Abstract
The proliferative response of podocytes to injury determines the histological phenotype. Moreover, an apparent lack of podocyte proliferation may underlie the development of glomerulosclerosis. Podocyte proliferation is closely linked with its state of differentiation. However, the mechanisms regulating these processes are not fully elucidated. Because D-type cyclins have been shown to be important in the regulation of proliferation and differentiation, we examined their expression in podocytes in vitro and in vivo. The glomerular expression of cyclins D1 and D3 was examined in vitro in cultured immortalized podocytes by immunostaining and Western blot analysis, and in embryonic mice and rats, the passive Heymann nephritis model of experimental membranous nephropathy in rats, and human immunodeficiency virus (HIV)-transgenic mice. Kidneys from cyclin D1 knockout mice were also examined. Cyclin D1 was abundant in cultured proliferating podocytes, but not in quiescent differentiated podocytes. In contrast, cyclin D3 was abundant in differentiated, but not proliferating podocytes. Cyclin D1 was expressed in embryonic mouse and rat glomeruli during the S- and comma-shaped stages, and was absent in podocytes at the capillary loop stage and in mature rodent glomeruli. Cyclin D1 protein increased after injury in passive Heymann nephritis rats and in HIV-transgenic mice. Cyclin D3 was constitutively and specifically expressed in podocytes in normal rodent glomeruli, and decreases during dedifferentiation and proliferation in HIV-transgenic mice. Kidneys from cyclin D1−/− mice were normal with the podocytes expressing specific differentiation markers. Cyclin D1 is not necessary for the terminal differentiation of podocytes, and expression coincides with cell-cycle entry. In contrast, cyclin D3 expression coincides with podocyte differentiation and quiescence.
Podocytes are highly specialized, terminally differentiated cells that line the glomerular basement membrane. Critical functions include preventing proteinuria, and also to counteract the changes in pressure within the glomerular capillary tuft.1 Podocytes proliferate during glomerulogenesis until they develop their characteristic morphology during the capillary loop stage, when they exit the cell cycle and become quiescent.2 Recent studies have shown that podocytes do enter the cell cycle after injury, but their capacity to progress through the cell cycle and proliferate depends on the form of glomerular disease.3 For example, podocytes proliferate in collapsing focal segmental glomerulosclerosis (FSGS) and human immunodeficiency virus (HIV)-associated nephropathy, but do not proliferate in membranous nephropathy and classic FSGS.4–6
Proliferation is governed at the level of the cell cycle by specific cell-cycle regulatory proteins. This requires that cyclin-dependent kinases (Cdks) are activated by partner cyclins.7 In contrast, proliferation is inhibited by Cdk inhibitors that bind to and inactivate cyclin-Cdk complexes.8 D-type cyclins (D1, D2, and D3) complex with Cdk4 or Cdk6, which are essential for early G1 entry. Active cyclin D-Cdk4 and -Cdk6 complexes phosphorylate the retinoblastoma protein, which is required for progression past the restriction point that obligates the cell to finish the cell cycle.9
To examine the role of D-type cyclins in podocyte proliferation and differentiation, we studied the expression of cyclin D1 and cyclin D3 during glomerulogenesis, experimental podocyte injury, and in vitro. Our results showed that cyclin D1 staining coincided with proliferation, and cyclin D3 with differentiation.
Materials and Methods
Experimental Design
The expression of cyclin D1 and cyclin D3 was examined in podocytes in vitro and in vivo.
In Vivo Studies
Glomerulogenesis:
Podocytes proliferate during glomerulogenesis, and cease to proliferate on maturation.10 To examine the expression of cyclins D1 and D3 during glomerulogenesis, mouse embryonal kidneys were dissected at days E15, E18, and E21, and at birth (n = 6 per time point) and fixed in paraffin for immunostaining (see below).
Passive Heymann Nephritis (PHN) Model:
The PHN model of experimental membranous nephropathy was induced in male Sprague-Dawley rats (Simson, Gilroy, CA) weighing 180 to 200 g by intraperitoneal injection of sheep antibody to Fx1A (5 ml/kg body wt) prepared as previously described.11 Control animals were injected with normal sheep serum. PHN and control animals were sacrificed at days 3, 5, 10, and 30 (n = 6 per group at each time point). Previous studies have shown that giving basic fibroblast growth factor (bFGF) to PHN rats augments podocyte injury.12 To determine the effects of bFGF on the expression of D-type cyclins in rats with PHN, an additional six PHN rats were injected intravenously with human recombinant bFGF (5 μg/200 g animal; gift of M Reidy, Ph.D., University of Washington, Seattle, WA) on days 3 and 4 and sacrificed on day 5. A 24-hour urine collection was performed on control and PHN rats before sacrifice, and urine protein excretion was determined in control and PHN animals by the sulfasalicylic acid method as previously reported.13 At sacrifice, renal biopsies were taken from control and PHN rats, and glomeruli were isolated by the sieving method as we have previously reported.11
HIV Transgenic Mice:
We also studied HIV transgenic mice that are characterized by podocyte proliferation as previously reported.14 In brief, transgenic mice bearing a GAG-Pol deleted genome and control wild-type mice were studied at weeks 6, 10, and 35 (n = 4 per time point). Renal biopsies were paraffin-embedded for immunostaining.
Thy1 Nephritis:
As an additional control for cyclin D1 expression, we used the Thy1 model of experimental mesangial proliferative glomerulonephritis that was induced in 180 to 220 g male Wistar rats (Simson) by an intravenous injection of goat anti-thymocyte plasma (0.35 ml/100 g body wt).15 Rats were sacrificed on day 5 (n = 6), when renal biopsies were taken from each animal for immunostaining.
Cyclin D1−/− Mice:
Finally, to determine whether cyclin D1 is essential for podocyte differentiation, kidney tissue from newborn and 4-month-old adult cyclin D1−/− mice (gift of Dr. V. Fantl, Cancer Research UK, London Research Institute, London, UK) was analyzed for the expression of cyclin D1, cyclin D3, and podocyte-specific markers.16
In Vitro Studies
To determine the expression of cyclins D1 and D3 during proliferation and differentiation in cultured podocytes, we used conditionally immortalized mouse podocytes in vitro as reported previously.17 Podocytes were grown in uncoated culture plates at 33°C in the presence of 20 U/ml mouse recombinant interferon-γ (Sigma Chemical Co., St. Louis, MO) to enhance expression of a thermosensitive T antigen. Under these conditions, cells proliferate and are undifferentiated. To induce differentiation and reduce proliferation, podocytes were grown at 37°C in the absence of interferon-γ for 14 days.
For Western blot analysis, protein was isolated both from undifferentiated (and proliferating) podocytes, and from podocytes 3 and 5 days after the induction of differentiation and quiescence.
Immunostaining
Renal biopsies from mice during glomerulogenesis, rats with PHN and Thy1 nephritis, HIV-transgenic mice, and cyclin D1−/− mice were fixed in formalin, embedded in paraffin, and cut into 4-μm-thick sections. Indirect immunoperoxidase immunostaining was performed as previously reported using the following primary antibodies incubated overnight at 4°C: cyclin D1 (1:1000, mouse monoclonal, clone DCS-6; Oncogene, Boston, MA), cyclin D1 (1:600, mouse monoclonal, MS-210; Neomarkers, Fremont, CA), cyclin D1 (1:100, mouse monoclonal, clone A-12; Santa Cruz, Santa Cruz, CA), cyclin D3 (1:300, mouse monoclonal, clone DCS-22; Neomarkers), WT-1 (1:1200, rabbit polyclonal, clone C-19; Santa Cruz), Synaptopodin (1:50, gift of Dr. Peter Mundel, Departments of Medicine and Anatomy, Albert Einstein College, Bronx, NY) and Glepp1 (1:50; gift of Dr. Roger Wiggins, Department of Internal Medicine, University of Michigan, Ann Arbor, MI). Controls for this step included omitting the primary antibody and substituting the primary antibody with preimmune mouse serum, and tissue from cyclin D1−/− mice to ensure antibody specificity for cyclin D1.
Tissue sections were boiled in citric acid (10 mmol/L, pH 6.0) for 10 minutes to unmask the antigens. Nonspecific background staining was reduced with the avidin/biotin blocking kit (SP-2001; Vector Laboratories, Inc., Burlingame, CA) and Background Buster (Accurate Chemical and Scientific Corp., Westbury, NY). A biotinylated horse anti-mouse secondary antibody (1:400, BA-2001; Vector Laboratories, Inc.) was then added, and detection was performed using the ABC-Kit (Vector Laboratories, Inc.). Black nuclear staining was detected using diaminobenzidine (Sigma Chemical Co.) with nickel as a chromogen.
In PHN rats, detection of sheep IgG and C5b-9 in glomeruli was performed by direct (sheep IgG) and indirect (C5b-9) immunofluorescence staining on 4-μm frozen sections fixed in ether/alcohol as described elsewhere.11 Sections were stained with fluorescein-conjugated rabbit anti-sheep IgG (Organon Teknika Corp., West Chester, PA) or biotinylated 2A1, a murine monoclonal antibody to rat C5b-9, followed by fluorescein-conjugated streptavidin (Amersham, Arlington Heights, IL). Periodic acid-Schiff staining was performed as previously reported.11
Quantitation of Immunostaining
The glomerular expression of each cell-cycle protein was graded semiquantitatively in a blinded manner in PHN and control rats at each time point. Thirty glomerular cross sections were evaluated in individual samples by counting the number of cells staining positive for each antigen. Mean values per time point were calculated and the results were expressed as the number of cells staining positive per 30 glomerular cross sections.
Western Blot Analysis and Immunoprecipitation
Glomeruli were isolated from the renal cortex of PHN and control rats by differential sieving as previously described.11 Glomerular cells were disrupted by a combination of freezing/thawing and sonication. To extract glomerular protein, glomeruli were resuspended in a buffer containing 1% Triton, 10% glycerol, 20 mmol/L HEPES, 100 mmol/L NaCl with a mixture of protease inhibitors. After centrifugation at 14,000 rpm for 10 minutes, protein concentration was measured by BCA protein assay (Pierce, Rockford, IL). Glomerular protein extracts (20 to 50 μg) were separated under reduced conditions on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. Protein was transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) by electroblotting and blocked in 5% nonfat dried milk for 30 minutes before incubation with antibodies to cyclin D1 (1:200, clone DCS-6; Oncogene) or cyclin D3 (1:200, clone DCS-22; Neomarkers). An alkaline phosphatase-conjugated secondary antibody was used (Promega, Madison, WI) with BCIP/NBT (Sigma) as substrate. To determine whether cyclin D1 and/or D3 co-immunoprecipitated with CDK4, 200 μg of glomerular protein from PHN day 5 and control animals were incubated with 4 μl of antibody against cdk4 (mouse monoclonal, clone DCS-35; Chemicon Int., Temecula, CA) at 4°C for 1 hour and precipitated after incubation with Protein G Sepharose 4 Fast Flow (Amersham Pharmacia) for another hour. The pellet was boiled in sodium dodecyl sulfate-buffer and the supernatant transferred to a 15% sodium dodecyl sulfate-gel. This experiment was repeated three times.
Immunofluorescence in Cultured Cells
For immunostaining, podocytes grown under proliferating (undifferentiated) and quiescent (differentiated) conditions, were fixed for 20 minutes in methanol:acetone (1:1) at −20°C, air-dried for 15 minutes, rehydrated with phosphate-buffered saline, and permeabilized with 0.1% Nonidet P-40. Cells were stained with monoclonal antibodies for cyclin D1 (1:20, clone DCS-6; Oncogene) and cyclin D3 (1:40, clone DCS-22; Neomarkers) overnight. This was followed by a biotinylated horse anti-mouse secondary antibody (1:100; Vector Laboratories, Inc.) the next day and then a fluorescein-conjugated streptavidin (1:100; Amersham Biosciences Corp., Piscataway, NJ). Controls for this step included omitting the primary antibody. Immunostaining was analyzed by fluorescence microscopy.
Results
Differential Expression of D-Type Cyclins During Glomerulogenesis
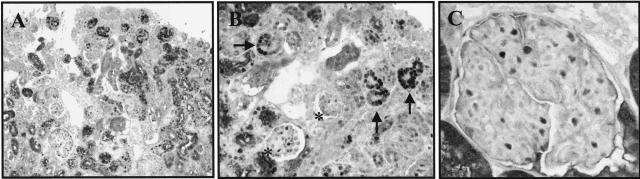
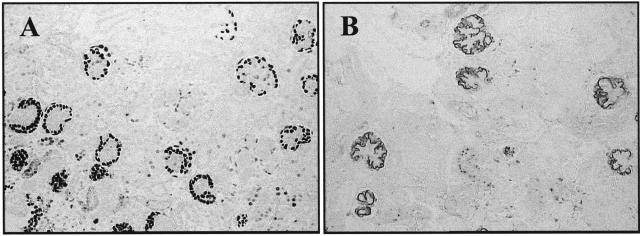
To determine the expression of early G1-phase cell-cycle proteins during glomerulogenesis, the expression of cyclin D1 and cyclin D3 were measured in embryonal mice by immunohistochemistry. Staining for cyclin D1 was abundant in podocyte precursor cells in renal vesicles, and the comma- and S-shaped bodies (Figure 1, A and B). In contrast, Figure 1 shows that cyclin D1 staining was not detected in a typical podocyte localization during the capillary loop stage (Figure 1B, asterisks), nor in the normal mature rat glomerulus (Figure 1C). Similar results were obtained with two different antibodies recognizing cyclin D1.
Figure 1.
Immunostaining for cyclin D1. A: Low-magnification view of embryonic mouse kidney at E21, showing positive cyclin D1 immunostaining in the immature cortical glomeruli. B: Higher power view showing that cyclin D1 is detected in vesicular-, comma-, and S-shaped bodies (arrows). In capillary loop glomeruli, positive staining in a nonpodocyte-specific distribution can be seen (asterisks). C: Cyclin D1 staining is not detected in the normal rat glomerulus.
Our results showed that the glomerular expression of cyclin D3 was the mirror image of cyclin D1. Figure 2A shows that cyclin D3 staining was absent in immature glomeruli, and was first detected at the capillary loop stage, and this was in a typical podocyte distribution (Figure 2B, arrows). Cyclin D3 staining was detected in podocytes in adult rats (Figure 2C) and mice (not shown), but was not detected in glomerular endothelial and mesangial cells.
Figure 2.
Cyclin D3 immunostaining. A: Low-magnification view of embryonal mouse kidney at E21, showing that cyclin D3 immunostaining was not detected in the outer cortical immature glomeruli, but was present in the deeper cortex in more mature glomeruli. B: Higher power view showing that cyclin D3 is detected in mature glomeruli in a typical podocyte localization (arrows). No cyclin D3 can be detected in vesicular-, comma-, and S-shaped bodies. C: Cyclin D3 is constitutively expressed in podocytes in normal adult rat glomeruli.
Cyclin D1, but Not Cyclin D3, Is Increased in PHN
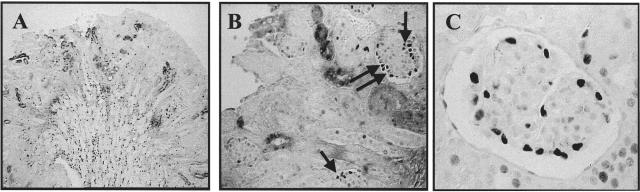
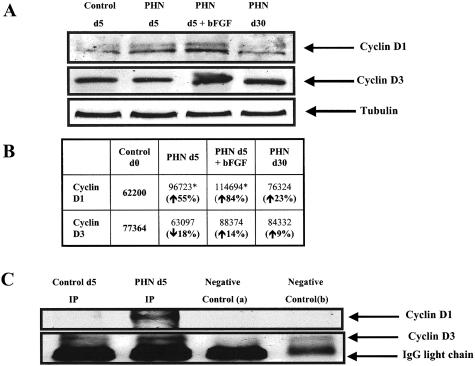
To determine whether C5b-9-induced podocyte injury increased cyclin D1 levels, we used the passive Heymann nephritis (PHN) model of experimental membranous nephropathy. Cyclin D1 immunostaining increased specifically in podocytes at PHN day 5 (Figure 3A). The number of podocytes staining positive for cyclin D1 was further augmented by giving bFGF to PHN rats (Table 1). Cyclin D1 immunostaining was also detected on day 30, although the peak was day 5 (Table 1). The increase in cyclin D1 protein levels was confirmed by Western blot analysis performed on isolated glomeruli from day 5 PHN rats (Figure 4).
Figure 3.
Immunostaining for cyclins D1 and D3 in PHN rats and HIV-transgenic mice. A: Immunostaining for cyclin D1 was increased in PHN rats at day 5, and this was in a typical podocyte localization. B: Cyclin D1 staining increased in HIV-transgenic mice at 6 weeks. C: Cyclin D3 immunostaining was detected in podocytes of PHN rats at day 5, and was unchanged compared to normal. D: In HIV-transgenic mice, podocyte dedifferentiation and proliferation was associated with a decrease in cyclin D3 staining.
Table 1.
Quantitation of Cyclin D1 and Cyclin D3 in PHN
| Control day 5 (number of positive cells/30 glomeruli) | PHN day 5 (number of positive cells/30 glomeruli) | PHN day 5 + bFGF (number of positive cells/30 glomeruli) | PHN day 30 (number of positive cells/30 glomeruli) | |
|---|---|---|---|---|
| Cyclin D1 | 6.5 ± 2.1 | 69.8 ± 17.9* | 104.3 ± 16.1*† | 37.8 ± 7.9 |
| Cyclin D3 | 127.7 ± 17 | 132.4 ± 18.4 | 130.3 ± 32.6 | 137.3 ± 15.3 |
The number of glomerular cells staining positive for each D-type cyclin was quantitated in control and PHN rats. The values expressed are the mean ± SD from six animals at each time point.
P < 0.05 compared to control animals.
P < 0.01 compared to control animals.
Figure 4.
Western blot analysis for cyclin D1 and cyclin D3 on glomerular protein from PHN rats. A: There was an increase in cyclin D1 protein expression in glomerular protein from day 5 PHN animals (lane 2) compared to control (lane 1), and the increase was more pronounced in PHN rats given bFGF (lane 3). Lane 4 shows that cyclin D1 levels normalized by day 30 PHN. Cyclin D3 protein levels were not changed in PHN. B: Quantification of the Western blots further shows that cyclin D1 levels increase in PHN, however, cyclin D3 levels did not change significantly. C: Co-immunoprecipitating glomerular protein from PHN day 5 rats with an antibody to cdk4 showed a positive signal for cyclin D1 (lane 2), which was absent in glomerular protein from control rats (lane 1).
Cyclin D3 is present in normal adult rat podocytes (Figure 2C). In contrast to an increase in cyclin D1, there were no statistical differences in the number of podocytes staining positive for cyclin D3 in PHN rats (Figure 3C and Table 1). This result was confirmed by Western blot analysis (Figure 4A).
Cyclin D1 is the catalytic partner for CDK4.18 We next determined if cyclin D1 complexed with CDK4 in PHN rats by performing immunoprecipitation studies on glomerular protein. Figure 4C demonstrates that cyclin D1 did not immunoprecipitate with CDK4 in control animals (lane 1). In contrast, cyclin D1 co-immunoprecipitated with Cdk4 in PHN rats at day 5 (lane 2). Interestingly cyclin D3 did not complex with Cdk4 in control and PHN animals (Figure 4).
Switch from Cyclin D3 to Cyclin D1 in HIV
Previous studies have shown that podocytes dedifferentiate and proliferate in HIV-transgenic mice19 a finding analogous to that seen during glomerulogenesis. We examined the expression of cyclin D1 and cyclin D3 in mice transgenic for HIV. There was an increase in cyclin D1 immunostaining during the acute inflammatory phase, which is characterized by podocyte proliferation in 6-week-old HIV mice, and the increase localized specifically to podocytes (Figure 3B). In contrast, cyclin D1 staining was not detected in 12-week-old HIV-transgenic mice when proliferation was absent in scarred glomeruli (not shown).
In contrast to the increase in cyclin D1, there was a marked decrease in cyclin D3 in podocytes in HIV mice at 6 weeks, and the decrease persisted at 12 weeks (Figure 3D). The decrease in cyclin D3 preceded podocyte proliferation because otherwise unaffected appearing podocytes had already lost cyclin D3 expression.
Switch from Cyclin D1 to Cyclin D3 During Differentiation in Podocytes in Vitro
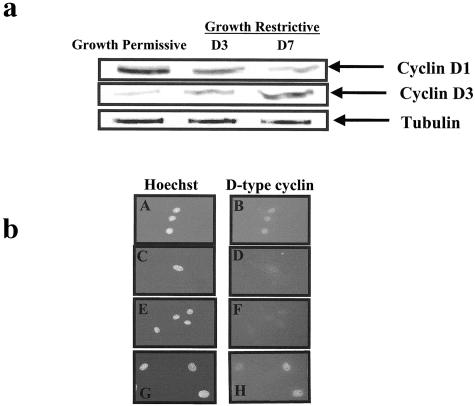
Conditionally immortalized mouse podocytes are characterized by proliferation when grown in the presence of interferon at 33°C, and quiescence when grown without interferon at 37°C. The switch from a proliferating to a quiescent phenotype was associated with a decrease in cyclin D1 protein levels (Figure 5a). In contrast, the onset of the differentiated phenotype coincided with a marked increase in cyclin D3 (Figure 5a). The changes in cyclin D1 and D3 protein levels measured by Western blot analysis were confirmed by immunofluorescent staining (Figure 5b).
Figure 5.
A: Western blot analysis for cyclin D1 and cyclin D3 in conditionally immortalized podocytes in vitro. Cyclin D1 was abundant in proliferating podocytes grown under permissive conditions. The levels of cyclin D1 decreased when podocytes were grown under restrictive conditions, and this coincided with a decrease in proliferation, and the development of a differentiated phenotype. There was a progressive increase in cyclin D3 levels when grown under restrictive conditions, compared to permissive conditions. Tubulin was used to assure equal loading. b: Immunofluorescence for cyclin D1 and cyclin D3 in conditionally immortalized podocytes. Cyclin D1 stains positive in proliferating podocytes grown under permissive conditions (b), and is barely detected under growth restrictive conditions (D). Hoechst staining was used as a nuclear counterstain (A and C). Cyclin D3 staining was not detected in proliferating podocytes (F), but was abundant in quiescent and differentiated podocytes grown under growth restrictive conditions (H). Hoechst staining was used as a nuclear counterstain (E, G).
Cyclin D−/− Mice Have Normal Glomerular Architecture
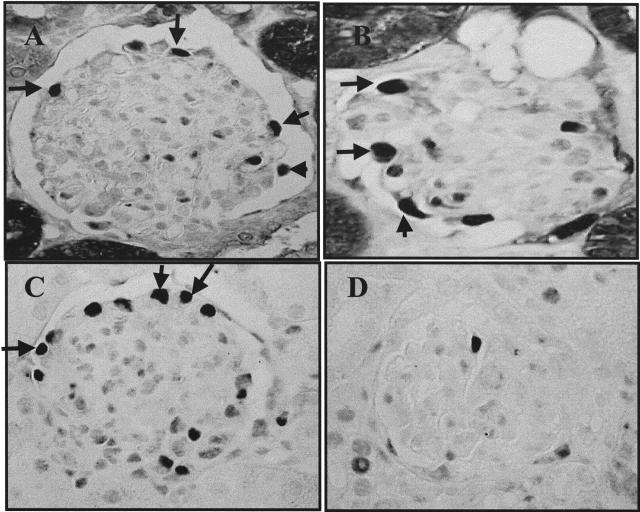
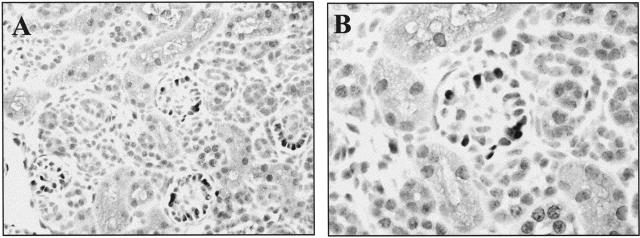
Cyclin D1−/− mice have a reduced birth weight and remain small during subsequent growth to adulthood compared to control wild-type mice.16,20 Our results showed that periodic acid-Schiff staining did not reveal any abnormalities in renal architecture (not shown). Moreover, immunostaining for markers of podocyte maturity, including WT-1 (Figure 6A), synaptopodin (Figure 6B), and Glepp1 (not shown) were normal in cyclin D1−/− mice, and similar to control cyclin D1+/+ mice. Finally, cyclin D3 immunostaining was normal in cyclin D1−/− mice (not shown). These results show that cyclin D1 alone is not required for normal glomerular development and podocyte differentiation.
Figure 6.
Detection of podocyte-specific proteins in cyclin D1−/− mice. Podocytes from adult cyclin D1−/− mice express WT-1 (A) and synaptopodin (B) markers of mature podocytes.
Specificity of Antibodies
The antibodies used to detect cyclin D1 and cyclin D3 are commercially available mouse monoclonal antibodies. However, a potential problem is that D-type cyclins may cross-react with one another, because of partial sequence similarities between the three cyclin D isoforms.21 Accordingly, to ensure antibody specificity, we performed the following experiments. First, we used tissue from the Thy1 model of glomerulonephritis as a positive control for proliferating mesangial cells. Mesangial cell proliferation in this model was associated with an increase in cyclin D1 staining in mesangial cells at day 5 (data not shown). In contrast, cyclin D3 staining did not change in proliferating mesangial cells (not shown). Second, we used kidneys and spleens from cyclin D1 knockout (−/−) mice as negative controls for cyclin D1 staining. As expected, staining for cyclin D1 was absent in tissue from these −/− mice (data not shown). However, cyclin D3 staining was detected in podocytes in cyclin D1−/− mice. Third, we stained embryonal kidneys from cyclin D1 knockout (−/−) mice with a commercially available mouse monoclonal antibody used by other groups to stain kidneys. Our results showed that the Santa Cruz cyclin D1 antibody stained podocytes from cyclin D1−/− mice (Figure 7, A and B). Thus, this antibody must cross-react with an antigen on podocytes that is not cyclin D1. Fourth, Western blot analysis showed a double band for cyclin D1 at 36 kd (corresponding to the active phosphorylated and inactive unphosphorylated forms), which migrate slower than cyclin D3 at 34 kd (not shown). Fifth we repeated the immunostaining and Western blot analysis with a second and different monoclonal antibody that also recognizes cyclin D1, and the results were identical to those using the first antibody. Finally Cdk4 has been reported to be the catalytic subunit of cyclin D1. We showed that cyclin D1, and not cyclin D3, co-immunoprecipitated with Cdk4 in glomerular protein from PHN rats, but not from controls (Figure 4C).
Figure 7.
Specificity of Santa Cruz cyclin D1 antibody. Cyclin D−/− mice immunostained with the cyclin D1 antibody from Santa Cruz showed positive staining in a podocyte distribution. A: Low-power field; B: high-power field.
Discussion
Mature podocytes are terminally differentiated and quiescent cells.1 However, the proliferative response determines the nature of the glomerular lesion after injury in glomerular disease. In classic FSGS, membranous nephropathy and minimal change disease, podocytes do not proliferate.22 In contrast, podocytes proliferate in HIV nephropathy, cellular FSGS, and collapsing FSGS.4,5 Thus, the mechanisms regulating podocyte proliferation are of importance. The results in this study show for the first time that cyclin D1 is not essential for podocyte development, but levels increase in the podocyte in response to injury. We show that in contrast, the expression of cyclin D3 coincides with a differentiated and quiescent podocyte phenotype.
The D-type cyclins (D1, D2, and D3) have a molecular weight of 34 to 36 kd, and share 57% identity throughout the entire coding region.21 Despite this, the expression of D-type cyclins differs from one cell type to another. Furthermore, the function of D-type cyclins may not be similar.18 Our first major finding was that podocyte differentiation in vitro and in vivo coincided with a decrease in cyclin D1 staining. The decrease in cyclin D1 expression was first detected at the capillary loop stage during glomerulogenesis. Similarly, cyclin D1 expression decreased when cultured mouse podocytes were switched from growth-permissive cell culture conditions (proliferative phenotype) to growth-restrictive conditions (quiescent phenotype). Moreover, our data showed that cyclin D1 knockout mice had a normal differentiated podocyte phenotype, despite being smaller than control wild-type mice.16,20 We also examined the expression of cyclin D1 in two models of podocyte injury. Cyclin D1 levels increased in PHN rats and in HIV-transgenic mice, and the increase coincided with entry into the cell cycle.
The classic role for cyclin D1 is to initiate cell cycle entry in early G1.23 Cyclin D1 levels increase transiently in response to mitogenic stimuli. Cyclin D1 binds to, and activates Cdk4 and Cdk6, and thus is critical for the phosphorylation of the retinoblastoma protein and cell cycle progression.18 Indeed, our results showed that after podocyte injury in PHN rats and HIV-transgenic mice, cyclin D1 immunostaining increased significantly. This was associated with cell cycle entry, as judged by markers of DNA synthesis. Taken together, our data suggest that cyclin D1 is present in immature and proliferating podocytes during glomerulogenesis, and that exit from the cell cycle on differentiation is accompanied by reduced expression. However, cell cycle entry after podocyte injury coincided with an increase in cyclin D1 staining. As would be expected cyclin D1 co-immunoprecipitated with Cdk4 in PHN animals but not in control animals. The immunoprecipitation band was identical with the upper phosphorylated and so active form of cyclin D1. Taken together, this data shows that cyclin D1 is not required for normal podocyte differentiation, but rather, levels increase on cell cycle entry.
Our data differs from that of Barasoni and colleagues19 and Nagata and colleagues,2 who showed that cyclin D1 staining increases during the capillary loop stage, and that cyclin D1 is present in normal rodent glomeruli in podocytes. We asked if the difference in our studies was because of antibody specificity, and we therefore extensively characterized the antibodies used in the current study, confirming that both cyclin D1 antibodies were indeed specific. We wondered if the Santa Cruz anti-cyclin D1 antibody used by other groups to identify cyclin D1 was cross-reacting with another antigen on podocytes. Indeed, our data showed that podocytes in cyclin D1−/− mice stained positive using the Santa Cruz antibody. Staining was also positive in cyclin D1+/+ mice using the Santa Cruz antibody. The antigen used to develop this antibody was full-length cyclin D1 of human origin. Therefore, because of the high homology of the different cyclin D isoforms, one interpretation of the studies by Barasoni and colleagues19 and Nagata and colleagues2 is that the Santa Cruz antibody cross-reacts with cyclin D2 and/or D3. This possibility was also raised by the manufacturer.
Barasoni and colleagues19 and Nagata and colleagues,2 suggested that cyclin D1 is required for normal podocyte differentiation. Our data also contradict this, because cyclin D1−/− mice do not have any abnormalities in podocyte differentiation. Park and colleagues24 have shown a role for cyclin D1 after induction of experimental tubular ischemia coinciding with tubular cell proliferation.
Our second major finding in this study was that cyclin D3 expression correlated with podocyte differentiation. Cyclin D3 staining was first detected on acquiring a mature phenotype during glomerulogenesis. Moreover, cyclin D3 levels increased in cultured podocytes on differentiation. These results are therefore the mirror image to cyclin D1. Our data also showed that cyclin D3 levels decreased in proliferating podocytes in HIV-transgenic mice, but not in podocytes in PHN rats, where podocytes maintain their differentiated phenotype.
A role for cyclin D3 has previously been shown in nonrenal cell differentiation. Cyclin D3 increases during myoblast transformation to quiescent myotubes,25,26, and cyclin D3 is required for megakaryocytopoiesis.27,28 In skeletal muscle and sqamous epithelia, proliferation is associated with increased cyclin D1 levels, whereas differentiation is associated with cyclin D3 in these cell types.29 Thus, our data in podocytes are similar to other nonrenal cell types. Taken together, our results are consistent with the notion that cyclin D3 may be critical in podocyte differentiation and therefore the maintenance of podocyte quiescence. We await the possibility to examine cyclin D3−/− mice when available.
Based on this study, we propose that cyclin D1 increases during the proliferative phase of glomerulogenesis, in proliferating podocytes in vitro, and during cell cycle re-entry after injury. We propose that the switch from cyclin D1 to cyclin D3 occurs during podocyte maturation, which may be necessary to maintain podocytes in quiescence. To proliferate, cyclin D1 levels must increase, and cyclin D3 levels decrease. This study therefore shows novel roles for D-type cyclins in podocytes.
Acknowledgments
We thank Vera Fantl, Ph.D., Cancer Research UK, London Research Institute, for providing the cyclin D1−/− mice.
Footnotes
Address reprint requests to Stuart J. Shankland M.D., Division of Nephrology, University of Washington School of Medicine, Box 356521, Seattle, WA 98195. E-mail: stuartjs@u.washington.edu.
Supported by the Public Health Service (grants DK34198, DK52121, DK51096, and DK56799) and the George M. O’Brien Kidney Center (grant DK47659).
References
- Kriz W, Hackenthal E, Nobiling R, Sakai T, Elger M. A role for podocytes to counteract capillary wall distention. Kidney Int. 1994;45:369–376. doi: 10.1038/ki.1994.47. [DOI] [PubMed] [Google Scholar]
- Nagata M, Nakayama K-I, Terada Y, Hoshi S, Watanabe T. Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol. 1998;153:1511–1520. doi: 10.1016/s0002-9440(10)65739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankland SJ, Al-Douahji M. Cell cycle regulatory proteins in glomerular disease. Exp Nephrol. 1999;7:207–211. doi: 10.1159/000020603. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. 2000;58:137–143. doi: 10.1046/j.1523-1755.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D’Agati V, Alpers CE. Differential expression of CDK-inhibitors in human glomerular disease: role in podocyte proliferation and differentiation. Kidney Int. 2000;58:674–683. doi: 10.1046/j.1523-1755.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Sluss HK, Sherr CJ, Matsushime H, Kato J, Livingston DM. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Saxen L. Cambridge: Cambridge University Press,; Organogenesis of the Kidney. 1997:pp 1–165. [Google Scholar]
- Shankland SJ, Floege J, Thomas SE, Nangaku M, Hugo C, Pippin J, Henne K, Hockenberry DM, Johnson RJ, Couser WG. Cyclin kinase inhibitors are increased during experimental membranous nephropathy: potential role in limiting glomerular epithelial cell proliferation in vivo. Kidney Int. 1997;52:404–413. doi: 10.1038/ki.1997.347. [DOI] [PubMed] [Google Scholar]
- Floege J, Kriz W, Schulze M, Susani M, Kerjaschki D, Mooney A, Couser WG, Koch KM. Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephrology. J Clin Invest. 1996;96:2809–2819. doi: 10.1172/JCI118351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley GM, Benson ES. Philadelphia: WB Saunders,; Examination of the Urine. 1974 [Google Scholar]
- Kajiyama W, Kopp JB, Marinos NJ, Klotman PE, Dickie P. Glomerulosclerosis and viral gene expression in HIV-transgenic mice: role of nef. Kidney Int. 2000;58:1148–1159. doi: 10.1046/j.1523-1755.2000.00271.x. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Hugo C, Coats SR, Nangaku M, Pichler RH, Gordon KL, Pippin J, Roberts JM, Couser WG, Johnson RJ. Changes in cell cycle protein expression during experimental mesangial proliferative glomerulonephritis. Kidney Int. 1996;50:1230–1239. doi: 10.1038/ki.1996.432. [DOI] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Bruggeman LA, Mundel P, D’Agati VD, Klotman PE. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 2000;58:173–181. doi: 10.1046/j.1523-1755.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Menninger J, Beach D, Ward DC. Molecular cloning and chromosomal mapping of CCND genes encoding human D-type cyclins. Genomics. 1992;13:575–584. doi: 10.1016/0888-7543(92)90127-e. [DOI] [PubMed] [Google Scholar]
- Kriz W. Progressive renal failure—inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant. 1996;11:1738–1742. [PubMed] [Google Scholar]
- Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Park SK, Kang MJ, Kim W, Koh GY. Renal tubule regeneration after ischemic injury is coupled to the up-regulation and activation of cyclins and cyclin dependent kinases. Kidney Int. 1997;52:706–714. doi: 10.1038/ki.1997.386. [DOI] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Spicer DB, Lassar AB. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- Kiess M, Gill RM, Hamel PA. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation of myoblasts into quiescent myotubes. Oncogene. 1995;10:159–166. [PubMed] [Google Scholar]
- Wang Z, Zhang Y, Kamen D, Lees E, Ravid K. Cyclin D3 is essential for megakaryocytopoiesis. Blood. 1995;86:3783–3788. [PubMed] [Google Scholar]
- Zimmet JM, Toselli P, Ravid K. Cyclin D3 and megakaryocyte development: exploration of a transgenic phenotype. Stem Cells. 1998;16(Suppl 2):97–106. doi: 10.1002/stem.5530160713. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Strauss M, Bartek J. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene. 1998;17:1027–1037. doi: 10.1038/sj.onc.1202016. [DOI] [PubMed] [Google Scholar]