Abstract
Genetic loss of surface Fas antigen expression leads to reduced apoptosis of myeloid and lymphoid progenitor cells, and a propensity to develop autoimmunity and myeloid leukemia in mouse models. Oncogenic p21ras decreases surface Fas antigen expression and renders fibroblasts resistant to Fas mediated apoptosis. Neurofibromin, which is encoded by NF1, is a GTPase activating protein that negatively regulates p21ras activity. NF1 loss leads to deregulation of p21ras-effector pathways, which control myeloid cell survival. Heterozygous inactivation of Nf1 increases mast cell numbers in Nf1 +/− mice, and enhances mast cell survival in response to c-kit ligand (kit-L). Here, we show that Nf1-deficient mast cells have reduced surface Fas antigen expression in response to kit-L and are resistant to Fas ligand-mediated apoptosis. Using genetic intercrosses between Nf1 +/− and class I A-PI-3K-deficient mice, we demonstrate that hyperactivation of the p21ras-class IA PI-3K pathway is the mechanism for this phenotype. Finally, we demonstrate that mast cells from both Fas antigen-deficient mice and Nf1 +/− mice are resistant to apoptosis following kit-L withdrawal in vivo. Thus, therapies designed to decrease p21ras activity and up-regulate Fas antigen expression may limit the pathological accumulation of myeloid cells in disease states where p21ras is hyperactivated.
Mast cells are important mediators of host immune responses and are active cellular participants in allergic inflammation, neurodegenerative disorders, and some cancers.1,2 Local cutaneous mast cell numbers increase during inflammatory processes, tissue repair, and certain tumor microenvironments.1–5 The mechanisms involved in regulating mast cell numbers following these initiating events are not understood, but are likely complex and dependent on local paracrine or autocrine signals, which activate programmed cell death. Several in vitro studies suggest that Fas antigen (Apo-1/CD95) may directly or indirectly regulate the apoptotic program in mast cells.6–8 Further, Fas antigen-deficient mice (lpr/lpr) have significantly increased numbers of myeloid precursors in both the bone marrow and spleen.9 However, the role of Fas antigen signaling in regulating mast cell numbers in vivo in murine models of mast cell hyperplasia has not been investigated.
Mutations in the NF1 tumor suppressor gene cause neurofibromatosis type 1, a pandemic autosomal dominant genetic disorder with an incidence of 1:3500. Neurofibromin, the protein encoded by NF1, functions as a GTPase activating protein (GAP) for p21ras by accelerating the hydrolysis of active p21ras-GTP to inactive p21ras-GDP.10,11 Individuals with NF1 and genetically engineered mice harboring mutations at the Nf1 locus have a number of myeloid cell abnormalities.3,4,12–23 Specifically, NF1 patients develop neurofibromas,24 which are infiltrated with a high density of degranulating mast cells, and recent genetic studies in mice suggest that Nf1 +/− mast cells may play a central role in initiating tumorigenesis.25–28 Further, mice transplanted with Nf1 nullizygous fetal liver hematopoietic stem cells uniformly develop a myeloid leukemia that is highly reminiscent of the juvenile myelomonocytic leukemia observed in children with NF1.19,20,23 Currently, the biochemical and cellular mechanisms underlying the expansion of both myeloid progenitors and mast cells in NF1 patients is poorly understood.
Fas signaling is tightly regulated by several mechanisms including expression of proteins that directly bind Fas or inhibit caspase activity and mitochondrial events.29,30 Recent studies in non-myeloid cells demonstrate that Fas antigen expression is regulated by downstream p21ras signaling pathways.31–33 Specifically, oncogenic p21ras signaling through the phosphoinositide-3-kinase (PI-3K) pathway has been reported to down-regulate Fas antigen expression in immortalized fibroblasts, which renders them resistant to Fas ligand-mediated apoptosis.32 In support of these data, bone marrow-derived mast cells (BMMCs) derived from SH2-containing inositol 5-phosphatase (SHIP)-deficient mice, which have increased PI-3K activation, are insensitive to Fas ligand-mediated cell death.34
The murine c-kit receptor and its ligand, kit-L, are components of a signaling pathway that promote mast cell proliferation and survival.3 We recently demonstrated that Nf1 heterozygous BMMCs have increased p21ras activity and PI-3K activation both at baseline and in response to stimulation with kit-L.3,4 As loss of neurofibromin leads to deregulated p21ras signaling and increased activation of the p21ras-classIA- PI-3K pathway, we tested whether Nf1-deficient BMMCs would have a reduction in Fas ligand-mediated apoptosis and Fas antigen expression. Here, using genetic intercrosses between Nf1 +/− and class IA-PI-3 kinase-deficient mice, we provide genetic and biochemical evidence to demonstrate that Nf1-deficient BMMCs have decreased Fas antigen expression and Fas-ligand mediated apoptosis via hyperactivation of the p21ras-class IA PI-3K signaling pathway. Further, we demonstrate that mast cells from both Fas antigen-deficient mice (lpr/lpr) and Nf1 +/− mice are resistant to apoptosis following kit-L withdrawal in vivo.
Materials and Methods
Animals
Nf1 +/− mice were obtained from Dr. Tyler Jacks at the Massachusetts Institute of Technology (Cambridge, MA) in a C57BL/6.129 background and back-crossed for 13 generations into the C57BL/6J strain. p85α +/− mice were obtained in a C57BL/6J.129 background from Dr. Lewis Cantley at Harvard University (Boston, MA) and back-crossed for 10 generations into a C57BL/6J strain. lpr/lpr mice were obtained in a C57BL/6J background from the Jackson Laboratory (Bar Harbor, ME). Multiple FO founders were used to generate mast cells from embryonic day 13.5 fetal liver from the four F2 Nf1 and p85α genotypes as outlined: FO: Nf1 +/−; +/+ X +/+; p85α +/−. F1: Nf1 +/−; p85α +/− X +/+; p85α +/−. F2: Nf1 +/−; +/+, +/+; p85α −/−, Nf1 +/−; p85α −/−, +/+; +/+. The Nf1 and p85α alleles were genotyped by polymerase chain reaction (PCR) as previously described.4,35 These studies were conducted with a protocol approved by the Indiana University Laboratory Animal Research Center.
Mast Cell Cultures
Bone marrow-derived mast cells (BMMCs) or fetal liver mast cells (FLMCs) from embryonic day 13.5 fetal liver were established exactly as previously described.3,4,36 BMMCs and FLMCs were cultured as previously described with minor modifications, and the homogeneity of BMMCs and FLMCs was determined by Giemsa staining.3,4,37 Aliquots of cells were also stained alcian blue and safranin to confirm that they were mast cells. Furthermore, fluorescence activated cytometeric analysis (FACS) (Becton Dickinson, San Jose, CA) revealed similar forward and side light scatter characteristics and the same percentage of c-kit+ expression in BMMCs and FLMCs for all murine experimental genotypes (data not shown). For assays examining the expression of cellular surface Fas antigen expression or Fas ligand-mediated cell death, 1 × 106 BMMCs or FLMCs were cultured in RPMI media containing 1% L-glutamine (BioWhittaker, Walkersville, MD) in the absence of both growth factors and serum for 16 hours. Mast cells were then cultured in RPMI media containing 10% fetal calf serum (Hyclone, Logan UT), 1% L-glutamine, 2% penicillin/streptomycin (1 mmol/L/ml) (BioWhittaker) and 100 ng/ml of recombinant murine kit-L (Peprotech, Rocky Hill, NJ) for 72 hours. Cells were then examined for expression of Fas antigen or assayed for Fas ligand-mediated apoptosis.
Flow Cytometric Analysis for Fas Antigen Expression
Surface Fas antigen expression was evaluated by fluoresence cytometry. Mast cells were stained for 30 minutes at 4°C with 1 μg/ml of FITC-conjugated anti-Fas monoclonal antibody (Jo-2) (Pharmingen, San Diego, CA) or an isotype control. Cells were washed three times with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) (Sigma, St. Louis, MO) and analyzed by FACS.
Assay for FasL-Induced Cell Death
Following 72 hours of kit-L stimulation (100 ng/ml) as described above, Fas ligand-mediated apoptosis of mast cells was induced by the addition of 100 ng/ml of recombinant human Fas ligand (Upstate Biotech, Waltham, MA). Apoptotic cells were identified by examining DNA fragmentation using the TUNEL method (Roche, Boulder, CO) as previously described.38 Following 16 hours of incubation with recombinant human Fas ligand, TUNEL-positive cells were enumerated by fluorescence cytometry.
Generation of Recombinant Retroviral Plasmids
Previously developed recombinant retrovirus constructs were used in these studies.37 The internal sequences of these constructs are under the transcriptional control of the myeloproliferative sarcoma retrovirus promoter. Constructs also contain a puromycin resistance gene, pac, which is under the transcriptional control of the phosphoglycerate kinase (PGK) promoter. Three viruses were used in these experiments: a virus expressing the full-length NF1 GTPase activating related domain (NF1 GRD) and pac (MSCV-NF1 GRD-pac); a virus expressing a GAP-inactive mutant of the NF1 GRD that harbors a known human mutation in the arginine finger loop (R1276P)39 and pac (MSCV-1276P NF1 GRD-pac); and a virus expressing the selectable marker gene alone (MSCV-pac).37
Retroviral Transduction of BMMCs or FLMCs
The transduction protocol has been previously described and was used here with minor modifications.37 Briefly, embryonic day 13.5 fetal liver cells recovered from genotyped livers or bone marrow mast cells were pre-stimulated for 48 hours in liquid cultures of RPMI containing 20% fetal bovine serum (Hyclone) supplemented with kit-L (100 ng/ml) and interleukin-6 (IL-6) (200 U/ml) (Peprotech). Cells were transduced on mitomycin C-treated E86 producer cells in the presence of kit-L, IL-6, and polybrene (5 μg/ml) for 48 hours. Transduced cells were then cultured under conditions to promote mast cell growth as described previously and as above.37
Detection of p21ras-GTP Levels
BMMCs were deprived of serum and growth factors for 24 hours and stimulated with 10 ng/ml of kit-L for 5 minutes. p21ras activation was subsequently determined using p21ras activation assay kits (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer’s protocol and as described previously.4
PI-3 Kinase and ERK Inhibition
BMMCs were preincubated with 5 μmol/L of LY294002 (Sigma), 50 μmol/L of PD98059 (Cell Signaling, Beverly, MA), or DMSO vehicle control for 2 hours. Following pre-incubation, 100 ng/ml of kit-L was added to cultures for up to 72 hours as described above. Following treatment with the inhibitors or vehicle alone, cells were analyzed for surface Fas antigen expression.
In Vivo Cell Survival Assay
Under light avertin anesthesia, micro-osmotic pumps (Alzet, Cuppertino, CA) containing kit-L were placed under the dorsal back skin of WT, Nf1 +/−, or Fas antigen-deficient (lpr) mice. Osmotic pumps released a continuous infusion of kit-L at a rate of 20 μg/kg/day for 7 days. Mice were sacrificed and skin samples were recovered at the site of kit-L release at various times following pump depletion. Dorsal skin was stained with a drop of India ink at the point of exit of kit-L from the osmotic pump before pump removal. Three-cm sections of skin marked with India ink were removed, fixed in buffered formalin, and processed in paraffin-embedded sections. Specimens were stained with hematoxylin-eosin to assess routine histology and with Giemsa to identify mast cells. Cutaneous mast cells were quantitated in a blinded fashion by counting 2 mm2 sections in proximity to the India ink stain. Mast cells undergoing apoptosis were detected using a DermaTACS in situ apoptosis detection kit (Trevigen, Gaithersburg, MD), which is based on DNA-end labeling using terminal deoxynucleotidyl transferase (TdT) and modified nucleotides. Detection of incorporated molecules is achieved using a chromogenic substrate with a horseradish peroxidase detection system. Reactions were performed without the labeling enzyme as a negative control.
Immunofluorescence
Skin sections were deparaffinized in xylene for 15 minutes then rehydrated in dilutions of ethanol (100%, 95%, 70%) and PBS for 5 minutes each. Sections were then blocked in 10% goat serum plus 3% BSA for 20 minutes and incubated with anti-Fas (Jo2) antibody at a dilution of 1:1000 or isotype control (1:1000) for 1 hour at room temperature. In situ immunofluorescence was assess using a Zeiss Axioskope 20 immunofluorescent microscope system (Upstate Biotech, Waltham, MA).
Results
Loss of Nf1 Results in Resistance to Fas Ligand-Mediated Apoptosis and a Reduction in Surface Fas Antigen Expression
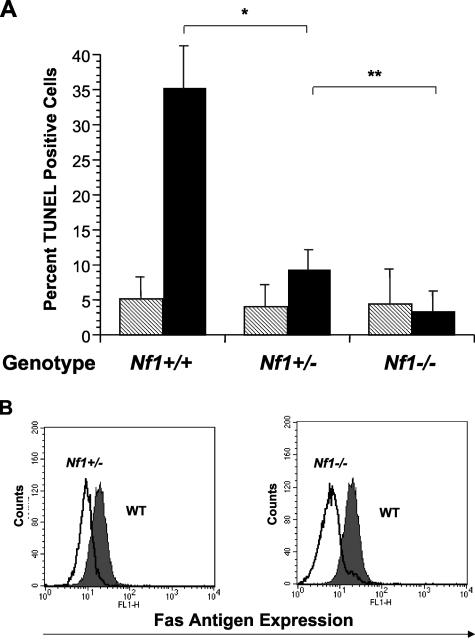
Loss of Nf1 increases p21ras activity in Nf1 +/− mast cells and Nf1 −/− bone marrow cells.4,19,20 To examine whether loss of Nf1 renders mast cells resistant to Fas ligand-mediated apoptosis, WT, Nf1 +/− and Nf1 −/− BMMCs were cultured in kit-L (100 ng/ml) for 72 hours and then stimulated with recombinant Fas ligand (100 ng/ml) or vehicle control for 16 hours. Following culture, the percentage of cells undergoing apoptosis was determined by examining DNA fragmentation using the TUNEL method. Nf1 +/− and Nf1 −/− mast cell cultures contained fewer TUNEL-positive cells compared to WT cultures in an Nf1 gene dose-dependent manner (Figure 1A). Importantly, mast cell cultures treated with vehicle alone did not show differences in TUNEL-positive cells in the three genotypes tested (Figure 1A). To ensure that differences in cell survival were not explained by alterations in c-kit receptor expression, we quantified surface expression of c-kit on WT, Nf1 +/−, and Nf1 −/− BMMCs. No differences in c-kit expression were observed between the three experimental genotypes (data not shown).
Figure 1.
A–B: Neurofibromin-deficient mast cells are resistant to Fas ligand-mediated apoptosis and have reduced surface Fas antigen expression. A: WT, Nf1 +/−, or Nf1 −/− mast cells were cultured in serum-enriched medium and kit-L for 72 hours. Following culture, cells were stimulated with either Fas ligand (black bars) or vehicle (hatched bars). The percentage of cells undergoing apoptosis was determined by FACS analysis using the TUNEL method. Results represent the mean ± SEM of five independent experiments. * P < 0.03 for comparison of Fas ligand-treated versus vehicle-treated mast cell cultures within each experimental genotype by Student’s paired t-test. ** P < 0.05 for comparison of Fas ligand-treated Nf1 +/− mast cells versus Fas ligand-treated Nf1 −/− mast cells by Student’s paired t-test. B: WT, Nf1 +/−, or Nf1 −/− mast cells were cultured in serum-enriched medium and kit-L for 72 hours and analyzed for expression of surface Fas antigen following culture. The dark profile represents Fas antigen expression by WT mast cells and the overlays represent Fas antigen expression by Nf1 +/− and Nf1 −/− cells in the left and right panels, respectively. Data are representative of five other independent experiments with similar results.
One potential explanation for resistance to Fas ligand-mediated apoptosis in Nf1-deficient mast cells is that surface Fas antigen expression is altered. Thus, we tested whether loss of neurofibromin decreases Fas antigen expression in response to kit-L. One × 106 WT, Nf1+/−, or Nf1 −/− BMMCs were placed in RPMI serum-enriched cultures containing 100 ng/ml of kit-L for 72 hours. Following culture, cells were stained with an anti-Fas antibody and surface Fas antigen expression was determined by FACS analysis. Nf1 +/− and Nf1 −/− cells expressed diminished levels of Fas antigen compared to WT BMMCs (Figure 1B). However, no differences in surface Fas expression between the experimental genotypes were observed before stimulation with kit-L (data not shown). Thus, loss of neurofibromin imparts resistance to Fas ligand-mediated apoptosis in Nf1-deficient mast cells that is directly linked with reduced Fas antigen expression.
Expression of NF1 GAP Related Domains into Nf1 −/− Mast Cells Increases Fas Antigen Expression, and Restores Fas Ligand-Mediated Apoptosis.
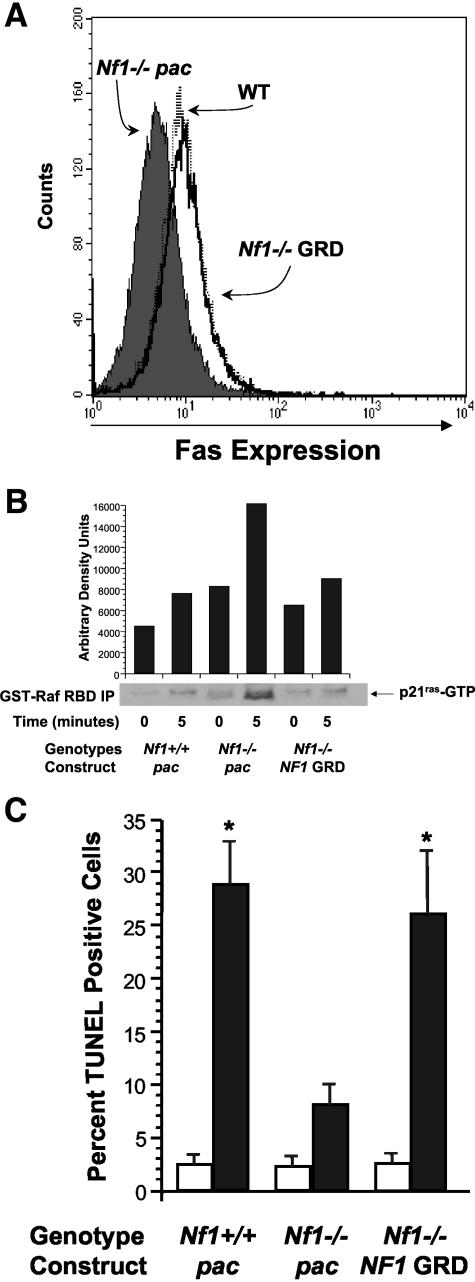
Expression of recombinant NF1 GAP related domains (GRDs) in primary Nf1-deficient cells restores the activation of the p21ras-Raf-Mek-ERK effector pathway to WT levels.37 To determine whether reduced surface Fas antigen expression in Nf1-deficient cells is a function of increased p21ras activity, WT and Nf1 −/− mast cells were transduced with a recombinant retrovirus encoding full length NF1 GRD and a selectable marker gene, pac. Following transduction and puromycin selection, mast cells were cultured in serum-enriched medium containing 100 ng/ml of kit-L for 72 hours, and surface Fas antigen expression was examined using fluorescence cytometry. In contrast to Nf1 −/− mast cells expressing pac alone, expression of NF1 GRD in Nf1 −/− mast cells increased Fas antigen expression to WT levels (Figure 2A). A similar correction was observed when NF1 GRD was expressed in Nf1 +/− mast cells. To test whether increased Fas antigen expression correlated with decreased p21ras activity in Nf1 −/− mast cells, we examined p21ras-GTP levels in NF1 GRD transduced cells. Expression of NF1 GRD into Nf1 −/− mast cells, but not the control sequences (pac), reduced p21ras activity to WT levels (Figure 2B). However, expression of a known human mutant NF1GRD cDNA (R1276P), which contains a single point mutation that reduces NF1 GAP activity by 8000-fold,39 neither restored Fas antigen expression nor increased Fas-induced apoptosis in Nf1 −/− mast cells (data not shown). Thus, increased p21ras activity is directly linked to reduced Fas antigen expression in Nf1-deficient cells.
Figure 2.
A–C: Expression of the NF1GRD in Nf1 −/− mast cells increases surface Fas antigen expression and Fas ligand-mediated apoptosis. A: Cell surface Fas antigen expression in Nf1 −/− mast cells transduced with a recombinant retrovirus expressing the NF1GRD. Following transduction with a recombinant retrovirus expressing either the NF1GRD or a puromycin-resistant gene (pac) and selection in puromycin, WT and Nf1 −/− cells were cultured in serum-enriched media and kit-L for 72 hours and surface Fas antigen expression was measured by FACS. The dark profile depicts Fas antigen expression on Nf1-deficient cells transduced with the control virus and the open overlay represents Fas expression by cells transduced with NF1 GRD sequences. The dashed overlay represents Fas expression by WT cells transduced with a retrovirus expressing pac only. Data are representative of five other independent experiments with similar results. B: p21ras-GTP levels in Nf1 −/− mast cells transduced with a recombinant retrovirus encoding either the NF1GRD or pac and WT mast cells transduced with a retrovirus encoding pac only. After transduction and selection in puromycin, mast cells were serum starved in the absence of growth factors for 24 hours and then stimulated with kit-L for 5 minutes. p21ras-GTP levels were quantitated as described in Materials and Methods. Data are representative of five other independent experiments with similar results. C: Fas ligand-mediated apoptosis in Nf1 −/− mast cells transduced with a recombinant retrovirus encoding the NF1GRD. Following transduction with a recombinant retrovirus encoding either the NF1GRD or a puromycin-resistant gene (pac) and selection in puromycin, WT and Nf1 −/− cells were cultured in serum-enriched media and kit-L for 72 hours. Following culture, cells were stimulated with either Fas ligand (black bars) or vehicle (white bars). The percentage of cells undergoing apoptosis was determined by FACS analysis using the TUNEL method. Results represent the mean ± SEM of five independent experiments. * P < 0.05 for comparison of Fas ligand-treated and vehicle-treated mast cell cultures within each experimental genotype by Student’s paired t-test.
To test whether expression of NF1 GRD sequences into Nf1 −/− cells would increase Fas ligand-mediated apoptosis to WT levels, Nf1 −/− BMMCs transduced with either the NF1 GRD or the reporter construct sequences only (pac) and WT cells were cultured in kit-L (100 ng/ml) for 72 hours and then stimulated with recombinant Fas ligand (100 ng/ml) or vehicle control for 16 hours. Following culture, the percentage of cells undergoing apoptosis was determined by examining DNA fragmentation using the TUNEL method. Consistent with a restoration of surface Fas antigen expression, Nf1 −/− mast cells transduced with the NF1 GRD were sensitive to Fas ligand-mediated apoptosis (Figure 2C).
Genetic Inactivation of Class IA- PI-3K Activity in Nf1-Deficient Mast Cells Restores Fas Antigen Expression and Sensitizes Cells to Fas Ligand-Mediated Apoptosis
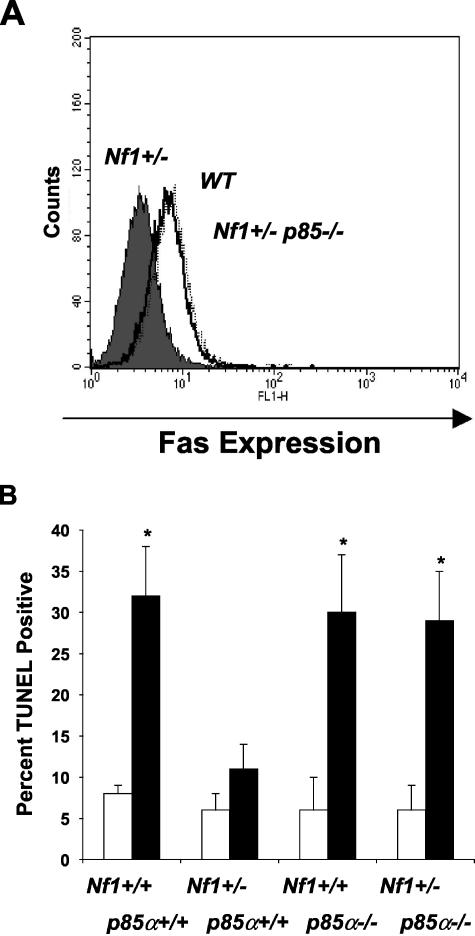
Given the biochemical link between p21ras activity and Fas antigen expression in neurofibromin-deficient cells, we next sought to identify which p21ras effector pathways regulate Fas antigen expression. Based on previous studies in immortalized fibroblasts31 and the fact neurofibromin-deficient mast cells have increased PI-3 kinase and ERK activity in response to kit-L,4 we initially tested whether pharmacological inhibition of the PI-3K or the p21ras-Raf-Mek-ERK pathway would alter Fas antigen expression in WT, Nf1 +/−, or Nf1 −/− mast cells by using specific inhibitors of PI-3K (LY294002), and MEK (PD98059). BMMCs were pre-incubated with serum-enriched media and either LY294002 (5 μmol/L), PD98059 (50 μmol/L), or vehicle control for two hours, and kit-L was then added to all cultures. Following 72 hours of culture, surface Fas antigen expression was analyzed by FACS. As was previously observed, Fas antigen expression was reduced in neurofibromin-deficient cells treated with vehicle control compared to WT cells (data not shown). While pretreatment with PD98059 did not restore surface Fas antigen expression in Nf1-deficient mast cells, preincubation of Nf1 +/− and Nf1 −/− mast cells with LY294002 significantly increased Fas antigen expression (data not shown). To more rigorously test whether class IA PI-3 kinase alters surface Fas antigen expression in Nf1 +/− mast cells, we intercrossed Nf1 +/− mice with mice deficient in the p85α regulatory subunit of class IA PI-3K (p85α−/−). While we were not able to generate Nf1 −/−, p85α −/− mast cells secondary to the lethality of these embryos, we did establish Nf1 +/−, p85α −/− mast cells along with the appropriate experimental controls from embryonic day 13.5 fetal liver to test our hypothesis. Consistent with our prior observations using similar culture conditions, Nf1 +/− BMMCs expressed low levels of Fas antigen. However, Nf1 +/−; p85α −/− BMMCs had Fas antigen expression comparable to WT controls (Figure 3A) and were sensitive to Fas ligand-mediated apoptosis (Figure 3B). Thus, these results show that increased Fas antigen expression and resistance to Fas ligand-mediated apoptosis in Nf1 +/− mast cells is mediated by increased activation of the p21ras-class IA-PI-3K pathway.
Figure 3.
A–B: Genetic inhibition of class IA PI-3 kinase increases surface Fas antigen expression and Fas ligand-mediated apoptosis in neurofibromin-deficient mast cells. A–B: Effect of genetic inactivation of p85α on Fas antigen expression and Fas ligand-mediated apoptosis in neurofibromin-deficient mast cells. FLMCs from the four F2 Nf1 and p85α experimental genotypes were cultured for 72 hours in serum-enriched medium and kit-L. Following culture, an aliquot of cells was examined for cell surface Fas antigen expression and the remaining cells were stimulated with either Fas ligand or DMSO vehicle control for 16 hours. Following stimulation with Fas ligand, the percentage of apoptotic cells was examined by FACS using the TUNEL method. The dark profile in B depicts Fas expression on Nf1 +/− mast cells, and the open overlay represents Fas expression on Nf1 +/−; p85α −/−-deficient cells. The dashed overlay represents Fas expression on WT mast cells. Fas expression on WT and p85α −/− mast cells was not different (data not shown). In C, results represent the mean ± SEM of five independent experiments. * P < 0.05 for comparison of Fas ligand-treated (dark bars) and vehicle-treated mast cell cultures (open bars) within each experimental genotype by Student’s paired t-test.
Mast Cells from Nf1 +/− Mice Are Resistant to Apoptosis following kit Ligand Withdrawal and Do Not Express Fas Antigen after Stimulation with kit-L in Vivo
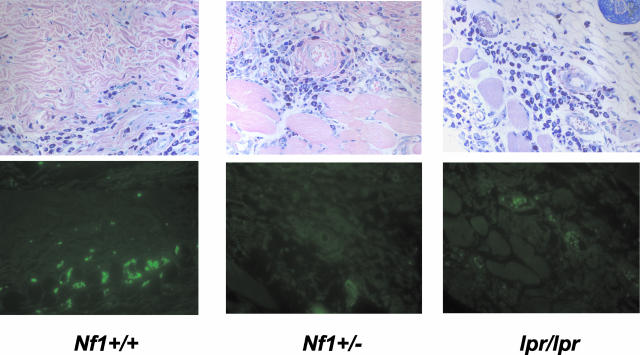
Injection of polyethylene glycol conjugated kit-L into the skin of WT mice or continuous infusion of kit-L via a subcutaneous microsmotic pump results in mast cell accumulation at the site of injection that is associated with local mast cell proliferation.4,40,41 To test whether heterozygous inactivation of Nf1 would alter Fas antigen expression in vivo in response to kit-L, we administered kit-L to WT, Nf1 +/−, and Fas antigen-deficient mice (lpr mice) via microsmotic pumps and examined Fas antigen expression by immunofluorescence. Micro-osmotic pumps were filled with a dose of kit-L to administer 20 μg/kg/day for 7 days and placed into the dorsal back skin. This dose of kit-L induces an approximate fivefold and sevenfold increase in the number of mast cells that accumulate in WT and Nf1 +/− mice, respectively.4 In situ immunofluorescence was performed on skin sections taken from mice of the various genotypes following 7 days of kit-L administration. Skin sections were stained with an FITC-conjugated anti-Fas antigen monoclonal antibody (Jo2) or an isotype control, and immunofluorescence on cutaneous mast cells of WT, Nf1 +/−, and lpr/lpr mice was assessed. Figure 4 demonstrates that mast cells present in skin samples from WT mice express Fas antigen. However, Fas expression could not be detected in either Nf1 +/− or lpr/lpr cutaneous mast cells (Figure 4). Mast cells in adjacent skin samples did not stain positive when immunofluorescence was performed with an isotype antibody control (data not shown). Thus, these studies verify that the biochemical mechanisms identified in vitro for decreased Fas antigen expression in neurofibromin-deficient mast cells are valid in an experimental model of mast cell stimulation with kit-L in vivo.
Figure 4.
In vivo expression of Fas antigen in Nf1-deficient and Fas antigen-deficient (lpr/lpr) mice. Osmotic pumps were loaded with kit-L or PBS and placed in the subcutaneous mid-dorsum of WT, Nf1 +/−, and lpr/lpr mice to locally administer 20 μg/kg/day of kit-L for 7 days. Skin sections were taken from mice at the point of pump insertion at the end of 7 days and stained with a FITC-conjugated antibody against Fas antigen. A representative skin section from each experimental group stained with either Giemsa to identify mast cells (top panel) or a FITC-conjugated antibody against Fas antigen to identify mast cells expressing Fas antigen (bottom panel) is shown. Data are representative of five other independent experiments with similar results.
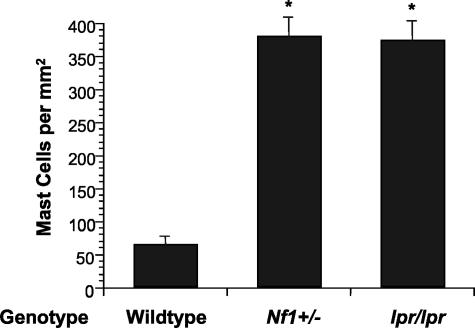
Finally, to genetically test the role of Fas antigen expression in regulating mast cells numbers following kit-L withdrawal in vivo, we administered kit-L to WT, Nf1 +/−, and Fas antigen-deficient mice (lpr mice) via micro-osmotic pumps and examined cutaneous mast cell numbers following pump depletion. Withdrawal of kit-L after continuous administration to WT mice has previously been shown to rapidly reduce mast cell numbers by inducing mast cells apoptosis.40 Micro-osmotic pumps were filled with a dose of kit-L to administer 20 μg/kg/day for 7 days and placed into the dorsal back skin. Pumps were removed 5 days following pump depletion and skin biopsies were taken from the site of pump insertion and stained with Giemsa to identify mast cells. Administration of kit-L induced a profound increase in mast cell numbers in mice of each genotype, as previously described4 (data not shown). However, while the number of mast cells returned to baseline in WT mice 5 days after pump depletion, Nf1 +/− mice retained elevated numbers of mast cells 5 days following pump depletion (Figure 5). Interestingly, lpr/lpr mice retained similar numbers of mast cells following pump depletion as Nf1 +/− mice. Thus, decreased Fas antigen expression on Nf1 +/− and lpr/lpr mast cells is associated with resistance to mast cell apoptosis following kit-L withdrawal in vivo.
Figure 5.
The effects of loss of Nf1 or Fas antigen on accumulation of cutaneous mast cells in response to local administration and withdrawal of kit-L in vivo. A: Osmotic pumps were loaded with kit-L or PBS and placed in the subcutaneous mid-dorsum of WT, Nf1 +/−, and lpr/lpr mice to locally administer 20 μg/kg/day of kit-L for 7 days. Skin sections were taken from mice 5 days after pump depletion and stained with Giemsa to identify mast cells. Cutaneous mast cells were quantitated by counting 2 mm2 sections. Data represent the represent the mean ± SEM of five independent experiments. *P < 0.03 for numbers of cutaneous mast cell in Nf1 +/− and lpr/lpr mice compared to WT mice by Student’s paired t-test.
Discussion
Individuals with NF1 and genetically engineered mice harboring mutations at the Nf1 locus have a number of myeloid cell abnormalities.3,4,12–23 Children with NF1 have a 500-fold increased incidence of developing myeloid leukemia associated with a loss of the normal NF1 allele.13,14,18 Though Nf1 −/− mice die in utero from complex cardiovascular abnormalities, adoptive transfer of Nf1 −/− fetal liver stem cells into WT recipients results in the development of a myeloid leukemia that is highly reminiscient of the human disease.19,20,22,23 The development of neurofibromas, which are complex tumors composed of multiple cell types, is a hallmark of neurofibromatosis type 1.24 Mast cells infiltrate plexiform and cutaneous neurofibromas in humans28 as well as murine models of cutaneous tumors25,42 where they secrete proteins that can remodel the extracellular matrix and initiate angiogenesis.1,2,24,42 Consistent with these observations in NF1 patients, we have recently shown that Nf1 +/− mast cells have increased proliferation4 and migration12, in response to kit-L and that Nf1 +/− mice have increased numbers of cutaneous and peritoneal mast cells. Thus, identification of the biochemical mechanisms responsible for the expansion of Nf1-deficient myeloid cells is critical for understanding disease pathogenesis and the rational design of experimental therapeutics.
Neurofibromin, the protein encoded by NF1, functions as a GAP for p21ras by accelerating the hydrolysis of active p21ras-GTP to inactive p21ras-GDP.10,11,43 We and others have shown that neurofibromin-deficient myeloid progenitors and mast cells have increased p21ras activity in response to multiple hematopoietic cell growth factors, which is directly linked to increased proliferation.4,19–21,37 While most studies have investigated which downstream p21ras effectors are altered in enhancing proliferation, the role of NF1 in regulating myeloid cell survival is incompletely understood. These studies are important since growth factor regulation of both apoptosis and proliferation not only plays a critical role in normal hematopoiesis but can also contribute to leukemogenesis and tumor formation when one or more elements fail to function properly.
One of the most potent activators of programmed cell apoptosis in both hematopoietic and non-hematopoietic cell lineages is the death receptor, Fas antigen.32 Interestingly, recent studies have shown that oncogenic p21ras inhibits the expression of Fas antigen and renders p21ras-transformed cells resistant to Fas-mediated apoptosis.31,32 The role of Fas signaling in regulating hematopoietic cell numbers has been described in both myeloid and lymphoid cell lineages.9,44 lpr/lpr mice have increased numbers of both myeloid and lymphoid progenitors assayed in bone marrow and spleen compared to wild-type controls.9 Moreover, Fas gene mutations have been implicated in development and progression of myelomas, lymphoid leukemias, and lymphomas.45–47 Given that p21ras activation has been linked to Fas antigen expression and that Fas signaling is important for regulating myeloid cell numbers in vivo, we designed studies to test whether Fas antigen expression was altered in neurofibromin-deficient mast cells.
Using genetic intercrosses between Nf1 +/− and class1A-PI3-kinase-deficient mice, we provide several lines of evidence to demonstrate that Nf1-deficient mast cells have decreased Fas antigen expression and Fas-ligand-mediated apoptosis via hyperactivation of the p21ras-class1A-PI3-K pathway. These results are consistent with studies in SHIP −/− mast cells, which also have increased PI-3K-Akt activity and are insensitive to Fas-mediated apoptosis.34 Expression of the NF1 GRD in neurofibromin-deficient mast cells restores p21ras activity to wild-type levels, increases Fas antigen expression and restores sensitivity to Fas ligand. Similar results were observed when pharmacological or genetic inhibition of PI-3K was tested in neurofibromin-deficient mast cells. Importantly, we have also identified a role for Fas ligand-mediated cell death in resolving mast cell numbers in vivo. The resolution kinetics of mast cells following kit-L-induced accumulation are similar in Nf1 +/− mice and lpr/lpr mice and cutaneous mast cells sampled from Nf1 +/− mice do not express detectable levels of Fas antigen in vivo. Finally, while the primary focus of these studies was to determine whether Fas antigen expression was altered in neurofibromin-deficient mast cells, we have initiated studies to test whether Fas antigen expression is decreased in myeloid progenitors harvested from mice transplanted with Nf1 −/− fetal liver hematopoietic stem cells. In preliminary studies, we have observed that Fas antigen expression is decreased in phenotypically defined populations of hematopoietic cells (ScaI+ lin−)21 that are highly enriched for primitive and mature populations of myeloid hematopoietic progenitors (data not shown). Taken together, these results argue that reduced Fas antigen expression contributes at least in part to increased numbers of cutaneous mast cells in Nf1 +/− mice and demonstrate that alterations in p21ras activity can alter Fas antigen expression in primary, non-transformed myeloid cells.
The development of myeloid leukemias and the contribution of mast cells to neurofibroma formation in NF1 patients likely involves increased proliferation and resistance to apoptosis of neurofibromin-deficient cells. Targeting the biochemical pathways responsible for both of these cellular phenotypes is an attractive strategy for treating NF1 patients. Recently, inhibition of oncogenic p21ras by farnesyltransferase inhibitors has been shown to up-regulate Fas antigen expression under both basal growth conditions and in the presence of growth factors.31 Currently, several different farnesyltransferase inhibitors have entered clinical testing in a number of disease states where p21ras is hyperactivated, including NF1 patients with myeloid leukemia. In evaluating the clinical efficacy of these trials and in dissecting the pathogenic mechanisms for the accumulation of mast cells in neurofibromas, it will be important to test whether Fas antigen expression is altered in primary cells harvested from patient specimens. Given that increased PI3-kinase activation is responsible for the reduction in Fas antigen expression in neurofibromin-deficient cells, pharmacological inhibition of PI3-K may also decrease mast cell numbers within neurofibromas or potentially be useful in treating myeloid leukemias in NF1 patients. Finally, since NF1 patients have a wide variety of clinical manifestations including bone abnormalities, learning disorders, vascular abnormalities, and brain tumors, it will be interesting to test whether Fas signaling is altered in other neurofibromin-deficient cell lineages.
Acknowledgments
We thank Marsha Hippensteel for administrative support and Dr. Lewis Cantley for generous supply of p85α +/− mice.
Footnotes
Address reprint requests to D. Wade Clapp, Indiana University School of Medicine, Herman B. Wells Center for Pediatric Research, 1044 W. Walnut St., R4/408, Indianapolis, IN 46202. E-mail: dclapp@iupui.edu.
Supported by grants 1 K08 CA096579–01 (to D.A.I.), D.A.I. is a recipient of a Basil O’Connor Award from the March of Dimes (5FY2003162), NIDDK P30 DK49218 (to D.A.I. and D.W.C.), Department of Defense (DOD) grant DAMD17-01-1-0711 (to D.A.I. and D.W.C.), 2 R01 CA74177-06 (to D.W.C.). H.H. is a Howard Hughes Medical Institute Medical Student Training Fellow recipient.
K.H. and D.A.I. contributed equally to this work.
References
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23–F26. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DA, Yang FC, Travers JB, Wenning MJ, Hiatt K, New S, Hood A, Shannon K, Williams DA, Clapp DW. Genetic and biochemical evidence that haploinsufficiency of the Nf1 tumor suppressor gene modulates melanocyte and mast cell fates in vivo. J Exp Med. 2000;191:181–188. doi: 10.1084/jem.191.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DA, Hiatt K, King AJ, Fisher L, Shivakumar R, Derstine C, Wenning MJ, Diaz B, Travers JB, Hood A, Marshall M, Williams DA, Clapp DW. Hyperactivation of p21ras and the hematopoietic-specific Rho GTPase, Rac2, cooperate to alter the proliferation of neurofibromin-deficient mast cells in vivo and in vitro. J Exp Med. 2001;194:57–70. doi: 10.1084/jem.194.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JJ, Klein KA, Neuberger TJ, Leftwich JA, Westin EH, Kauma S, Fletcher JA, DeVries GH, Huff TF. Role for the stem cell factor/KIT complex in Schwann cell neoplasia and mast cell proliferation associated with neurofibromatosis. J Neurosci Res. 1994;37:415–432. doi: 10.1002/jnr.490370314. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Wagelie-Steffen AL, von Stebut E, Metcalfe DD. Fas (CD95, APO-1) antigen expression and function in murine mast cells. J Immunol. 1997;159:4006–4014. [PubMed] [Google Scholar]
- Wagelie-Steffen AL, Hartmann K, Vliagoftis H, Metcalfe DD. Fas ligand (FasL, CD95L, APO-1L) expression in murine mast cells. Immunology. 1998;94:569–574. doi: 10.1046/j.1365-2567.1998.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman CF, 2nd, Jacobs-Helber SM, Mirmonsef P, Gillespie SR, Bouton LA, Collins HA, Sawyer ST, Shelburne CP, Ryan JJ. Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J Exp Med. 2000;192:1093–1103. doi: 10.1084/jem.192.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E, Moreau G, Arnould A, Vasseur F, Khodabaccus N, Dy M, Ezine S. Increased fetal and extramedullary hematopoiesis in Fas-deficient C57BL/6-lpr/lpr mice. Blood. 1999;94:2613–2621. [PubMed] [Google Scholar]
- Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK, Culver M, Carey JC, Copeland NG, Jenkins NA. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Yang F-C, Ingram DA, White H, Mead L, Chen S, Wenning MJ, Atkinson S, Kapur R, Williams DA, Clapp DW. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for NF1+/−-mast cells. J Clin Invest. 2003;112:1851–1861. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur GM. The NF1 gene in myelopoiesis and childhood myelodysplastic syndromes. N Engl J Med. 1994;330:637–638. doi: 10.1056/NEJM199403033300912. [DOI] [PubMed] [Google Scholar]
- Side L, Taylor B, Cayouette M, Conner E, Thompson P, Luce M, Shannon K. Homozygous inactivation of the NF1 gene in bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders. N Engl J Med. 1997;336:1713–1720. doi: 10.1056/NEJM199706123362404. [DOI] [PubMed] [Google Scholar]
- Neubauer A, Shannon KM, Liu E. Mutations of the ras proto-oncogenes in childhood monosomy 7. Blood. 1991;77:594–598. [PubMed] [Google Scholar]
- Shannon KM, Watterson J, Johnson P, O’Connell P, Lange B, Shah N, Steinherz P, Kan YW, Priest JR. Monosomy 7 myeloproliferative disease in children with neurofibromatosis, type 1: epidemiology and molecular analysis. Blood. 1992;79:1311–1318. [PubMed] [Google Scholar]
- Shannon KM, O’Connell P, Martin GA, Paderanga D, Olson K, Dinndorf P, McCormick F. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. N Engl J Med. 1994;330:597–601. doi: 10.1056/NEJM199403033300903. [DOI] [PubMed] [Google Scholar]
- Emanuel PD, Bates LJ, Zhu SW, Castleberry RP, Gualtieri RJ, Zuckerman KS. The role of monocyte-derived hemopoietic growth factors in the regulation of myeloproliferation in juvenile chronic myelogenous leukemia. Exp Hematol. 1991;19:1017–1024. [PubMed] [Google Scholar]
- Largaespada DA, Brannan CI, Jenkins NA, Copeland NG. Nf1 deficiency causes Ras-mediated granulocyte/macrophage colony stimulating factor hypersensitivity and chronic myeloid leukaemia. Nat Genet. 1996;12:137–143. doi: 10.1038/ng0296-137. [DOI] [PubMed] [Google Scholar]
- Bollag G, Clapp DW, Shih S, Adler F, Zhang Y, Thompson P, Lange BJ, Freedman MH, McCormick F, Jacks T, Shannon K. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in hematopoietic cells. Nat Genet. 1996;12:144–148. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Vik TA, Ryder JW, Srour EF, Jacks T, Shannon K, Clapp DW. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J Exp Med. 1998;187:1893–1902. doi: 10.1084/jem.187.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Taylor B, Shannon K, Clapp DW. Quantitative effects of Nf1 inactivation on in vivo hematopoiesis. J Clin Invest. 2001;108:709–715. doi: 10.1172/JCI12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DA, Wenning MJ, Shannon K, Clapp DW. Leukemic potential of doubly mutant Nf1 and Wv hematopoietic cells. Blood. 2003;101:1984–1986. doi: 10.1182/blood-2002-08-2635. [DOI] [PubMed] [Google Scholar]
- Riccardi VM. Baltimore: The John Hopkins University Press,; NeurofibromatosisPhenotype, Natural History, and Pathogenesis. 1992 [Google Scholar]
- Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin ME, Jacks T. Thinking beyond the tumor cell: Nf1 haploinsufficiency in the tumor environment. Cancer Cell. 2002;1:408–410. doi: 10.1016/s1535-6108(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Hirota S, Nomura S, Asada H, Ito A, Morii E, Kitamura Y. Possible involvement of c-kit receptor and its ligand in increase of mast cells in neurofibroma tissues. Arch Pathol Lab Med. 1993;117:996–999. [PubMed] [Google Scholar]
- Tibbetts MD, Zheng L, Lenardo MJ. The death effector domain protein family: regulators of cellular homeostasis. Nat Immunol. 2003;4:404–409. doi: 10.1038/ni0503-404. [DOI] [PubMed] [Google Scholar]
- Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- Zhang B, Prendergast GC, Fenton RG. Farnesyltransferase inhibitors reverse Ras-mediated inhibition of Fas gene expression. Cancer Res. 2002;62:450–458. [PubMed] [Google Scholar]
- Peli J, Schroter M, Rudaz C, Hahne M, Meyer C, Reichmann E, Tschopp J. Oncogenic Ras inhibits Fas ligand-mediated apoptosis by down-regulating the expression of Fas. EMBO J. 1999;18:1824–1831. doi: 10.1093/emboj/18.7.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalas W, Kisielow P, Strzadala L. Inhibition of MEK induces fas expression and apoptosis in lymphomas overexpressing Ras. Leuk Lymphoma. 2002;43:1469–1474. doi: 10.1080/1042819022386815. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont DJ, Penninger M. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, Yazicioglu MN, Ingram DA, McCarthy JB, Borneo J, Williams DA, Kapur R. Genetic evidence for convergence of c-Kit- and {α}4 integrin-mediated signals on class IA PI-3kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood. 2003;101:4725–4732. doi: 10.1182/blood-2002-08-2521. [DOI] [PubMed] [Google Scholar]
- Serve H, Yee NS, Stella G, Sepp-Lorenzino L, Tan JC, Besmer P. Differential roles of P13-kinase and kit tyrosine 821 in kit receptor-mediated proliferation, survival, and cell adhesion in mast cells. EMBO J. 1995;14:473–483. doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt K, Ingram DA, Zhang Y, Bollag G, Clapp DW. Neurofibromin GTPase-activating protein-related domains restore normal growth in Nf1 −/− cells. J Biol Chem. 2001;276:7240–7245. doi: 10.1074/jbc.M009202200. [DOI] [PubMed] [Google Scholar]
- Haneline LS, Broxmeyer HE, Cooper S, Hangoc G, Carreau M, Buchwald M, Clapp DW. Multiple inhibitory cytokines induce deregulated progenitor growth and apoptosis in hematopoietic cells from Fac−/− mice. Blood. 1998;91:4092–4098. [PubMed] [Google Scholar]
- Klose A, Ahmadian M, Schuelke M, Scheffzek K, Hoffmeyer S, Gewles A, Schmitz F, Kaufmann D, Peters H, Wittinghofer A, Nurnberg P. Selective disactivation of neurofibromin GAP activity in neurofibromatosis type 1 (NF1). Hum Mol Genet. 1998;7:1261–1268. doi: 10.1093/hmg/7.8.1261. [DOI] [PubMed] [Google Scholar]
- Iemura A, Tsai M, Ando A, Wershil B, Galli S. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol. 1994;144:321–328. [PMC free article] [PubMed] [Google Scholar]
- Tsai M, Shih L-S, Newlands GFJ, Takeishi T, Langley K, Zsebo K, Miller H, Geissler EN, Galli S. C-kit ligand SCF, induces the development of connective tissue-type and mucosal mast cells in vivo: analysis by anatomical distribution, histochemistry, and protease phenotype. J Exp Med. 1991;174:125–131. doi: 10.1084/jem.174.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Raymond W, Bergers G, Webster M, Behrendtsen O, Z W, Caughey G, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag G, McCormick F. NF is enough of GAP. Nature. 1992;356:663–664. doi: 10.1038/356663a0. [DOI] [PubMed] [Google Scholar]
- Alenzi FQ, Marley SB, Lewis JL, Chandrashekran A, Warrens AN, Goldman JM, Gordon MY. A role for the Fas/Fas ligand apoptotic pathway in regulating myeloid progenitor cell kinetics. Exp Hematol. 2002;30:1428–1435. doi: 10.1016/s0301-472x(02)00957-8. [DOI] [PubMed] [Google Scholar]
- Landowski TH, Qu N, Buyuksal I, Painter JS, Dalton WS. Mutations in the Fas antigen in patients with multiple myeloma. Blood. 1997;90:4266–4270. [PubMed] [Google Scholar]
- Beltinger C, Kurz E, Bohler T, Schrappe M, Ludwig WD, Debatin KM. CD95 (APO-1/Fas) mutations in childhood T-lineage acute lymphoblastic leukemia. Blood. 1998;91:3943–3951. [PubMed] [Google Scholar]
- Tamiya S, Etoh K, Suzushima H, Takatsuki K, Matsuoka M. Mutation of CD95 (Fas/Apo-1) gene in adult T-cell leukemia cells. Blood. 1998;91:3935–3942. [PubMed] [Google Scholar]