as the reduced level of circulating RANTES after 1 or 2 months of treatment suggests that this IKK2 inhibitor may act by modulating the transcription of genes encoding the expression of chemokines. This is supported by the fact that when AS602868 is administered to collagen-induced-arthritic mice, the decrease in disease incidence and severity is accompanied by a reduction in cytokines and chemokines production (Leceta J, Juarranz NJ, Delgado M, Dreano M, Abad C, Martinez C, submitted).
The possibility that treatment with IKK2 inhibitor decreases the transcription of HIV genes is weak. Indeed, the HIV-LTR, well known to contains functional NF-κB response elements,37,38 has been replaced by the CD4 promoter in the CD4C/HIV1-transgenic model. Therefore, the effect of the IKK2 inhibitor is likely not mediated by a regulation of the transgene expression. Further experiments are warranted to delineate the mechanism of action of the inhibitor in this model. It might be hypothesized that expression of the regulatory HIV proteins in CD4C/HIV transgenic mice, such as Nef that is a known inducer of NF-κB activation39 leads to the NK-κB-mediated release of pro-inflammatory cytokines as well as the release of RANTES.40
The fact that the IKK2 inhibitor was not sufficient to totally arrest CD45+ cell infiltration in kidney and consequently disease progression, suggests that to block the disease progression a multi-therapy approach may need to be undertaken.
In summary, we further document that the disease developed by CD4C/HIV1 transgenic mice shares many similarities with the AIDS syndrome and we show that recruitment and accumulation of leukocytes within diseased organs is key event in disease progression. Most importantly, we show that the oral administration of an IKK2 inhibitor markedly attenuated the renal pathology and wasting and prolonged survival. The therapeutic use of IKK inhibitor may therefore constitute a novel clinical approach for treating HIV1-associated inflammatory disorders.
Acknowledgments
We thank Dr. Kinsey Maundrell for helpful discussions, Suzanne Herren, Edith Magnenat, and Elisabeth Vial-Knecht for technical help, Roberto Lia for animal care assistance, and Dr. Arthur Roach for critical reading of the manuscript. We also thank Ched Grimshaw and Shripad Bhagwat from Celgene-Signal.
Footnotes
Address reprint requests to Dr. Y. Sagot, Serono Pharmaceutical Research Institute, 14 Chemin des Aulx, 1214 Plan les Ouates, Switzerland. E-mail: Yves.Sagot@serono.com.
References
- Wheeler DA, Gibert CL, Launer CA, Muurahainen N, Elion RA, Abrams DI, Bartsch GE. Weight loss as a predictor of survival and disease progression in HIV infection: Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:80–85. doi: 10.1097/00042560-199805010-00012. [DOI] [PubMed] [Google Scholar]
- Rao TK. Human immunodeficiency virus infection and renal failure. Infect Dis Clin North Am. 2001;15:833–850. doi: 10.1016/s0891-5520(05)70175-6. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Mulligan K. Weight loss and wasting in patients infected with human immunodeficiency virus. Clin Infect Dis. 2003;36:S69–S78. doi: 10.1086/367561. [DOI] [PubMed] [Google Scholar]
- Schwenk A, Beisenherz A, Romer K, Kremer G, Salzberger B, Elia M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active anti-retroviral treatment. Am J Clin Nutr. 2000;72:496–501. doi: 10.1093/ajcn/72.2.496. [DOI] [PubMed] [Google Scholar]
- Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- Ross MJ, Klotman PE, Winston JA. HIV-associated nephropathy: case study and review of the literature. AIDS Patient Care STDS. 2000;14:637–645. doi: 10.1089/10872910050206559. [DOI] [PubMed] [Google Scholar]
- Chang HR, Dulloo AG, Bistrian BR. Role of cytokines in AIDS wasting. Nutrition. 1998;14:853–863. doi: 10.1016/S0899-9007(98)00108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna Z, Kay DG, Cool M, Jothy S, Rebai N, Jolicoeur P. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J Virol. 1998;72:121–132. doi: 10.1128/jvi.72.1.121-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DG, Yue P, Hanna Z, Jothy S, Tremblay E, Jolicoeur P. Cardiac disease in transgenic mice expressing human immunodeficiency virus-1 nef in cells of the immune system. Am J Pathol. 2002;161:321–335. doi: 10.1016/S0002-9440(10)64184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus DD, Destree AT, Mazzola LM, McCormack TA, Dick LR, Xu B, Huang JQ, Pierce JW, Read MA, Coggins MB, Solomon V, Goldberg AL, Brand SJ, Elliott PJ. A new model of cancer cachexia: contribution of the ubiquitin-proteasome pathway. Am J Physiol. 1999;227:E332–E341. doi: 10.1152/ajpendo.1999.277.2.E332. [DOI] [PubMed] [Google Scholar]
- Frelin C, Imbert V, Griessinger E, Loubat A, Dreano M, Peyron JF. AS602868, a pharmacological inhibitor of IKK2, reveals the apoptotic potential of TNF-α in Jurkat leukemic cells. Oncogene. 2003;22:8187–8194. doi: 10.1038/sj.onc.1206963. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-κB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- Moriuchi H, Moriuchi M, Fauci AS. Nuclear factor-κ B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997;158:3483–3491. [PubMed] [Google Scholar]
- Coodley GO, Loveless MO, Merrill TM. The HIV wasting syndrome: a review. J Acquir Immune Defic Syndr. 1994;7:681–694. [PubMed] [Google Scholar]
- Grunfeld C, Feingold KR. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- Baracos VE, DeVivo C, Hoyle DH, Goldberg AL. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995;268:E996–E1006. doi: 10.1152/ajpendo.1995.268.5.E996. [DOI] [PubMed] [Google Scholar]
- Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol. 1999;276:C1132–C1138. doi: 10.1152/ajpcell.1999.276.5.C1132. [DOI] [PubMed] [Google Scholar]
- Costelli P, Baccino FM. Mechanisms of skeletal muscle depletion in wasting syndromes: role of ATP-ubiquitin-dependent proteolysis. Curr Opin Clin Nutr Metab Care. 2003;6:407–412. doi: 10.1097/01.mco.0000078984.18774.02. [DOI] [PubMed] [Google Scholar]
- Griggs RC, Moxley RT, III, Forbes GB. 3-methylhistidine excretion in myotonic dystrophy. Neurology. 1980;30:1262–1267. doi: 10.1212/wnl.30.12.1262. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Thakur P, Katoch SS. β adrenoceptor agonists, clenbuterol, and isoproterenol retard denervation atrophy in rat gastrocnemius muscle: use of 3-methylhistidine as a marker of myofibrillar degeneration. Jpn J Physiol. 2003;53:229–237. doi: 10.2170/jjphysiol.53.229. [DOI] [PubMed] [Google Scholar]
- Rovin BH, Dickerson JA, Tan LC, Hebert CA. Activation of nuclear factor-κ B correlates with MCP-1 expression by human mesangial cells. Kidney Int. 1995;48:1263–1271. doi: 10.1038/ki.1995.410. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Cancer anorexia and cachexia. Nutrition. 2001;17:438–442. doi: 10.1016/s0899-9007(01)00506-8. [DOI] [PubMed] [Google Scholar]
- Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest. 1996;97:1447–1453. doi: 10.1172/JCI118566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovera M, Garcia-Martinez C, Agell N, Lopez-Soriano FJ, Authier FJ, Gherardi RK, Argiles JM. Ubiquitin and proteasome gene expression is increased in skeletal muscle of slim AIDS patients. Int J Mol Med. 1998;2:69–73. [PubMed] [Google Scholar]
- Smit E, Skolasky RL, Dobs AS, Calhoun BC, Visscher BR, Palella FJ, Jacobson LP. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987–1999. Am J Epidemiol. 2002;156:211–218. doi: 10.1093/aje/kwf039. [DOI] [PubMed] [Google Scholar]
- Macallan DC. Wasting in HIV infection and AIDS. J Nutr. 1999;129:238S–242S. doi: 10.1093/jn/129.1.238S. [DOI] [PubMed] [Google Scholar]
- Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li JS. Role of NF-κB and cytokine in experimental cancer cachexia. World J Gastroenterol. 2003;9:1567–1570. doi: 10.3748/wjg.v9.i7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudrier J, Weng X, Kay DG, Pare G, Calvo EL, Hanna Z, Kosco-Vilbois MH, Jolicoeur P. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-γ and IL-6. Immunity. 2001;15:173–185. doi: 10.1016/s1074-7613(01)00177-7. [DOI] [PubMed] [Google Scholar]
- Chad DA, Smith TW, Blumenfeld A, Fairchild PG, DeGirolami U. Human immunodeficiency virus (HIV)-associated myopathy: immunocytochemical identification of an HIV antigen (gp 41) in muscle macrophages. Ann Neurol. 1990;28:579–582. doi: 10.1002/ana.410280418. [DOI] [PubMed] [Google Scholar]
- Polo S, Veglia F, Malnati MS, Gobbi C, Farci P, Raiteri R, Sinicco A, Lusso P. Longitudinal analysis of serum chemokine levels in the course of HIV-1 infection. AIDS. 1999;13:447–454. doi: 10.1097/00002030-199903110-00002. [DOI] [PubMed] [Google Scholar]
- Bailey JL, Mitch WE. Metabolic acidosis as a uremic toxin. Semin Nephrol. 1996;16:160–166. [PubMed] [Google Scholar]
- Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- Lopez-Franco O, Suzuki Y, Sanjuan G, Blanco J, Hernandez-Vargas P, Yo Y, Kopp J, Egido J, Gomez-Guerrero C. Nuclear factor-κ B inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol. 2002;161:1497–1505. doi: 10.1016/s0002-9440(10)64425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Asin S, Bren GD, Carmona EM, Solan NJ, Paya CV. NF-κB cis-acting motifs of the human immunodeficiency virus (HIV) long terminal repeat regulate HIV transcription in human macrophages. J Virol. 2001;75:11408–11416. doi: 10.1128/JVI.75.23.11408-11416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varin A, Manna SK, Quivy V, Decrion AZ, Van Lint C, Herbein G, Aggarwal BB. Exogenous Nef protein activates NF-κ B, AP-1, and c-Jun N-terminal kinase and stimulates HIV transcription in promonocytic cells: role in AIDS pathogenesis. J Biol Chem. 2003;278:2219–2227. doi: 10.1074/jbc.M209622200. [DOI] [PubMed] [Google Scholar]
- Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]