Abstract
Tissue-nonspecific alkaline phosphatase (TNAP) hydrolyzes the mineralization inhibitor inorganic pyrophosphate (PPi). Deletion of the TNAP gene (Akp2) in mice results in hypophosphatasia characterized by elevated levels of PPi and poorly mineralized bones, which are rescued by deletion of nucleotide pyrophosphatase phosphodiesterase 1 (NPP1) that generates PPi. Mice deficient in NPP1 (Enpp1−/−), or defective in the PPi channeling function of ANK (ank/ank), have decreased levels of extracellular PPi and are hypermineralized. Given the similarity in function between ANK and NPP1 we crossbred Akp2−/− mice to ank/ank mice and found a partial normalization of the mineralization phenotypes and PPi levels. Examination of Enpp1−/− and ank/ank mice revealed that Enpp1−/− mice have a more severe hypermineralized phenotype than ank/ank mice and that NPP1 but not ANK localizes to matrix vesicles, suggesting that failure of ANK deficiency to correct hypomineralization in Akp2−/− mice reflects the lack of ANK activity in the matrix vesicle compartment. We also found that the mineralization inhibitor osteopontin (OPN) was increased in Akp2−/−, and decreased in ank/ank mice. PPi and OPN levels were normalized in [Akp2−/−; Enpp1−/−] and [Akp2−/−; ank/ank] mice, at both the mRNA level and in serum. Wild-type osteoblasts treated with PPi showed an increase in OPN, and a decrease in Enpp1 and Ank expression. Thus TNAP, NPP1, and ANK coordinately regulate PPi and OPN levels. The hypomineralization observed in Akp2−/− mice arises from the combined inhibitory effects of PPi and OPN. In contrast, NPP1 or ANK deficiencies cause a decrease in the PPi and OPN pools that leads to hypermineralization.
Bone mineralization is a tightly controlled process, the initial stages of which begin in chondrocyte- and osteoblast-derived matrix vesicles (MVs).1,2 MVs contain calcium and inorganic phosphate ions (Pi) and it is within the MVs that the first crystals of hydroxyapatite are formed. These crystals grow and are exposed to the extracellular environment because of protrusion through the MV membrane. Exposure of the hydroxyapatite crystals to the extracellular milieu further enables growth and proliferation of the crystals.3–5 Inorganic pyrophosphate (PPi) antagonizes the ability of Pi to crystallize with calcium to form hydroxyapatite and thereby suppresses hydroxyapatite crystal propagation. For normal mineral deposition to proceed, a tight balance is required between the levels of extracellular Pi and PPi.
Three molecules have been identified as central regulators of extracellular PPi and Pi levels (Table 1), ie, tissue-nonspecific alkaline phosphatase (TNAP), which hydrolyzes PPi,6–10 nucleotide pyrophosphatase phosphodiesterase 1 (NPP1), which generates PPi from nucleoside triphosphates,11–13 and the multiple-pass transmembrane protein ANK, which mediates intracellular to extracellular channeling of PPi.14,15
Table 1.
Glossary of the Genes and Proteins Studied in This Article
| Gene symbol | Protein symbol | Common name; synonyms |
|---|---|---|
| Akp2 | TNAP | Tissue-nonspecific alkaline phosphatase; TNSALP |
| Enpp1 | NPP1 | Nucleotide pyrophosphatase phosphodiesterase-1; PC-1 |
| Ank (wild-type allele) and ank (mutated allele) | ANK | Ankylosis protein |
| Opn | OPN | Osteopontin |
TNAP is an important promoter of mineralization because it catalyzes the hydrolysis of PPi thereby decreasing the concentrations of this calcification inhibitor, while concomitantly increasing the levels of Pi. Mice in which the TNAP gene has been inactivated (Akp2−/−) mimic the most severe form of hypophosphatasia, a disease characterized by rickets, osteomalacia, spontaneous bone fractures, and increased PPi levels.16,17 Akp2−/− skeletal preparations show poor mineralization in the parietal bones, scapulae, vertebral bones, and ribs.18–20
NPP1 serves as a physiological inhibitor of calcification, at least in part by generating PPi.11–13,21 In human infants, severe NPP1 deficiency states were recently linked to a syndrome of spontaneous infantile arterial and periarticular calcification.22,23 NPP1 knockout mice (Enpp1−/−) also known as tiptoe walking (ttw/ttw) mice, spontaneously develop progressive ankylosing intervertebral and peripheral joint hyperostosis and articular cartilage calcification.24–29 Despite the different manner in which NPP1 and ANK supply PPi to bone matrix, a similar phenotype is associated with a naturally occurring truncation mutation of the C-terminal cytosolic domain of ANK that appears to attenuate PPi channeling in ank/ank mutant mice.14,15,30
The TNAP-, NPP1-, and ANK-deficient mice all have altered levels of PPi and thus, these mice are valuable tools to further understand the function of PPi in the process of bone mineralization. Central to our understanding of this process is not only how osteoblasts and chondrocytes make and dispose of PPi, but what downstream effects PPi has on bone forming cells, in particular osteoblast gene expression. We have previously shown that crossbreeding the Akp2−/− and the Enpp1−/− mice rescues the PPi levels of the single-deficient animals, resulting in a correction of the mineralization defects.10 Given the similarity in function of NPP1 and ANK, and also the similarity in phenotype of the deficient mice, here we investigated whether simultaneously affecting TNAP and ANK function would also ameliorate the mineralization defects of the single-deficient mice as previously observed for the [Akp2−/−; Enpp1−/−] double deficiency. We also examined the effects of PPi on osteoblastic gene expression. It is well established that during the process of normal bone differentiation and subsequent deposition of mineral, a number of osteoblast marker genes are expressed in a defined spatial and temporal manner. We have recently shown that osteopontin (OPN), a putative inhibitor of mineralization,31–33 is down-regulated in the hypermineralized Enpp1 and ank/ank mice.29 Here we investigated the changes in OPN expression in the single- and double-knockout mice and the effects of TNAP-, ANK-, and NPP1-mediated alterations in PPi levels on both OPN expression and hydroxyapatite deposition. Our data have enabled us to build a model of the concerted action of these three molecules in controlling normal mineralization that also explains the pathological abnormalities in hypo- and hypermineralization disorders.
Materials and Methods
Reagents
All routine chemicals were of an analytical grade from Sigma (St. Louis, MO), unless otherwise indicated.
Generation and Maintenance of Akp2−/−, Enpp1−/−, and [Akp2−/−; ank/ank] Mice
The generation and characterization of Akp2−/−, Enpp1−/−, ank/ank, and [Akp2−/−; Enpp1−/−] mice has been reported previously.10,19,20,26 Mice carrying the ank mutation14,15 were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred into the Akp2−/− strain to generate [Akp2−/−; ank/ank] double-deficient mice. To determine genotypes, genomic DNA was isolated from tails and analyzed using polymerase chain reaction (PCR) protocols.10,29 Southern blots were used to confirm the double-knockout genotypes.
Tissue Preparation and Morphological Analysis
Whole-mount skeletal preparations were prepared by removal of skin and viscera of mice followed by a 1-week immersion in 100% ethanol, followed by 100% acetone. Samples were then transferred to a 100% ethanol solution containing 0.01% Alizarin Red S, 0.015% Alcian Blue 8GX, and 0.5% acetic acid for 3 weeks. Samples were then destained with 1% (v/v) KOH/50% glycerol solution. Cleared samples were stored in 100% glycerol. For analysis of mineral deposition, the lumbar spines from 10-day-old mice were fixed in 10% neutral buffered formalin for 5 days. After washing in phosphate-buffered saline-based sucrose solutions, samples were embedded in optimal cutting temperature (OCT) solution. Sections (16 μm) were stained using the von Kossa procedure as previously described.34 The degree of mineralization of the spines was quantified by examining the vertebral apophyses of the von Kossa-stained lumbar sections from each respective genotype. The number of ossification centers containing visible mineral deposits (stained black/brown) versus those containing no deposits, was determined using low (×16) magnification. Three sections, each containing a minimum of five vertebrae, were counted per mouse and the percentage of mineralized apophyses was plotted as a function of the Akp2 and ANK genotypes. The number of mice examined for each genotype were: [Akp2+/+; Ank/Ank], n = 2; [Akp2+/+; Ank/ank], n = 7; [Akp2+/+; ank/ank], n = 2; [Akp2+/−; Ank/Ank], n = 8; [Akp2+/−; Ank/ank], n = 9; [Akp2+/−; ank/ank], n = 3; [Akp2−/−; Ank/Ank], n = 2; [Akp2−/−; Ank/ank], n = 9; and [Akp2−/−; ank/ank], n = 2. For immunohistochemical analysis, mouse skeletal tissues were dissected and fixed in 10% neutral buffered formalin for 2 days and then decalcified in 4% hydrochloric acid, processed for histology, and embedded in paraffin. For detection of OPN, sections were deparaffinized, blocked with 10% goat serum for 20 minutes, and incubated overnight at 4°C with rabbit polyclonal antibody to OPN (Chemicon, Temecula, CA). Washed sections were incubated for 1 hour at 22°C with biotinylated goat anti-rabbit IgG followed by a 1-hour incubation with peroxidase-conjugated avidin. Peroxidase activity was detected using the Fast DAB staining kit (Sigma), according to the manufacturer’s instructions.
Isolation and Culture of Primary Calvarial Osteoblasts
Mouse calvarial cells were isolated from 3-day-old mice through sequential collagenase digestion, as previously described.10 Calvarial cells of the same genotype were pooled and plated at a density of 4 × 104 cells/cm2 in α-MEM (Life Technologies, Inc., Grand Island, NY), supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, and 1% l-glutamine. The cells were incubated in a humidified atmosphere of 5% CO2 in air at 37°C. The medium was completely replaced every third day. For studies under mineralizing conditions, media were supplemented with β-glycerophosphate (10 mmol/L) and l-ascorbic acid (50 μg/ml).
Analysis of PPi Levels
PPi levels were determined by differential adsorption on activated charcoal of UDP-D-[6-3H]glucose (Amersham Biosciences, Piscataway, NJ) from the reaction product of 6-phospho[6-3H]gluconate, as previously described.12,13 To determine intracellular PPi, washed cells were heated at 65°C for 45 minutes, washed again, and lysed in 1% Triton X-100, 1.6 mmol/L MgCl2, 0.2 mol/L Tris, pH 8.1 (lysis buffer).12 Extracellular PPi was determined from conditioned media treated in the same manner.12 We determined cell protein, and specific activity of nucleosidetriphosphate pyrophosphohydrolase and alkaline phosphatase activities as described.13
Western Blot Analysis of NPP1 and ANK Localization
Primary calvarial osteoblasts were treated with mineralization media for 14 days and the cell-associated MVs were collected by collagenase digestion for 2 hours at 37°C. The supernatant was collected and initially centrifuged at 20,000 × g for 20 minutes at 4°C to pellet cellular debris. This was followed by centrifugation at 100,000 × g for 1 hour to isolate the MV fraction, which was resuspended in Hanks’ balanced salt solution. Fifty μg of protein was used for Western blotting from both the MV fraction as well as the cell lysates collected in the first centrifugation. Western blot analysis was performed as previously described using rabbit anti-mouse ANK,35 and rabbit anti-mouse NPP1.15
RNA Isolation and Reverse Transcriptase (RT)-PCR
Total RNA was isolated from osteoblasts using 0.5 ml of Trizol (Life Technologies)/35-mm dish and was reverse-transcribed using the Titanium One-Step RT-PCR kit (Invitrogen, San Diego, CA) according to the manufacturer’s instructions. The primers used were as follows: Mouse Opn (5′-CTCCCGGAGAAAGTGACTGA-3′, 5′-GACCTCAGAAGATGAACTAT-3′), mouse Akp2 (5′-AGTCCGTGGGCATTGTGACTA-3′, 5′-TGCTGCTCCACTCACGTC GAT-3′), mouse Ank (5′-CTAGCAGGGTTTGTGGGAGAA-3′, 5-TTTATGAAGCAGGGG CGTGAA-3), mouse Enpp1 (5′-GCTACAGCTTTCTAGCCATGA-3′, TTAATCCAAGCC CAGGTCCTT-3′). Mouse β-actin primers were used as a loading control. The PCR products were separated by electrophoresis on 1% agarose gels and were visualized by ethidium bromide staining with UV light illumination.
OPN Enzyme-Linked Immunosorbent Assay
To measure OPN protein levels, we developed an enzyme-linked immunosorbent assay based on a previously published method36 using plates coated with a monoclonal antibody to native OPN (Chemicon) using a 1:2500 dilution of OPN antibody in 100 μl/well 0.1 mol/L sodium bicarbonate, pH 9.0, at 4°C overnight. Wells were blocked with 10 mmol/L Tris, 150 mmol/L NaCl, and 0.05% Tween-20, pH 8.0, for 1 hour at 22°C. Samples, diluted in 1% bovine serum albumin, 10 mmol/L Tris, and 150 mmol/L NaCl, pH 8.0, were added to the wells and incubated for 1 hour at 37°C. Recombinant murine OPN was used as standard. Washed wells were incubated sequentially for 1 hour at 37°C with rabbit anti-OPN (1:1000, Chemicon), biotinylated goat anti-rabbit IgG (1:1000), and streptavidin conjugated with AP (1:500 dilution). Color was developed using p-nitrophenylphosphate and read at 405 nm.
Results
Crossbreeding Akp2−/− Mice to ank/ank Mutant Mice Partially Rescues the Mineralization Defects Observed in the Single Mutants
We have previously shown that the simultaneous deletion of the Akp2 and Enpp1 genes can rescue the mineralization defects of both the Akp2 and Enpp1 single-knockout mice.10 The transmembrane protein ANK acts as a transport channel for PPi, and therefore has a similar function to NPP1 in that it also increases extracellular PPi levels. Truncation mutation of the C-terminal intracellular domain of ANK in ank/ank mutant mice, results in a hypermineralized phenotype, called murine progressive ankylosis14,15 and these mice show remarkable similarity in phenotype to the NPP1-deficient mice.
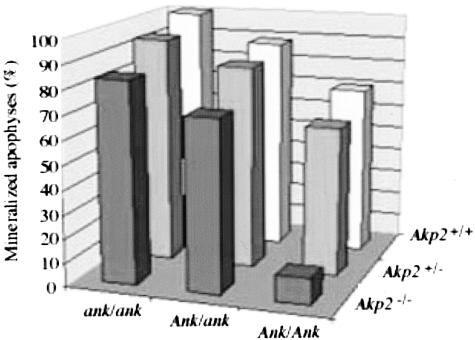
Given this similarity in function between ANK and NPP1, we examined whether a double deficiency in both the Ank and Akp2 genes could rescue the mineralization defects of both the Akp2−/− and ank/ank mutant mice. To this end we crossbred ank/ank mutant mice to Akp2+/− heterozygote mice to obtain [Akp2−/−; ank/ank] double-mutant mice. We examined the degree of mineralization in the spines of [Akp2−/−; ank/ank] mice using von Kossa staining to detect mineral deposition because we have previously shown10 that the vertebral apophyses provide a sensitive indication of the extent of hypo- and hypermineralization. We calculated the percentage mineralization of the apophyses in both the single- and double-mutant animals (Figure 1). As expected, Akp2−/− mice showed a clear depression in the level of mineralization of the vertebrae, with only 10% of the vertebral apophyses mineralized. The ank/ank mutant animals had an increased level of mineralization in the spine, with 100% mineralization in comparison to 70% in wild-type controls. Mice doubly deficient in both Akp2 and Ank, showed an improvement in the levels of abnormal mineralization (80%) in comparison to the Akp2−/− single-knockout (10%) and the ank/ank mutant mice (100%). In Akp2−/− animals the level of mineralization was ameliorated by mutation of just one Ank allele in these mice as the [Akp2−/−; Ank/ank] mice showed normalized levels of mineralization, ∼70%, ie, control levels. In the [Akp2−/−; ank/ank] double-mutant animals there remained a higher degree of mineralization than in control mice, but the percentages were lower than in the ank/ank mutant animals. Thus, we conclude that crossbreeding Akp2−/− mice to ank/ank mice results in a partial rescue of the abnormal mineralization of both single-mutant mice.
Figure 1.
[Akp2−/−; ank/ank] mice display a partial correction in mineralization defects. The degree of mineralization in the vertebral apophyses of the various single- and double-knockout animals was determined by von Kossa staining of spine sections as described in Materials and Methods. The percentage of mineralized apophyses is plotted as a function of Akp2 and Ank genotypes.
PPi Levels Are Partially Corrected in the [Akp2−/−; ank/ank] Double-Deficient Mice
Given the partial correction of the mineralization defects that we observed in [Akp2−/−; ank/ank] mice, we next examined whether the levels of PPi were also affected in these animals. In both the Akp2−/− mice and the ank/ank mutant animals, PPi levels are altered (Figure 2). In Akp2−/− mice elevated levels of PPi result from the lack of the pyrophosphatase activity of TNAP whereas in ank/ank mutant mice there is a decrease in extracellular levels of PPi because of the absence of ANK-mediated transport of PPi across the plasma membrane. The ank/ank mutant mice although displaying decreased levels of extracellular PPi, have increased levels of intracellular PPi.
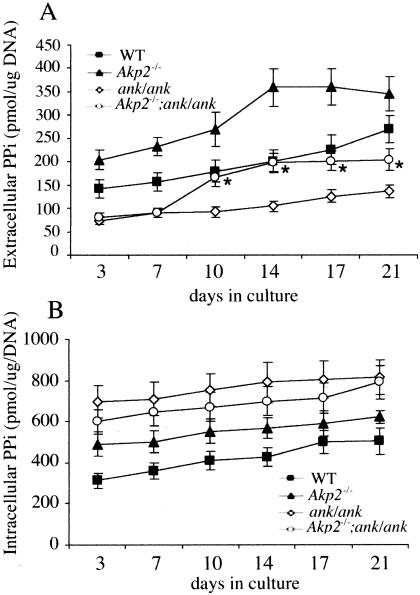
Figure 2.
Intracellular levels of PPi are normalized in [Akp2−/−; ank/ank] mice. Primary osteoblasts were cultured in the presence of mineralizing media, ie, β-glycerophosphate (10 mmol/L) and l-ascorbic acid (50 μg/ml). At the indicated days, extracellular (A) and intracellular (B) PPi levels were determined as described in the Materials and Methods. n = 9; *, P < 0.05 relative to Akp2−/− and ank/ank.
We determined the profile of both intra- and extracellular PPi levels from calvarial osteoblasts isolated from the respective knockout or mutant mice throughout the course of a 21-day bone nodule assay (Figure 2, A and B). Interestingly, we found that the extracellular levels of PPi were significantly normalized in the [Akp2−/−; ank/ank] osteoblasts during the latter stages of differentiation. However, for intracellular levels of PPi, although there is a tendency toward normalization, the changes are not statistically significant.
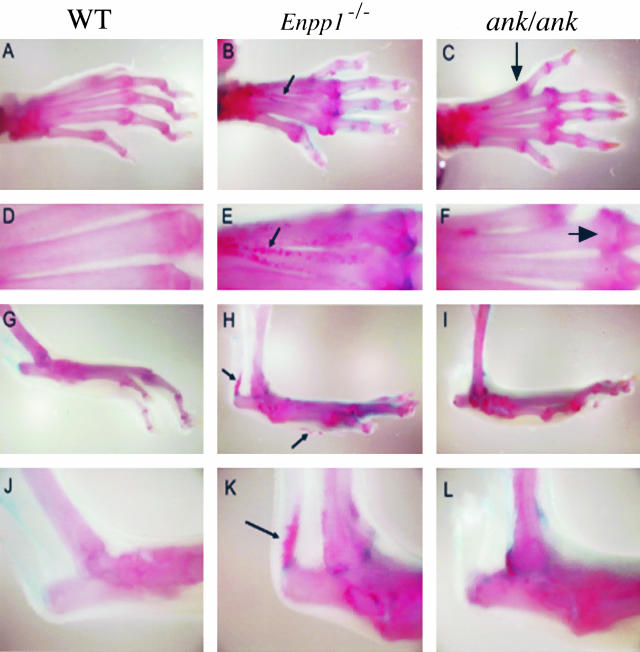
It seems, from the partial correction in both skeletal mineralization and PPi concentrations, that the Ank and Enpp1 deficiencies may not be identical because crossbreeding ank/ank mice to Akp2−/− mice does not result in the same degree of phenotypic rescue observed in the [Akp2−/−; Enpp1−/−] double-knockout mice. This led us to compare more closely the degree of mineralization in both the Enpp1−/− and ank/ank mutant animals. Whole mount examination of their Alizarin Red-stained skeletons consistently revealed subtle differences in the soft tissue ossification phenotypes of these mice. It appears that the Enpp1-knockout mice have a more severe phenotype than ank/ank mutant mice (Figure 3). The Enpp1−/− mice display soft tissue mineralization in the metatarsal bones (Figure 3, B and E) as well as ossification of the Achilles tendon (Figure 3, H and K), whereas the ank/ank mutants, although clearly displaying hyperossification (Figure 3C), do not have the same degree of hypermineralization as observed in the Enpp1−/− mice.
Figure 3.
Whole mount skeletal preparations of wild-type (WT), Enpp1−/−, and ank/ank mice. The specimens were stained with Alizarin Red. Enpp1−/− mice display a more severe hyperossification phenotype than ank/ank mice. C: Large arrows indicate the increased amount of mineral in the phalanges of the ank/ank mice. Small arrows indicate the areas of soft tissue mineralization in the metatarsal bones of Enpp1−/− mice (B, E), ossification of the Achilles tendon is also observed (H, K).
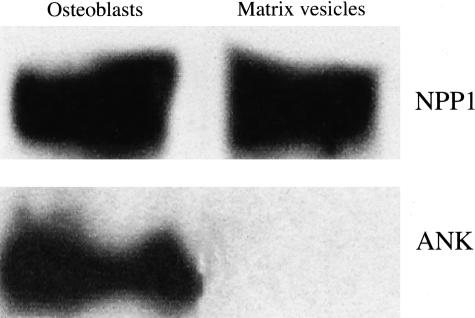
To further examine the differences between NPP1 and ANK, we crossbred Enpp1−/− and ank/ank mice. We surmised that if these molecules act on separate pathways, [Enpp1−/−; ank/ank] double-deficient mice would have a greater degree of hypermineralization than the single-mutant animals. Indeed, [Enpp1−/−; ank/ank] double-deficient mice display a greater degree of paraspinal ligament ossification than the single-deficient mice as determined by von Kossa staining of the spines (Figure 4). This implies that NPP1 and ANK have distinct effects on extracellular PPi concentrations, and therefore on mineralization. This was confirmed by examination of the ANK and NPP1 localization in osteoblasts and MVs. Western blot analysis of ANK and NPP1 localization revealed that both are present in osteoblasts but only NPP1 is present in MVs (Figure 5). This suggests that the localization of NPP1 and ANK to different microenvironments might account for the different mineralization abnormalities and degree of rescue observed in the Enpp1−/− and ank/ank models.
Figure 4.
Mineral deposition in [Enpp1−/−; ank/ank] double-deficient mice. Nondecalcified lumbar spine sections were stained using the von Kossa technique to observe mineral deposition. Areas of excess mineral deposition are observed (arrows) in the Enpp1−/−, ank/ank, and [Enpp1−/−; ank/ank] panels. There is more severe vertebral joint ossification in the double mutant mice than that observed in single-deficient mice.
Figure 5.
Localization of ANK and NPP1 in osteoblasts and MVs. Protein extracts of wild-type osteoblast lysates and osteoblast-derived MVs were used to perform Western blot analysis of ANK and NPP1 as described in Materials and Methods. NPP1 was detected in both osteoblast lysates and MVs, but ANK was only detected in osteoblast lysates.
Osteopontin Expression Is Affected in Akp2−/− and ank/ank Mutant Osteoblasts
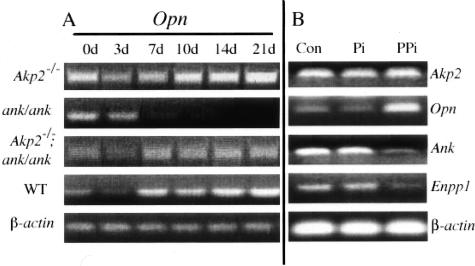
We next investigated the downstream events associated with either an increase or a decrease of extracellular PPi. The mineralization defects observed in the Akp2-, Enpp1-, and Ank-deficient mice suggest a defect in the osteoblast compartment, because it is in the osteoblast that the process of bio-mineralization is initiated. During the process of normal bone mineralization a number of osteoblast marker genes are expressed in a well-defined spatial and temporal manner. We have previously shown that one of these markers, OPN, which is an inhibitor of mineralization,31,37 is depressed at the mRNA level in osteoblasts isolated from Enpp1−/− mice.29 We therefore examined the expression of Opn in calvarial osteoblast cells isolated from Akp2−/− and ank/ank mice as well as the [Akp2−/−; ank/ank] double-deficient mice (Figure 6A). We found that throughout the 21-day period of differentiation in a bone nodule assay, Opn levels were altered in both the Akp2−/− and the ank/ank mice in comparison to wild-type controls. In Akp2−/− calvarial osteoblasts, Opn expression was markedly increased throughout the course of the differentiation period. In contrast, in ank/ank osteoblasts cells, Opn expression was comparable to that of wild-type cells during the initial stages of differentiation, up to day 3, but was completely shut-off during the later stages. Interestingly, we observed a normalization of Opn expression in [Akp2−/−; ank/ank] mice, although the levels were not restored to those of wild-type controls. The partial normalization in Opn levels in the [Akp2−/−; ank/ank] osteoblasts parallels the partial correction observed in the mineralization defects and PPi concentrations.
Figure 6.
Expression of Opn in Akp2−/−, ank/ank, and [Akp2−/−; ank/ank] mice. A: Primary osteoblasts from each respective genotype were cultured in the presence of β-glycerophosphate (10 mmol/L) and l-ascorbic acid (50 μg/ml). At the indicated days total RNA was extracted and RT-PCR analysis was performed to determine the expression pattern of osteopontin (Opn). β-actin was used as a loading control for all genotypes, the wild-type (WT) levels of β-actin are shown. B: Wild-type primary osteoblasts were cultured in the presence or absence of Pi (500 pmol/L) or PPi (500 pmol/L), after 24 hours total RNA was isolated and RT-PCR analysis was performed for Akp2, Opn, Ank, and Enpp1 expression. β-actin was used as a loading control.
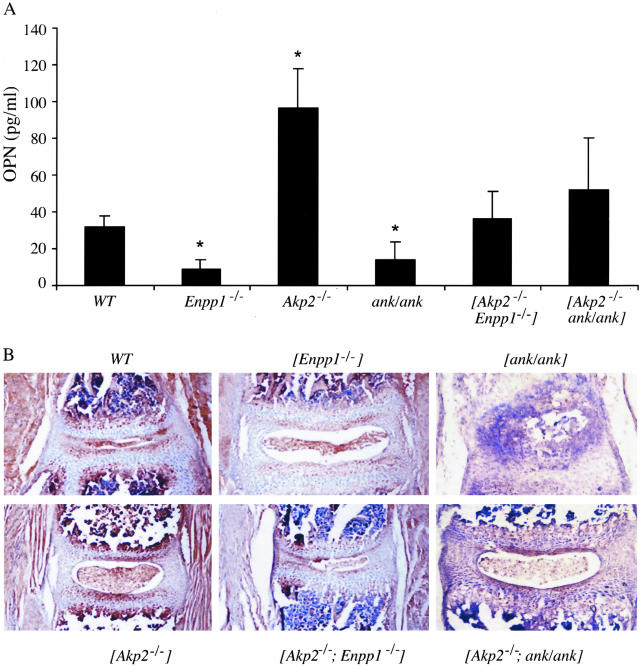
We next investigated whether this effect on Opn expression in the Akp2−/− and ank/ank mice was because of changes in the concentrations of PPi given that these mice have elevated (Akp2−/−), or depressed (ank/ank), levels of PPi. To this end we determined whether in fact PPi is able to modulate Opn expression in osteoblasts (Figure 6B). Addition of exogenous PPi to wild-type calvarial osteoblasts caused an increase in Opn mRNA levels in response to PPi. This effect is because of PPi, and not its hydrolysis product Pi because at these concentrations Pi did not affect OPN expression in the wild-type osteoblast cells. Interestingly, PPi levels affect not only Opn expression, but also Enpp1 and Ank expression. In response to PPi treatment, both Enpp1 and Ank expression are depressed, thus suggesting that both NPP1 and ANK can influence Opn expression through their regulation of PPi concentrations. These effects on Opn expression were not only observed at the mRNA level, but were also reflected in changes in serum levels of circulating OPN in the various single- and double-mutant mouse models (Figure 7A). This analysis revealed a similar pattern of OPN changes as observed at the mRNA level in that serum levels of OPN were elevated in Akp2−/− mice and depressed in ank/ank mice. In the [Akp2−/−; ank/ank] double-knockout mice we observed a normalization of serum OPN levels. Furthermore, we measured serum OPN in the Enpp1−/− mice and observed a similar reduction in concentration as that observed in the ank/ank mice. As with the [Akp2−/−; ank/ank] mice, we observed a significant correction in serum OPN levels in the [Akp2−/−; Enpp1−/−] double-knockout mice. In addition to serum level of OPN, we also looked at the in situ localization of OPN in bone. Immunohistochemical analysis of spine sections from these mice revealed changes in OPN expression that paralleled the mRNA data as well as the changes in serum levels of OPN (Figure 7B). Akp2−/− mice display an increase in OPN expression in the vertebral apophyses in comparison to wild-type animals, whereas in analogous locations in Enpp1−/− and ank/ank mice, OPN expression is lower compared to wild-type controls. The [Akp2−/−; Enpp1−/−] and [Akp2−/−; ank/ank] mice display OPN levels comparable to control animals (Figure 7B).
Figure 7.
OPN levels in Akp2−/−, Enpp1−/−, and ank/ank mice. A: The serum OPN levels from the indicated mice were measured using an enzyme-linked immunosorbent assay technique as described in Materials and Methods. Serum OPN levels are depressed in Enpp1−/− and ank/ank mice, and are elevated in Akp2−/− mice. OPN levels are normalized in the [Akp2−/−; Enpp1−/−] and [Akp2−/−; ank/ank] mice. n = 5; *, P > 0.05 relative to wild-type. B: Immunohistochemical analysis of the distribution of OPN in the wild-type (WT), Enpp1−/−, ank/ank, Akp2−/−, [Akp2−/−; Enpp1−/−] and [Akp2−/−; ank/ank] mice vertebral apophyses using rabbit polyclonal anti-OPN as described in Materials and Methods. Original magnifications, ×20.
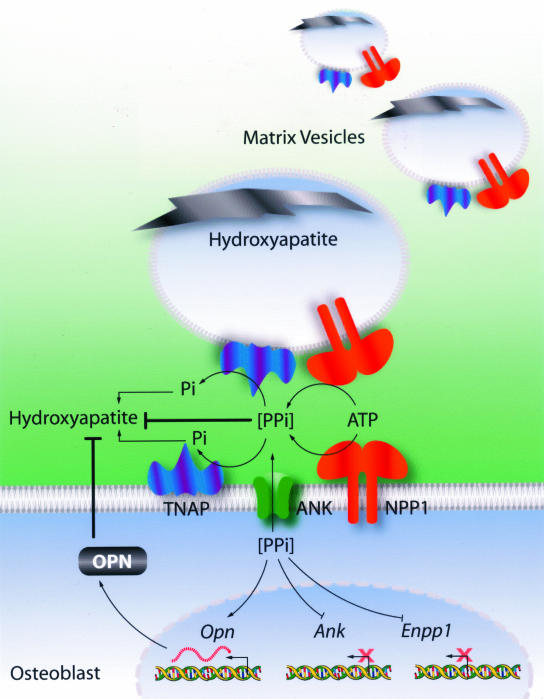
Thus, we conclude that the phenotype of the hypomineralized Akp2−/− mice, and the hypermineralization abnormalities observed in the Enpp1 and ank/ank mutant mice are because of the effects of not one, but at least two mineralization inhibitors (Figure 8). OPN has previously been shown to inhibit hydroxyapatite crystal formation and it is thought that it physically limits crystal growth in bone. We show here that in mice lacking TNAP, there is an increase in OPN levels that results from the increased transcription of the Opn gene induced by elevated concentrations of extracellular PPi. Thus, hypomineralization in the Akp2−/− mice likely ensues as a result of the combined effect of abnormally high levels of both of these mineralization inhibitors, ie, PPi and OPN. In mice lacking NPP1, and in ANK-deficient animals, the depressed levels of PPi lead to reduced levels of OPN, and the decrease in the levels of both mineralization inhibitors leads to an overall increase in mineralization.
Figure 8.
Diagrammatic representation of the roles of TNAP, ANK, NPP1, PPi, and OPN in the regulation of hydroxyapatite deposition. Both NPP1 and ANK raise extracellular levels of PPi while TNAP is required for depletion of the PPi pool. Both TNAP and NPP1 are functional in MVs whereas ANK is not, therefore NPP1 plays a more crucial role in PPi production than ANK. As a result, the absence of NPP1 in Enpp1−/− mice results in a more severe phenotype than in ank/ank mice. A negative feedback loop exists in which PPi, produced by NPP1 and transported by the channeling action of ANK, inhibits expression of the Enpp1 and Ank genes. In addition, PPi induces expression of the Opn gene and production of OPN, which further inhibits mineralization. In the absence of TNAP, high levels of PPi inhibit mineral deposition directly and also via its induction of OPN expression. The combined action of increased concentrations of PPi and OPN causes hypomineralization. In the absence of NPP1 or ANK, low levels of PPi, in addition to a decrease in OPN levels, leads to hypermineralization. This model clearly points to NPP1 and ANK as therapeutic targets for the treatment of hypophosphatasia. Similarly, targeting TNAP function can be useful in the treatment of hypermineralization abnormalities caused by altered PPi metabolism.
Discussion
The restriction of mineral deposition to bone tissues and the regulation of the mineralization process are crucial in maintaining a healthy skeleton. Central in maintaining normal mineralization are Pi and PPi, because mineral deposition is dependent on a balance between the intra- and extracellular levels of these compounds. Impaired hydroxyapatite deposition results in diseases such as hypophosphatasia, in which bones lack mineral because of an excess of the mineralization inhibitor PPi. Conversely a deficiency in PPi results in ectopic calcification and soft tissue mineralization. The Akp2−/−, Enpp1−/− and ank/ank mice all have severe mineralization defects and associated abnormal PPi levels and as such, are valuable tools to examine the possible roles of these genes in controlling mineralization and the effects of PPi on this process.
We have previously shown that the simultaneous deletion of the Akp2 and Enpp1 genes normalizes PPi levels thereby causing a concomitant correction in the mineralization defects of the single-knockout animals.10 These data were significant in that they provided clear confirmation of the previously proposed pyrophosphatase role for TNAP in bone tissue6–9 but also pointed to both NPP1 and TNAP as therapeutic targets in the treatment of mineralization diseases such as hypophosphatasia and osteoarthritis. Given that the ANK protein functions like NPP1, albeit in a different manner, to increase extracellular PPi levels, we examined whether an analogous crossbreeding of ank/ank mice to Akp2−/− mice would correct the mineralization defects of the Akp2−/− and ank/ank single-deficient mice. We show here that the [Akp2−/−; ank/ank] mice displayed a partial correction in the hypo- and hypermineralization phenotypes as measured by the extent of mineralization in the vertebral apophyses of these mice. This partial normalization of mineralization was paralleled by a partial correction in PPi levels. Interestingly, we observed a normalization of extracellular PPi levels in the [Akp2−/−; ank/ank] mice, but the intracellular PPi concentrations in these mice remained abnormal. Presumably this residual abnormality in PPi levels prevented the complete normalization of mineralization in the double-knockout mice, illustrating the importance of a balance between the intra- and extracellular concentrations of PPi in regulating hydroxyapatite deposition. In this context a question that remains unanswered is how ank/ank osteoblasts are able to respond to a pulse of extracellular PPi with an increase in Opn expression, given the high concentration of intracellular PPi inherent in these cells.29
The difference in phenotypic abnormalities between the [Akp2−/−; Enpp1−/−] and the [Akp2−/−; ank/ank] mice led us to examine in detail the degree of hypermineralization in both the Enpp1−/− and ank/ank mutants as well as in the [Enpp1−/−; ank/ank] double-mutant mice. We determined that there are subtle differences in the hypermineralization defects between the ank/ank and Enpp1−/− mice and that the ectopic calcification and soft tissue ossification of the Enpp1−/− mice is more severe than that of the ank/ank mice. Furthermore, the degree of soft-tissue ossification was further increased in the [Enpp1−/−; ank/ank] double-mutants compared to the single-deficient mice, thus indicating that these two molecules act differently because the absence of both results in an additive effect on hypermineralization. The fact that NPP1 localizes to MVs but that ANK is excluded from this extracellular compartment further validates this conclusion.
It is within the membrane-limited microenvironment of the MV that initial mineral accumulation takes place, followed by propagation of the hydroxyapatite crystals that eventually burst through the MV membrane and become exposed to the extracellular matrix.1 It is well established that MVs are enriched in TNAP3,4 and it is now clear that TNAP is essential for the second phase of mineral deposition because MVs from hypophosphatasia patients,38 or from Akp2−/− mice,39 contain mineral that does not propagate. The absence of ANK in MVs suggests that its PPi-channeling function is not required for initiation and propagation of hydroxyapatite crystals and that it is TNAP and NPP1 that are responsible for this process. Thus, the absence of NPP1 in the MVs of Enpp1−/− osteoblasts results in a greater deficit in PPi levels than an absence of ANK, leading to the more severe hypermineralization observed in Enpp1−/− mice. The deficit in extracellular PPi production in ank/ank mice results only from the decreased activity of ANK in the osteoblasts. Presumably there is still a sufficient amount of PPi within the MVs of the ank/ank mice (provided by NPP1) such that the hypermineralization phenotype is not as severe as in Enpp1−/− mice. Furthermore these data explain the only partial rescue in the [Akp2−/−; ank/ank] mice because in these animals there is an excess PPi in the MV (because of NPP1) that will not be hydrolyzed in the absence of TNAP, ie, the levels of PPi in MVs are not normalized in the [Akp2−/−; ank/ank] mice, and thus full rescue does not ensue. In the [Akp2−/−; Enpp1−/−] mice however, there is no excess of PPi in the MVs and so mineral deposition proceeds normally.
Although the Akp2−/−, Enpp1−/−, and ank/ank mice all have severe mineralization phenotypes associated with abnormal PPi levels, it is not known precisely how PPi inhibits the mineralization capacity of osteoblasts. For osteoblasts to lay down mineral, a number of osteoblastic genes must be turned on and off in a well-defined sequence. Several of these characteristic matrix proteins can affect hydroxyapatite deposition and growth.40 One of these factors is OPN, a secreted glycoprotein that has been suggested to physically limit hydroxyapatite formation and deposition both in vivo31,37 and in vitro.41 We have recently shown that OPN may be a potential modulator of PPi effects.29 In this study we examined the effects of PPi on OPN expression in the three relevant mouse models of mineralization defects. In ank/ank and Enpp1−/− primary mouse osteoblasts, OPN expression is inhibited in comparison to control levels. We hypothesized that because TNAP has a crucial role in regulating PPi levels, OPN levels might also be altered in Akp2−/− mice. In fact, we found that Akp2−/− mice showed significant elevations in OPN expression at the mRNA level. A similar pattern in serum protein levels of OPN was observed, with both Enpp1−/− and ank/ank mice showing a decrease in serum OPN, whereas Akp2−/− mice had increased levels. Interestingly, OPN levels appeared normal in [Akp2−/−; Enpp1−/−] and [Akp2−/−; ank/ank] double homozygote mice, which paralleled either a full or partial normalization of mineralization defects and PPi levels, thus suggesting that PPi can regulate OPN expression. It should be noted that the range of OPN concentrations measured in this study (10 to 120 pg/ml) are lower than those reported in other studies (100 to 5000 ng/ml).42,43 This is most likely because of our using serum rather than plasma for our measurements, because it is known that serum levels of OPN are lower than plasma levels.36
To confirm that the depression of OPN in Enpp1−/− and ank/ank, and the increase in OPN in Akp2−/− mice is because of changes in PPi concentrations we examined the effects of exogenous addition of PPi to wild-type osteoblasts. Exogenous PPi resulted in an induction of Opn expression, and in addition, PPi down-regulated expression of both Enpp1 and Ank, although PPi treatment had no significant effect on Akp2 expression. We determined that this modulation of Opn expression is in fact because of PPi and not its hydrolysis product Pi. It has been previously shown that Pi can induce Opn expression,44 however in our study the end concentrations of Pi, resulting from the complete hydrolysis of the added PPi, are significantly lower (500 pmol/L) than that used in the study of Beck and colleagues44 (∼10 mmol/L). We chose a PPi concentration of 500 pmol/L because our previous data has revealed this concentration as the difference between wild-type and Enpp1−/− mice. Therefore, our data supports a direct regulation of Opn by PPi, the product of ANK and NPP1 function, and further suggests a negative feedback pathway by which PPi inhibits Ank and Enpp1 expression.
We therefore conclude that under normal conditions the concerted action of TNAP, NPP1, and ANK regulate intra- and extracellular PPi levels and that this tight regulation is required for controlled mineral deposition. Hypophosphatasia in the Akp2 knockout mice arises from deficits in TNAP activity, resulting in an increase in extracellular PPi levels and a concomitant increase in OPN levels; the combined effect of both these inhibitory factors leads to hypomineralization. This conclusion suggests the testable hypothesis that the simultaneous ablation of the Opn and the Akp2 gene should lead to an improvement in the hypomineralization abnormalities of the Akp2−/− mice. A comparison of the [Akp2−/−; Opn−/−], [Akp2−/−; Enpp1−/−], and [Akp2−/−; ank/ank] mice should give an indication as to the relative contributions of both OPN and PPi in establishing the rickets/osteomalacia phenotype of the Akp2−/− mice.
In turn, an NPP1 or ANK deficiency leads to a decrease in the PPi levels that cause a decrease in the OPN pool. Thus the simultaneous decrease of both these inhibitory factors, ie, PPi and OPN, results in the hypermineralization abnormalities in the Enpp1−/− and ank/ank mice. Because the mineralization defects in Akp2−/− mice, along with elevated PPi and OPN levels, are normalized to varying extents by ablation of either the NPP1 or ANK gene, we conclude that both NPP1 and ANK represent rational therapeutic targets for hypophosphatasia, a disease for which to date there is no treatment. In addition, our current data provide further proof that TNAP is a potential target for hypermineralization diseases such as osteoarthritis and ankylosis.
Acknowledgments
We thank Kenny Venegas, Sara Janke, and Jeffrey Nickel for maintenance and genotyping of mice; Kosi Gramatikoff for generating the illustration for Figure 8; and Dr. Agi Grigoriadis for critical reading of the manuscript.
Footnotes
Address reprint requests to Professor José Luis Millán, Ph.D., The Burnham Institute, 10901 North Torrey Pines Rd., La Jolla, CA 92037. E-mail: millan@burnham.org.
Supported by the National Institutes of Health (grants AR47908, DE12889, and P01 AGO-7996) and the Research Service of the Department of Veterans Affairs.
D.H. and L.H. contributed equally to this work.
References
- Anderson HC. Molecular biology of matrix vesicles. Clin Orthop Res. 1995;314:266–280. [PubMed] [Google Scholar]
- Boskey AL, Boyan BD, Schwartz Z. Matrix vesicles promote mineralization in a gelatin gel. Calcif Tissue Int. 1997;60:309–315. doi: 10.1007/s002239900234. [DOI] [PubMed] [Google Scholar]
- Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci USA. 1970;67:1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akisaka T, Gay CV. The plasma membrane and matrix vesicles of mouse growth plate chondrocytes during differentiation as revealed in freeze-fracture replicas. Am J Anat. 1985;173:269–286. doi: 10.1002/aja.1001730404. [DOI] [PubMed] [Google Scholar]
- Moss DW, Eaton RH, Smith JK, Whitby LG. Association of inorganic-pyrophosphatase activity with human alkaline-phosphatase preparations. Biochem J. 1967;102:53–57. doi: 10.1042/bj1020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeska RJ, Wuthier RE. Studies on matrix vesicles isolated from chick epiphyseal cartilage.. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim Biophys Acta. 1975;391:51–60. doi: 10.1016/0005-2744(75)90151-5. [DOI] [PubMed] [Google Scholar]
- Meyer JL. Studies on matrix vesicles isolated from chick epiphyseal cartilage. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Arch Biochem Biophys. 1984;231:1–8. doi: 10.1016/0005-2744(75)90151-5. [DOI] [PubMed] [Google Scholar]
- Rezende A, Pizauro J, Ciancaglini P, Leone F. Phosphodiesterase activity is a novel property of alkaline phosphatase from osseous plate. Biochem J. 1994;301:517–522. doi: 10.1042/bj3010517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessle L, Johnsson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millán JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R, Rosenbach M, Fong F, Goding J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1. Arthritis Rheum. 1994;37:934–941. doi: 10.1002/art.1780370624. [DOI] [PubMed] [Google Scholar]
- Johnson K, Vaingankar S, Chen Y, Moffa A, Goldring M, Sano K, Jin-Hua P, Sali A, Goding J, Terkeltaub R. Differential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondrocytes. Arthritis Rheum. 1999;42:1986–1997. doi: 10.1002/1529-0131(199909)42:9<1986::AID-ANR26>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Johnson K, Moffa A, Chen Y, Pritzker K, Goding J, Terkeltaub R. Matrix vesicle plasma membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J Bone Miner Res. 1999;14:883–892. doi: 10.1359/jbmr.1999.14.6.883. [DOI] [PubMed] [Google Scholar]
- Hakim FT, Cranley R, Brown KS, Eanes ED, Harne L, Oppenheim JJ. Hereditary joint disorder in progressive ankylosis (ank/ank) mice. I. Association of calcium hydroxyapatite deposition with inflammatory arthropathy. Arthritis Rheum. 1994;27:1411–1420. doi: 10.1002/art.1780271212. [DOI] [PubMed] [Google Scholar]
- Ho AM, Johnson MD, Kingsley DM. Role of mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–269. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocrine Rev. 1994;15:439–461. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- Whyte MP. The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. New York: MacGraw-Hill,; 2001:pp 5313–5329. [Google Scholar]
- Waymire KG, Mahuren JD, Jaje JM, Guilarte TR, Coburn SP, McGregor GR. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet. 1995;11:45–51. doi: 10.1038/ng0995-45. [DOI] [PubMed] [Google Scholar]
- Narisawa N, Frölander N, Millán JL. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, Waymire K, Narisawa S, Millán JL, MacGregor GR, Whyte MP. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- Rutsch R, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, Terkeltaub R. PC-1 Nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nürnberg P. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Okawa A, Nakamura I, Goto S, Moriya H, Nakamura Y, Ikegawa S. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- Sali A, Favaloro JM, Terkeltaub R, Goding JW. Germline deletion of the nucleoside triphosphate pyrophosphohydrolase (NTPPPH) plasma cell membrane glycoprotein-1 (PC-1) produces abnormal calcification of periarticular tissues. Vanduffel L, Lemmems R, editors. Maastricht: Shaker Publishing BV,; Ecto-ATPases and Related Ectoenzymes. 1999:pp 267–282. [Google Scholar]
- Johnson K, Pritzker K, Goding J, Terkeltaub R. The nucleoside triphosphate pyrophosphohydrolase isozyme PC-1 directly promotes cartilage calcification through chondrocyte apoptosis and increased calcium precipitation by mineralizing vesicles. J Rheumatol. 2001;28:2681–2691. [PubMed] [Google Scholar]
- Johnson K, Hashimoto S, Lotz M, Pritzker K, Goding J, Terkeltaub R. Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification. Arthritis Rheum. 2001;44:1071–1081. doi: 10.1002/1529-0131(200105)44:5<1071::AID-ANR187>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Johnson K, Goding J, Van Etten D, Sali A, Hu SI, Farley D, Krug H, Hessle L, Millán JL, Terkeltaub R. Linked deficiencies in extracellular inorganic pyrophosphate (PPi) and osteopontin expression mediate pathologic ossification in PC-1 null mice. J Bone Miner Res. 2003;18:994–1004. doi: 10.1359/jbmr.2003.18.6.994. [DOI] [PubMed] [Google Scholar]
- Nürnberg P, Theile H, Chandler D, Höhne W, Cunningham ML, Ritter H, Leschik G, Uhlmann K, Mischung C, Harrop K, Goldblatt J, Borochowitz ZU, Kotzot D, Westermann F, Mundlos S, Braun HS, Laing N, Tinschert S. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet. 2001;28:37–41. doi: 10.1038/ng0501-37. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Maresca M, Ulrich W, Doty SB, Butler WT, Prince CW. Osteopontinhydroxyapatite interactions in vitro. Inhibition of hydroxyapatite formation and growth in a gelatin gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Kyle CL, Goldberg HA. Modulation of crystal formation by bone phosphoproteins; structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem J. 1994;300:723–728. doi: 10.1042/bj3000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- Narisawa S, Wennberg C, Millán JL. Abnormal vitamin B6 metabolism in alkaline phosphatase knock-out mice causes multiple abnormalities, but not the impaired bone mineralization. J Pathol. 2001;193:125–133. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH722>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Hessle L, Wennberg C, Mauro S, Narisawa S, Goding J, Sano K, Millán JL, Terkeltaub R. Tissue-nonspecific alkaline phosphatase (TNAP) and plasma cell membrane glycoprotein-1 (PC-1) act as selective and mutual antagonists of mineralizing activity by murine osteoblasts. Am J Physiol. 2000;279:R1365–R1377. doi: 10.1152/ajpregu.2000.279.4.R1365. [DOI] [PubMed] [Google Scholar]
- Bautista DS, Saad Z, Chambers AF, Tonkin KS, O’Malley FP, Singhai H, Tokmakejian S, Bramwell V, Harris JF. Quantification of osteopontin in human plasma with an ELISA: basal levels in pre- and postmenopausal women. Clin Biochem. 1996;29:231–239. doi: 10.1016/0009-9120(96)84728-a. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Hsu HH, Morris DC, Fedde KN, Whyte PW. Matrix vesicle in osteomalacic hypophosphatasia bone contain apatite-like mineral crystals. Am J Pathol. 1997;151:1555–1561. [PMC free article] [PubMed] [Google Scholar]
- Anderson HC, Sipe JE, Hessle L, Dhamayamraju R, Atti E, Camacho NP, Millán JL. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL. Matrix proteins and mineralization: an overview. Connect Tissue Res. 1996;35:357–363. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317:59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt DT, Noda M, O’Regan AW, Pavlin D, Bergman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta K, Ishizuka T, Horita S, Hayashi T, Ajiro A, Uchida K, Honda K, Oba T, Kawashima A, Yumura W, Kabaya T, Akiba T, Nihei H. Soluble osteopontin and vascular calcification in hemodialysis patients. Nephron. 2001;89:455–458. doi: 10.1159/000046119. [DOI] [PubMed] [Google Scholar]
- Beck GR, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]