Abstract
The role of the extracellular matrix (ECM) in the tumor microenvironment is not limited to being a barrier against tumor invasion. The ECM is a reservoir of cell binding proteins and growth factors that affect tumor cell behavior. It is also substantially modified by proteases produced by tumor cells or stroma cells. As a result of the activity of these proteases, cell-cell and cell-ECM interactions are altered, new biologically active ECM molecules are generated, and the bioavailability and activity of many growth factors, growth factor receptors, and cytokines are modified. ECM-degrading proteases also play a critical role in angiogenesis, where they can act as positive as well as negative regulators of endothelial cell proliferation and vascular morphogenesis. This review article summarizes some of the most relevant findings made over the recent years that were discussed at a workshop organized by the Path B Study Section of the National Institutes of Health in October 2002.
The presence in a tumor mass of non-malignant cells located in close proximity with neoplastic cells has been well known. However it is only more recently that the active contribution of non-malignant cells to tumor progression has been fully recognized.1,2 Endothelial cells, fibroblasts, myoepithelial cells, pericytes, and inflammatory cells, present within the tumor stroma and at the invasive edge of tumors, constitute a microenvironment that can significantly affect the behavior of malignant cells. In addition to these normal cells, proteins of the extracellular matrix (ECM) form a non-cellular compartment to the tumor microenvironment that is extensively modified and remodeled by proteases either secreted by neoplastic and non-neoplastic cells or localized at the surface of cells. As a result of the activity of these proteases, important changes in cell-cell and cell-ECM interactions occur, and new signals are generated from the cell surface. These signals affect gene expression and ultimately influence critical cell behaviors such as proliferation, survival, differentiation, and motility. It is within this context that the Pathology B Study Section of the National Institutes of Health convened a workshop held at Hilton Head Island, South Carolina, in October 2002. This review summarizes some of the most relevant findings made over the recent years by investigators who have studied the role of adhesion molecules, their interaction with ECM proteins, and the contribution of ECM proteins and proteases in tumor progression.
Epithelial to Mesenchymal Transformation: From Cell-Cell to Cell-ECM Interaction
Epithelial to mesenchymal transformation (EMT) is a complex process in development by which epithelial cells lose their strong intercellular adhesion and their basolateral polarity to gain front-end back-end polarity, and the ability to migrate through the ECM.3 Not surprisingly, EMT is also among the first manifestations occurring during the transformation of epithelial cells into malignant adenocarcinoma. As cells lose cell-cell contact, new cell-ECM interactions are generated. As a consequence, several cell surface-associated adhesion molecules change function and are the source of signals that favor growth, motility, ECM degradation and metastasis. This concept was illustrated by several speakers, Drs. Arthur Mercurio (Beth Israel Deaconess Medical Center, Boston, MA), Sharon Stack (Northwestern University Medical School, Chicago, IL), Harold Chapman (UCSF, San Francisco, CA), Mary Zutter (Vanderbilt University School of Medicine, Nashville, TN), and Ruth Muschel (University of Pennsylvania, Philadelphia, PA).
Dr. Mercurio described a novel model system for studying the EMT of carcinoma that involves the use of colonic organoids. LIM 1863 organoids are well-differentiated colon carcinoma cells that exhibit a glandular morphology akin to the colonic epithelium and undergo a bona fide EMT conversion to a migratory monolayer phenotype in response to transforming growth factor-β (TGF-β). A product of activated macrophages, identified as tumor necrosis factor-α (TNF-α), accelerates the TGF-β-mediated EMT dramatically and results in the establishment of an autocrine loop of TNF-α that is dependent on ERK activation. These findings indicate that stromally derived TNF-α can synergize with TGF-β to modulate a critical step in colon carcinogenesis.4 Studies performed by Mercurio’s group and others have established an important role for the α6β4 integrin in the progression of many carcinomas.5,6 This integrin resides on the basal surface of epithelia where it anchors the epithelium to the underlying basement membrane in rigid structures termed hemidesmosomes. In response to EMT, α6β4 is mobilized from hemidesmosomes by a mechanism that involves serine phosphorylation of the β4 subunit and, as a consequence, it is translocated to the leading edge of invasive cells.7 In invasive carcinoma cells, α6β4 plays a dominant role in regulating signaling pathways that influence migration.8,9 One of the consequences of EMT shown by Mercurio’s group is a marked increase in the expression of vascular endothelial growth factor (VEGF). Interestingly, VEGF functions in an autocrine manner to sustain the survival of post-EMT cells. This autocrine loop involves the VEGF receptor neuropilin-1 that is expressed in many carcinomas. The hypoxic environment present within many solid tumors actually enhances the survival of post-EMT cells, in part, because it stimulates VEGF expression and promotes VEGF autocrine signaling.10 An important issue that arises from the contribution of VEGF autocrine signaling to tumor survival is an understanding of the mechanisms that regulate VEGF expression. Mercurio’s group reported that the α6β4 integrin can promote the survival of breast carcinoma cells in stress conditions,11 a finding that raised the possibility that a specific integrin, which has been implicated in cancer progression, could regulate VEGF expression. Subsequent studies detected a significant influence of α6β4 on VEGF translation via the PI3-K/mTOR pathway. These findings reveal a novel mechanism of tumor cell survival and they highlight the ability of a specific integrin to regulate protein translation.
To evaluate the potential functional link between adhesion and proteolysis, Dr. Stack presented data demonstrating that proteinase expression and subsequent tumor cell behavior are regulated via differential engagement of cell-matrix and cell-cell adhesion receptors. Inducing α3β1 integrin clustering in oral squamous cell carcinoma cells (OSCC) in culture, she observed an increase in the expression of both urokinase-type plasminogen activator (uPA) and matrix metalloproteinase-9 (MMP-9). Integrin-induced uPA expression required extracellular signal-regulated kinase (ERK1/2) activation and was accompanied by a dramatic redistribution of the uPA receptor (uPAR) to sites of integrin clustering.12 Control of proteinase expression by integrin was present in pre-malignant cells but lost in highly invasive OSCC cells, suggesting that this regulatory mechanism may contribute to the invasive phenotype.13 She also demonstrated a link between E-cadherin-mediated cell-cell adhesion and proteinase expression. Using a calcium-switch protocol to generate de novo E-cadherin junction assembly, she showed that the formation of new cell-cell contacts resulted in suppression of uPA and MMP-9 expression, while inhibition of junction assembly with function-blocking antibodies restored basal level expression of these proteinases. E-cadherin-mediated cell-cell adhesion increased both phosphoinositol-3 kinase (PI3K)-dependent Akt phosphorylation and epidermal growth factor receptor (EGFR)-dependent MEK/ERK activation.14 Blocking PI3K activity prevented proteinase down-regulation and delayed junction assembly. Prevention of junction assembly between dispersed cells resulted in enhanced uPA and MMP-9 expression and an increase in proteinase-dependent invasion. These data provide evidence for a novel bi-directional communication between proteinases and cadherins and suggest that preferential engagement of cell-ECM versus cell-cell adhesion receptors can result in differential activation of signaling pathways that positively or negatively modulate proteinase expression. However, as both normal and malignant epithelia exist in the tissue context wherein both cell-cell and cell-ECM adhesive contacts may be engaged, the future experimental challenge lies in delineating mechanisms of cross-talk between integrin- and cadherin-mediated signaling events that participate in regulation of pericellular proteolytic potential.
Dr. Chapman then demonstrated that the co-localization of uPAR and the integrin α3β1 has another important consequence on cell behavior.15 The integrin α3β1 is known to localize both at cell-cell contacts and focal contacts with laminin-5. Using a model of kidney epithelial cells from α3-null mice, he showed that sustained uPAR expression mediated loss of epithelial cell-cell contact and increased cell motility through a pathway requiring reconstitution of the cells with α3. His group made point mutants within an exposed β-propeller loop (residues 242–246) in the α3 subunit previously proposed to interact with uPAR, but outside the known laminin-binding region.16 Point mutation of a conserved His245 to Ala resulted in α3 incapable of binding uPAR. This mutant was fully capable of binding and signaling through laminin-5 engagement but had no response to uPAR co-expression. Expression of uPAR and wt α3β1 resulted in scattering of epithelial cells, increased src kinase activity, down-regulation of E-cadherin expression, and loss of E-cadherin/actin complexes. Inhibition of src kinase activity restored the epithelial cell phenotype. The data indicate that α3β1 regulates both cell-cell contact and matrix adhesion, but through distinct protein interaction sites within its β-propeller.17 TGF-β1, which promotes EMT, up-regulates uPAR independently of α3β1, but by doing so down-regulates E-cadherin and promotes cell-cell dissociation and cell scatter.
The α2β1 integrin, a collagen/laminin receptor, has been implicated in a number of complex biological processes including epithelial morphogenesis. Alterations in α2β1-integrin expression were identified in many poorly differentiated malignancies. Dr. Zutter presented her data on the recently published α2β1 integrin-deficient mouse.18 Mice deficient in the α2β1 integrin are viable, fertile, and develop normally with no excess lethality of the homozygotes. Although gross and histological evaluation of heart, lung, kidneys, gastrointestinal tract, pancreas, skin, and reproductive tracts revealed no abnormalities, mammary gland branching was abnormal.19 Quantitative analysis of mammary gland branching demonstrated that branching complexity of the mammary gland was markedly diminished in the α2-deficient animals. Additional studies are underway to define the impact on α2β1-integrin deficiency on the development and progression of cancer, and to understand the role that the integrin plays in branching morphogenesis.
Using vital fluorescent microscopy of isolated perfused lungs as a model to study the fate of tumor cells into the circulation, Dr. Muschel showed that tumor cells injected into the circulation rapidly attach to the pulmonary endothelium.20 This attachment is dependent on a contact between the α3β1 integrin and the ECM protein laminin 5 present in the vascular basement membrane and exposed intravascularly. Tumor cell attachment in the pulmonary vasculature is inhibited by blocking antibodies to α3 or β1 integrin subunits.20 Accordingly genetic deficiency of either the α3 or β1 integrin subunit has a similar negative effect on cell arrest into the pulmonary vasculature. After attachment, metastatic tumor cells proliferate but unexpectedly this proliferation first occurs within vascular channels, and extravasation rarely occurs. The α3β1 integrin which is commonly expressed in tumor cells appears thus as playing a critical role not only during the earliest steps of malignant transformation but also the latest steps like metastasis. It may be an important therapeutic target.
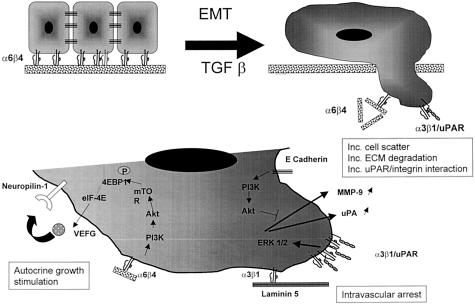
Thus, important changes in integrin interaction occur during EMT. Contact of integrins like α6β4 and α3β1 with the ECM down-regulates the expression of cell-cell adhesion molecules like E-cadherin and promotes the expression of proteases, growth factors, and protease receptors. Furthermore, the co-localization of uPAR and integrin permits a physical interaction between the subunit α3 and uPAR that further promotes EMT by down-regulating E-cadherin. Contact between α3β1 and the ECM protein laminin 5 is also critical for cell arrest in the vasculature. Altogether these changes shift the interactions that occur at the cell surface from cell-cell to cell-ECM and promote ECM degradation, invasion, and motility (Figure 1).
Figure 1.
Changes in cell-cell to cell-ECM interaction during EMT. Release of α4β6 integrin from hemidesmosomes (top left) allow interaction between α4β6 and ECM proteins that stimulate tumor cell proliferation by an autocrine mechanism that involves VEGF and its receptor neuropilin-1 (bottom left). Association between α3β1 and uPAR (top right) stimulates ERK1/2 and results in up-regulation of MMP-9 and uPA and promotes E-cadherin dissociation (bottom right). Interaction between α3β1 and laminin also promotes intravascular arrest of intravasated cells.
Galectin, a Multifunctional Protein with a Positive Role in Tumor Progression
Dr. Avraham Raz discussed the role of galectin, a superfamily of genes sharing conserved consensus sequences and β-galactoside-binding activities, and how their proteolytic processing by MMPs affects cellular adhesion and survival. His laboratory has focused its research efforts on galectin-3, and how it might influence cellular adhesion and survival of circulating cells, a major contributor to progression toward a metastatic state. Galectin-3 is an unusual protein, in that it is localized to and functions in the cytoplasm, cell membrane, nucleus, and the extracellular milieu. A notable feature of galectin-3 is its association with neoplastic transformation and cancer progression. The expression of galectin-3 is related to neoplastic transformation and progression toward metastasis in many cancers, including colon, stomach, thyroid, and breast.21 The introduction of galectin-3 cDNA into non-tumorigenic BT-549 cells resulted in the acquisition of anchorage-independent growth properties and tumorigenicity, suggesting a relationship between its expression and malignancy of human breast carcinoma cell lines. Consistently, down-regulation of galectin-3 expression in human breast and colon carcinoma cells by transfection with anti-sense cDNA resulted in a significant decrease in metastatic ability.22 These findings demonstrate an involvement of galectin-3 in malignant progression of carcinomas and suggest that it may serve as a potential molecular target for therapy of cells harboring overexpressed galectin-3. To address the possible molecular event(s) leading to augmentation of the metastatic behavior of carcinomas in response to altered galectin-3 expression, his laboratory investigated the role of galectin-3 in the cell-cell aggregation process by infecting galectin-3-null cells with galectin-3 cDNA. The cells then underwent carbohydrate-mediated homotypic cell aggregation. In addition, galectin-3-null human breast carcinoma cells transfected with galectin-3 cDNA acquired tumorigenicity in nude mice, the ability to invade through matrigel matrix and form adhesions to laminin and collagen IV but not to fibronectin. Furthermore, galectin-3 modulates the surface expression of some of the integrins specific for laminin, especially α6β1. These observations were further supported by inhibitory studies with simple competitive sugars such as modified citrus pectin (MCP). Citrus pectin is a branched complex polysaccharide polymer responsible for the texture of fruits and vegetables. Inoculation of B16-F1 cells with MCP resulted in a substantial inhibition of metastasis formation.23 Supplementation of MCP in the drinking water inhibited carbohydrate-mediated angiogenesis and the spontaneous metastasis of MAT-LyLu cells in rats and MDA-MB-435 human breast carcinomas in nude mice. Interestingly galectin-3 contains the same four amino acid motif (NWGR) conserved in the BH1 domain of bcl-2 gene family, a domain critical for bcl-2 anti-apoptotic activity.24 His laboratory and others have observed that galectin-3 is a novel anti-apoptotic gene product and that substitution of the Gly182 residue with Ala in the NWGR motif of galectin-3 abrogates its anti-apoptotic activity against cytotoxic drugs. Galectin-3 prevents anoikis in human breast epithelial cells, and galectin-3-mediated inhibition of anoikis may result from its ability to induce cell cycle arrest at late G1 through modulation of the expression of cyclins and their inhibitors.25
Redefining the Role of ECM-Degrading Proteases in Cancer
Our understanding of the role of proteases in cancer has substantially evolved over the last 5 years. The initial focus of our investigation was on their critical role in degrading the basement membrane to permit the penetration by tumor cells of surrounding connective tissues and blood vessels. We have now become aware that proteases, and in particular matrix metalloproteinases, can target many non-ECM proteins, including growth factors, growth factor receptors, cell-associated molecules, and cytokines. As a result, the activity of proteases in cancer is far more complex than initially anticipated and includes tumor promoting as well as tumor-suppressive effects. This aspect was discussed by Dr. Lynn Matrisian who described studies examining the substrates of MMP-7 that contribute to breast cancer tumor progression. MMP-7 is expressed by malignant breast epithelial cells, and overexpression of MMP-7 in transgenic mice results in hyperplastic nodules and an acceleration of neu-induced tumorigenesis26 The potential relevant substrates for this effect are complex. MMP-7 cleaves matrix components in the cellular microenvironment, resulting in visible disruption of basement membrane structures.27 MMP-7 can also cleave the cell adhesion molecule E-cadherin, resulting in a disruption of breast epithelial cell:cell junctions both in vitro and in vivo, increased saturation density, and increased cellular invasion.28 The effect of MMP-7 on breast epithelial cells can vary depending on the stage of tumor progression, which dictates the expression of tumor related cell-surface molecules. When MMP-7 is presented to pre-malignant breast epithelial cells that express both Fas and FasL, the result is an increase in cell death as a result of the solubilization of FasL and triggering of Fas-mediated apoptosis.28–30 However, when the same cells constitutively express MMP-7, the selection of a subpopulation of cells that demonstrate reduced sensitivity to Fas-mediated apoptosis occurs.30 The selection of apoptosis-resistant cells by constitutive expression of MMP-7 occurs both in vitro and in the involuting mammary gland in vivo.28 Thus the effects of MMP-7 on tumor progression can occur at the level of modulation of cellular adhesion, growth, invasion, and apoptosis, and can be mediated by substrates on the cell surface as well as in the cellular microenvironment (Figure 2).
Figure 2.
The effect of MMP-7 proteolytic activity on tumor behavior is a function of the substrate. Cleavage of Fas-L by MMP-7 releases cell-associated Fas-L and increases Fas-L/Fas interaction and promotes apoptosis in pre-malignant cells. Constitutive expression of MMP-7 by the same cells results in the selection of a subpopulation with reduced sensitivity to Fas-mediated apoptosis (top). Degradation of E-cadherin by MMP-7 promotes cell-cell dissociation, EMT, and motility (bottom). Degradation of basement membrane proteins by MMP-7 promotes invasion and metastasis.
Proteases are expressed in the extracellular milieu as inactive proforms that become activated through a variety of mechanisms that often involve a close collaboration among several families of proteases. Most antibodies available to identify proteases do not distinguish between the active and the inactive forms of these enzymes. Thus overexpression of these proteases, documented in tumors by immunohistochemistry, does not necessarily mean an increase in proteolytic activity.31 To be able to test the activity for these proteases in vivo or ex vivo becomes imperative. Dr. Bonnie Sloane discussed this issue and how the multidisciplinary and multi-institutional collaborative efforts of herself and colleagues with expertise on proteolytic enzymes in cancer and imaging modalities has begun to provide solutions to this critical issue. A consortium made of several investigators including Carl Blobel (Sloan-Kettering), Matthew Bogyo (Stanford), Thomas Bugge (NIDCR), Lisa Coussens (UCSF), Vincent Giranda (Abbott Laboratories), Thomas Meade (Northwestern), Lynn Matrisian (Vanderbilt), Christoph Peters (University of Freiburg), James Quigley (Scripps), Jeffrey Smith (Burnham), and Ching Tung (Harvard) was recently created. This group of investigators is working to: identify tumor proteases that play causal roles in malignant progression; identify stromal/inflammatory proteases that play causal roles in malignant progression; validate the identified proteases as potential targets for therapeutic intervention; develop functional assays for imaging proteolytic activity to analyze interactions among the cell types comprising a solid tumor; and develop functional assays for non-invasive imaging of the activity of the proteases that are validated as potential targets. The non-invasive assays are being designed to serve as surrogate endpoints for evaluating anti-protease therapies in vivo.
The importance of a more complete understanding of the roles of proteases in malignant progression has acquired urgency since the clinical trials using synthetic inhibitors of MMPs have not demonstrated that the inhibitors are efficacious. There are several possible explanations. For example, pre-clinical studies had indicated that MMP inhibitors (MMPIs) might be efficacious if used in prevention strategies or in early-stage cancers, yet not in the terminal-stage cancers which were used for the clinical trials. The disappointing results of the MMPI clinical trials are discussed in two recent reviews.31,32 A potentially confounding problem with the clinical trials is that they did not include a surrogate endpoint to demonstrate that the MMPIs had reached and reduced the activities of the targeted MMPs. The growing availability of probes for functional imaging of enzymatic activities using near infrared fluorescence and magnetic imaging resonance technologies suggested that a partnership of protease experts and imaging experts might produce non-invasive assays to assess protease activity in vivo as well as reductions in protease activity due to the presence of protease inhibitors. A major question in designing such probes is which proteases are appropriate targets for functional imaging. Similarly it is not yet clear which proteases are appropriate targets for inhibitor therapies. For the purposes of profiling protease expression, Dr. Sloane’s group is leading a Department of Defense Breast Cancer Center of Excellence (BCCOE) and has partnered with Affymetrix (Santa Clara, CA) to design a human-mouse protease microarray chip. This chip can be used to profile protease expression of human breast specimens and mouse models and determine the association of protease expression with specific stages of malignant progression. Furthermore, it can be used with xenograft mouse models to distinguish between the expression of proteases in the host tissue (mouse) and that in the tumor xenograft (human). At this time we are not able to profile expression of proteases by either proteomics or functional proteomics approaches, the latter of which would be particularly helpful in defining causal roles for proteases in malignant progression. The expression profiling of proteases in human breast specimens should serve to establish the validity of mouse models for the human disease and thus for their use in proof-of-principle studies to establish causal roles for candidate proteases. Such proof-of-principle studies will be performed in both transgenic models for mammary cancer and mammary fatpad orthotopic xenograft models. The transgenic models will be crossed with mice in which the candidate protease(s) has been ablated. The xenograft models will be used to directly compare the use of synthetic protease inhibitors for a candidate protease(s) and genetic ablation by implanting the tumors into the mammary fatpads of mice in which the candidate protease(s) has been deleted. The collaboration of investigators with mice deficient in a wide variety of proteases is critical to this effort and will allow us to use limited resources more cost-effectively.
Simultaneously, Dr. Sloane’s group is establishing technologies that allow imaging proteolytic activity in living cells and animals.33–35 These efforts have already demonstrated that co-culturing breast tumor cells and stromal fibroblasts of the same tissue origin significantly enhances degradation of the basement membrane protein type IV collagen. By using synthetic protease inhibitors, they have shown that MMPs, the serine protease plasmin and the cysteine protease cathepsin B, all contribute to this enhanced degradation of type IV collagen. These findings further substantiate the need for collaborative efforts among investigators with the expertise and tools to study proteases of multiple classes. Investigators with expertise in identifying peptide substrates with selectivity for the proteases identified as therapeutic targets in breast cancer are partnering with investigators with expertise in two non-invasive imaging modalities: near infrared fluorescence and magnetic resonance imaging. Probes using the peptide substrates are being validated by testing in mouse models crossed with mice deficient in the target protease(s). This is obviously a complex multidisciplinary and multi-institutional approach; however, collaborative efforts of many investigators with diverse expertise are needed if we are going to be able to integrate our findings, establish interactions among proteases, and ultimately be successful in identifying and validating proteases as therapeutic targets in breast cancer or any given cancer.
The Balance Between Proteases and Protease Inhibitors in Angiogenesis
The balance between proteases and protease inhibitors is a critical determinant in angiogenesis. It has been generally assumed that proteases are necessary to degrade the ECM and allow endothelial cells invading tissues and penetrating the tumor stroma. However the simplistic nature of this concept is now apparent as the function of several proteases and protease inhibitors in angiogenesis is appearing more complex than initially envisioned. Dr. Agnes Noel specifically discussed how critical the balance between proteases and protease inhibitors is in angiogenesis with data suggesting that both an absence or an excess of proteolysis could have a negative effect on angiogenesis.36 Recent data generated by her laboratory indicate a major role played by a membrane type (MT)-1 MMP during tumor angiogenesis. The overexpression of MT1-MMP in different cancer cell lines37 was associated with enhanced in vitro invasion and increased in vivo tumor growth and vascularization. MT1-MMP contributes to tumor angiogenesis through different mechanisms including at least: activation of αvβ3 integrin which protects endothelial cells from apoptosis; a fibrinolytic activity; a pericellular proteolysis that involves activation of pro-MMP-2; and a transcriptional regulation of VEGF expression.38 Consistent with the prevailing notion that angiogenesis requires protease-mediated proteolysis, natural protease inhibitors have been shown to suppress not only tumor invasion and metastasis, but also tumor growth and neovascularization in several tumor models.39 However, in some cases, a paradoxical association between a poor prognosis in patients with cancer and high levels of expression of protease inhibitors like tissue inhibitor of metalloproteinase-2 (TIMP-2) and plasminogen activator inhibitor-1 (PAI-1) has been reported for several cancer types.40 An increasing body of evidence suggests that TIMP-2 is a multifunctional protein having an inhibitory or stimulatory effect on tumorigenesis. TIMP-2 promotes the proliferation of some cell types and its anti-apoptotic effect may favor tumor expansion during the onset and early primary tumor growth. The anti-angiogenic activity of TIMP-2 is linked to a direct control of MMP proteolysis, an inhibitory effect on endothelial cell proliferation and/or a down-regulation of VEGF. However, besides its inhibitory activity, TIMP-2 plays a role in the process of proMMP-2 activation. According to the model of pro-MMP-2 activation proposed at present, the activation of proMMP-2 at the cell surface is regulated by a balance between MT1-MMP-TIMP-2 complex (“MMP-2 receptor”) and TIMP-2-free MT1-MMP (“pro-MMP-2 activator”). Therefore the effect of TIMP-2 on angiogenesis is dose-dependent with little TIMP-2 being required for MT1-MMP-mediated activation of proMMP-2 and excess TIMP-2 inhibiting MT1-MMP activity. A similar observation has been made with PAI-1 whose levels are paradoxically higher in more advanced stages of cancer. In PAI-1 knock-out mice there is for example a lack rather than an increase in angiogenic response in mice implanted with syngeneic malignant-transformed keratinocytes.41,42 The addition of PAI-1 to these mice by adenoviral transfer re-establishes angiogenesis. The reason for this paradoxical activity of PAI-1 is still not completely understood. As is the case for TIMP-2, PAI-1 is a multifunctional protein that in addition to inhibiting PA also interferes with vitronectin binding. Whereas these investigators have shown that the proangiogenic activity of PAI-1 is related to its anti-proteolytic activity, other investigators have shown that by inhibiting cell adhesion to vitronectin, PAI-1 promotes endothelial cell migration from vitronectin toward fibronectin.43 Altogether, these data highlight the importance of a balance between cell surface-associated proteases and their natural inhibitors during tumor angiogenesis.
Proteases also play a critical role in vascular mimicry, a process recently described by Dr. Mary Hendrix and collaborators (University of Iowa, Iowa City, IA). This group has observed that aggressive melanoma cells in three-dimensional matrices mimic embryonic vasculogenesis by forming ECM-rich, patterned tubular networks.44 Using microarray gene chip analyses, significant increases were observed in the expression of the γ2 chain of laminin 5 (Ln-5γ2), and MMP-1, -2, -9 and MT1-MMP in aggressive compared to poorly aggressive melanoma cells. These components co-localized with developing patterned networks, and anti-sense oligonucleotides to the Ln-5 γ2 chain (but not sense oligonucleotides), or antibodies to MMP-2 or MT1-MMP (but not MMP-9) inhibited the formation of these networks. Cultures that did not receive antibodies to either MMP-2 or MT1-MMP contained the Ln-5 γ2 chain promigratory cleavage fragments. Poorly aggressive melanoma cells seeded on collagen I matrices pre-conditioned by the aggressive cells formed tubular networks along the Ln-5 γ2 chain-enriched tracks deposited by the aggressive cells. These results suggest that increased expression of MMP-2 and MT1-MMP along with matrix deposition of the Ln-5 γ2 chain and/or its cleavage fragments is required for vasculogenic mimicry by aggressive melanoma cells.45 Furthermore, the apparent recapitulation of laminin-rich, patterned networks observed in aggressive melanoma patients’ tissue sections by aggressive melanoma tumor cells in three-dimensional culture may also serve as a model to help identify specific molecular targets which could function as templates for the coordinated migration of aggressive tumor cells and their proteolytic remodeling of the ECM, and may have profound implications for the development of novel therapies directed at the ECM to alter tumor progression. A more in-depth analysis of changes in differential gene expression of poorly aggressive melanoma cells grown on inductive matrices preconditioned by highly aggressive melanoma cells has revealed dramatic changes in the transdifferentiated molecular phenotype and increased migratory and invasive potential of the poorly aggressive melanoma cells.46 Furthermore, the inductive nature of the preconditioned matrix is abolished when the aggressive melanoma cells are initially treated with an MMP inhibitor before preconditioning the three-dimensional collagen gels. These observations also reflect the clinical challenge of detecting illusive tumor cells in their respective microenvironments, and present new possibilities for inhibiting inductive molecular signals in the ECM. Moreover, these results demonstrate the remarkable influence of the microenvironment on the transdifferentiation of melanoma cells, and may provide new perspectives on tumor cell plasticity for therapeutic exploitation.
The study of the source of some proteases and their critical substrate in angiogenesis has been the focus of Dr. Coussens’ laboratory. Using a transgenic model for squamous cell carcinoma in which the expression of the human papilloma virus (HPV16) is specifically induced in the epidermis under the control of the keratin promoter K14, she had shown that there is an increased expression of MMP-9 during the early stages of tumor development associated with the angiogenic switch necessary for tumor development.47 Double-transgenic mice overexpressing HPV but deficient in MMP-9 develop less but more aggressive tumors that can metastasize.48 Among the substrate for MMP-9 is VEGF, which is increasingly solubilized by MMP-9. In mice deficient in other murine-specific MMPs, she observed a significant increase in deposition of fibrillar collagen in the tumor stroma and a decrease in tumor formation suggesting that fibrillar collagen might inhibit tumor progression. Crossing K14HPV16 transgenic mice with transgenic mice carrying a mutation in the α1(I) collagen gene that causes the collagen to be resistant to collagenase-induced degradation at the helical locus, she then observed a significant inhibition of primary tumor formation as well as metastasis. These observations point to a potentially important anti-tumor activity of undegraded and/or more highly cross-linked fibrillar type I collagen in tumor progression.
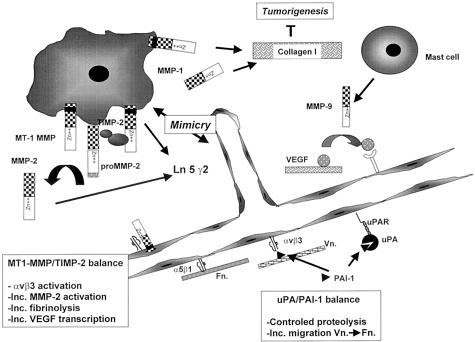
Thus proteases, protease inhibitors, and ECM proteins represent important components of the tumor microenvironment that control tumor vascularization and tumor progression. MMPs seem to have a critical role in increasing the solubility of some angiogenic factors like VEGF, and in proteolytically modifying ECM proteins like laminin 5 and fibrillar collagen, promoting not only cell migration but also cell plasticity and cell proliferation. Natural inhibitors of proteases in contrast can have a negative or positive effect on angiogenesis and their complex role will require further investigation (Figure 3).
Figure 3.
Complex interaction between proteases, protease inhibitors and ECM proteins in angiogenesis and tumorigenesis. The balance between MT1-MMP free of TIMP-2 and MT1-MMP/TIMP-2/proMMP-2 complex determines the degree of proMMP-2 activation, αvβ3 activation, pericellular fibrinolysis, and VEGF transcription (bottom left). The balance between uPA bound PAI-1 and vitronectin (Vn) bound PAI-1 controls uPA-mediated proteolysis. Vn bound PAI-1 promotes the migration of endothelial cells from Vn toward Fn (bottom right). MT1-MMP and MMP-2 cleave laminin 5 γ2 to generate cleavage products (Ln5 γ2 and Ln5 γ2x) which promote the migratory and invasive ability of melanoma cells expressing a vascular phenotype (vascular mimicry) (middle). MMP-9 produced by mast cells and inflammatory cells releases VEGF from the ECM and promotes VEGF/VEGFR interaction (top right). MT1-MMP and MMP-1 degrade interstitial type I collagen which when intact, inhibits tumorigenesis.
Conclusion
This workshop brought attention to several important novel concepts on the ECM and its remodeling by proteases during tumor progression. It is now clear that the proteolytic activity of ECM-degrading proteases generates biologically active protein fragments that can affect tumor cell proliferation, survival, and plasticity. We also became aware that ECM-degrading proteases like MMPs have more targets than just ECM proteins and can stimulate cell-cell dissociation, or cell apoptosis and endothelial cell proliferation by degrading molecules like E-cadherin, Fas-ligand, or by solubilizing VEGF. The existence of cell-associated receptors for proteases has for long been considered as a critical mechanism to concentrate proteolytic activity in the pericellular space. We now realize that these protease receptors, like uPAR, have a dual function and can physically interact with integrins and regulate gene expression. The contribution of stromal cells, endothelial cells, and inflammatory cells to the production of ECM-degrading proteases is also now well recognized. Natural inhibitors of ECM-degrading proteases can have a dual, positive and negative, regulatory function on angiogenesis, and the exact mechanisms involved require more investigation. These new observations are highly relevant as they may explain some of the recent failures of several inhibitors of MMPs in clinical trials. However to convince the pharmaceutical industry to resume clinical trials with MMP inhibitors will require addressing several important issues. First, it will be important to propose better and more sensitive endpoints than clinical progression based on survival or the size of a tumor. Since these inhibitors have a cytostatic rather than a cytotoxic activity, to be able to document their biological activity on angiogenesis or tumor proliferation using non-invasive imaging technologies in vivo will be critical. Second, to provide sensitive markers of the activity of these inhibitors in vivo will be essential, since what determines their administration in clinical trials is not a maximum tolerable dose (MTD) but rather an optimal biologically active dose. This will require the availability of sensitive, non-toxic, and non-invasive methods to monitor the effect of synthetic protease inhibitors on proteolytic activity in vivo. Finally, pre-clinical models that more closely mimic the interaction between neoplastic cells and stromal cells in cancer progression are needed. De novo transgenic mouse models should be preferred to xenotransplanted models as they better reproduce host-neoplastic cell interaction. Clearly, the field is at a crossroads where challenging questions meet exciting research opportunities supported by novel technologies.
Footnotes
Address reprint requests to Dr. Yves A. DeClerck, Division of Hematology-Oncology, Childrens Hospital Los Angeles, MS #54, 4650 Sunset Boulevard, Los Angeles, CA 90027. E-mail: declerck@hsc.usc.edu.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Bates RC, Mercurio AM. Tumor necrosis factor-α stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell. 2003;14:1790–1800. doi: 10.1091/mbc.E02-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion: lessons from the α6β4 integrin. Semin Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-κB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- Rabinovitz I, Toker A, Mercurio AM. Protein kinase C-dependent mobilization of the α6β4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol. 1999;146:1147–1160. doi: 10.1083/jcb.146.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- O’Connor KL, Shaw LM, Mercurio AM. Release of cAMP gating by the α6β4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J Cell Biol. 1998;143:1749–1760. doi: 10.1083/jcb.143.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- Bachelder RE, Ribick MJ, Marchetti A, Falcioni R, Soddu S, Davis KR, Mercurio AM. p53 inhibits α6β4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Cell Biol. 1999;147:1063–1072. doi: 10.1083/jcb.147.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Brown R, Jones JC, Ellerbroek SM, Stack MS. Urinary-type plasminogen activator (uPA) expression and uPA receptor localization are regulated by α3β1 integrin in oral keratinocytes. J Biol Chem. 2000;275:23869–23876. doi: 10.1074/jbc.M000935200. [DOI] [PubMed] [Google Scholar]
- Munshi HG, Ghosh S, Mukhopadhyay S, Wu YI, Sen R, Green KJ, Stack MS. Proteinase suppression by E-cadherin-mediated cell-cell attachment in pre-malignant oral keratinocytes. J Biol Chem. 2002;277:38159–38167. doi: 10.1074/jbc.M202384200. [DOI] [PubMed] [Google Scholar]
- Munshi HG, Wu YI, Ariztia EV, Stack MS. Calcium regulation of matrix metalloproteinase-mediated migration in oral squamous cell carcinoma cells. J Biol Chem. 2002;277:41480–41488. doi: 10.1074/jbc.M207695200. [DOI] [PubMed] [Google Scholar]
- Kugler MC, Wei Y, Chapman HA. Urokinase receptor and integrin interactions. Curr Pharm Des. 2003;9:1565–1574. doi: 10.2174/1381612033454658. [DOI] [PubMed] [Google Scholar]
- Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote β1 integrin function through interactions with integrin α3β1. Mol Biol Cell. 2001;12:2975–2986. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Tom C, Kugler M, Ching T, Kreidberg J, Wei Y, Chapman HA. Distinct ligand binding sites in integrin a3b1 regulate matrix adhesion and cell: cell contact. J Cell Biol. 2003;163:177–188. doi: 10.1083/jcb.200304065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Pappan LK, Grenache DG, Li Z, Tollefsen DM, Santoro SA, Zutter MM. The contributions of the α2β1 integrin to vascular thrombosis in vivo. Blood. 2003 doi: 10.1182/blood-2003-04-1323. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The α(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Song C, Grimes MM, Fu W, Dewhirst MW, Muschel RJ, Al Mehdi AB. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol. 2002;161:749–753. doi: 10.1016/S0002-9440(10)64233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara H, Honjo Y, Yoshii T, Akahani S, Yoshida J, Hattori K, Okamoto S, Sawada T, Raz A, Kubo T. Expression of galectin-3 in fine-needle aspirates as a diagnostic marker differentiating benign from malignant thyroid neoplasms. Cancer. 1999;85:2475–2484. [PubMed] [Google Scholar]
- Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–668. [PubMed] [Google Scholar]
- Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj J. 1994;11:527–532. doi: 10.1007/BF00731303. [DOI] [PubMed] [Google Scholar]
- Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel anti-apoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. [PubMed] [Google Scholar]
- Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem. 2002;277:6852–6857. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- Rudolph-Owen LA, Matrisian LM. Matrix metalloproteinases in remodeling of the normal and neoplastic mammary gland. J Mammary Gland Biol Neoplasia. 1998;3:177–189. doi: 10.1023/a:1018746923474. [DOI] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia. 2001;3:459–468. doi: 10.1038/sj.neo.7900190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys. 2002;408:155–161. doi: 10.1016/s0003-9861(02)00525-8. [DOI] [PubMed] [Google Scholar]
- Vargo-Gogola T, Fingleton B, Crawford HC, Matrisian LM. Matrilysin (matrix metalloproteinase-7) selects for apoptosis-resistant mammary cells in vivo. Cancer Res. 2002;62:5559–5563. [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Sameni M, Moin K, Sloane BF. Imaging proteolysis by living human breast cancer cells. Neoplasia. 2000;2:496–504. doi: 10.1038/sj.neo.7900116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameni M, Dosescu J, Sloane BF. Imaging proteolysis by living human glioma cells. Biol Chem. 2001;382:785–788. doi: 10.1515/BC.2001.094. [DOI] [PubMed] [Google Scholar]
- Sameni M, Dosescu J, Moin K, Sloane BF. Functional imaging of proteolysis: stromal and inflammatory cells increase tumor proteolysis. Mol Imaging. 2003;2:1–17. doi: 10.1162/15353500200303136. [DOI] [PubMed] [Google Scholar]
- Devy L, Blacher S, Grignet-Debrus C, Bajou K, Masson V, Gerard RD, Gils A, Carmeliet G, Carmeliet P, Declerck PJ, Noel A, Foidart JM. The pro- or anti-angiogenic effect of plasminogen activator inhibitor 1 is dose dependent. EMBO J. 2002;16:147–154. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Janssen M, Foidart JM, Noel A. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix Biol. 2003;22:55–61. doi: 10.1016/s0945-053x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM, Noel A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. EMBO J. 2002;16:555–564. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- Hajitou A, Sounni NE, Devy L, Grignet-Debrus C, Lewalle JM, Li H, Deroanne CF, Lu H, Colige A, Nusgens BV, Frankenne F, Maron A, Yeh P, Perricaudet M, Chang Y, Soria C, Calberg-Bacq CM, Foidart JM, Noel A. Down-regulation of vascular endothelial growth factor by tissue inhibitor of metalloproteinase-2: effect on in vivo mammary tumor growth and angiogenesis. Cancer Res. 2001;61:3450–3457. [PubMed] [Google Scholar]
- Remacle A, McCarthy K, Noel A, Maguire T, McDermott E, O’Higgins N, Foidart JM, Duffy MJ. High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int J Cancer. 2000;89:118–121. doi: 10.1002/(sici)1097-0215(20000320)89:2<118::aid-ijc3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, Fusenig NE, Weidle U, Carmeliet G, Loskutoff D, Collen D, Carmeliet P, Foidart JM, Noel A. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin: implications for anti-angiogenic strategies. J Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai C, Laug WE, Shimada H, Declerck PJ, Stins MF, Durden DL, Erdreich-Epstein A, DeClerck YA. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer Res. 2001;61:5587–5594. [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 γ2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–6327. [PubMed] [Google Scholar]
- Seftor EA, Meltzer PS, Schatteman GC, Gruman LM, Hess AR, Kirschmann DA, Seftor RE, Hendrix MJ. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44:17–27. doi: 10.1016/s1040-8428(01)00199-8. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]