Abstract
Calcification of necrotic tissue is frequently observed in chronic inflammation and atherosclerosis. A similar response of myocardium to injury, referred to as dystrophic cardiac calcinosis (DCC), occurs in certain inbred strains of mice. We now examined a putative inhibitor of calcification, osteopontin, in DCC after transdiaphragmal myocardial freeze-thaw injury. Strong osteopontin expression was found co-localizing with calcification in DCC-susceptible strain C3H/HeNCrlBr, which exhibited low osteopontin plasma concentrations otherwise. Osteopontin mRNA induction was 20-fold higher than in resistant strain C57BL/6NCrlBr, which exhibited fibrous lesions without calcification and little osteopontin expression. Sequence analysis identified several polymorphisms in calcium-binding and phosphorylation sites in osteopontin cDNA. Their potential relevance for DCC was tested in congenic mice, which shared the osteopontin locus with C57BL/6NCrlBr, but retained a chromosomal segment from C3H/HeNCrlBr on proximal chromosome 7. These mice exhibited strong osteopontin expression and DCC comparable to C3H/HeNCrlBr suggesting that a trans-activator of osteopontin transcription residing on chromosome 7 and not the osteopontin gene on chromosome 5 was responsible for the genetic differences in osteopontin expression. A known osteopontin activator encoded by a gene on chromosome 7 is the transforming growth factor-β1, which was more induced (3.5×) in C3H/HeNCrlBr than in C57BL/6NCrlBr mice.
Soft tissue deposition of calcium phosphate and hydroxyapatite in the absence of systemic calcium or phosphate imbalances is referred to as dystrophic calcification. Generally, this form of soft tissue mineralization is associated with cell death and necrosis in degenerative (eg, aging) or chronic inflammatory conditions. Cardiovascular calcification is particularly common and is correlated with the risk of cardiovascular events in affected patients.1–3 Accumulating evidence suggested that calcium deposition in the cardiovascular system is an actively regulated process: vascular cells may shed apoptotic bodies as membranous nucleators for mineralization,4 and secrete various noncollagenous bone matrix proteins such as calcium-binding Gla proteins, osteoprotegerin, and osteopontin (Opn).5,6 Targeted gene inactivation of the matrix Gla protein7 or osteoprotegerin8 caused spontaneous vascular calcification suggesting their involvement in this form of pathological calcification.
Opn, also known as Spp1 or Eta-1, is a secreted sialic acid-rich phosphoprotein, that appeared particularly interesting for studies aiming at the causes and mechanisms of dystrophic calcification. Firstly, Opn can bind hydroxyapaptite9 and was associated with cardiovascular calcification in human atherosclerosis,10–12 valvular calcification,13 and Mönckeberg’s disease.14 The biological function of Opn may be influenced by extensive posttranslational modification including glycosylation15 and transglutamination.16 Phosphorylation of Opn appeared to be required for inhibition of mineralization.17 Secondly, Opn has an Arg-Gly-Asp (RGD) amino acid sequence motif18 serving as a ligand for integrins, a family of cell surface receptors promoting cell adhesion and migration.19,20 Cells binding to Opn via the αVβ3 integrin include osteoclasts and macrophages.21 Thirdly, strong expression of Opn has been demonstrated in macrophages in necrotic lesions of human myocardial infarction and after myocardial freeze-thaw injury in rats.22
Dystrophic calcification of necrotic myocardium has been observed in some gene-targeted mouse models of cardiomyopathy23–25: in mice with inactivation of desmin, the authors reported strong Opn expression in co-localization with calcified myocardial necrosis, yet function and regulation of Opn were not elucidated further.25 Certain predisposed inbred mouse strains, including DBA/2, BALB/c, and C3H/He, also develop dystrophic peri-/myocardial calcification spontaneously or in response to high-fat/low-protein diets,26,27 viral myocarditis,28 or direct freeze-thaw injury.29 This recessive phenotype has been termed dystrophic cardiac calcinosis or DCC, yet pathogenetic mechanisms remained hardly understood.
In this study we investigated the development of DCC in predisposed C3H/He and resistant C57BL/6 mice after myocardial freeze-thaw injury. In particular, we sought to examine the tentative association of Opn and DCC suggested previously.22,25 We examined systemic and myocardial expression levels of Opn in a temporal and spatial distribution to determine genetic differences in regulation. A novel congenic mouse model was analyzed to test linkage of the structural Opn locus with its regulation and with the DCC phenotype.
Materials and Methods
Animals
Inbred C57BL/6 and C3H/He strains were purchased from Charles River (Sulzbach-Rosenberg, Germany). Congenic strains were bred in our own mouse colony: at the outset (C57BL/6 × C3H/He) F1 male mice were backcrossed to female C57BL/6 mice. Selected progeny was further backcrossed to female C57BL/6 mice for another nine generations with the aim to preserve (C57BL/6 × C3H/He) heterozygosity on proximal chromosome 7. This was assessed by genotyping of microsatellite markers D7Mit56, D7Mit247, D7Mit229, D7Mit82, D7Mit31, D7Mit40, and D7Mit332 as described previously.30 Further brother × sister matings produced a chromosomal segment with homozygous alleles of the susceptible donor strain C3H/He. A detailed histological and genetic characterization of congenic strain B6.C3-(D7Mit56-D7Mit230) is in preparation. Throughout the entire experiment all animals had free access to water and Altromin 1324 chow (Altromin, Lage, Germany), and were maintained with a 12-hour light:dark cycle.
Myocardial Freeze-Thaw Injury
At the age of 6 to 8 weeks myocardial freeze-thaw injury was produced in C3H/He, C57BL/6, and congenic strains essentially as described.29 In brief, the abdomen of an anesthetized animal (intraperitoneal injection of 250 mg/kg body weight of a 12.5-mg/ml 2,2,2-tri-bromo-ethanol solution, prepared freshly in 2-methyl-2-butanol) was opened in midline. The left lobe of the liver was gently flipped over to allow visualization of the beating heart through the translucid diaphragmal base. A blunt steel pin (5 mm in diameter), precooled in liquid nitrogen, was pressed gently for 10 seconds onto the diaphragm aiming at the heart. Then, the abdomen was closed using 7.0 suture material. At the indicated time points after myocardial injury mice were sacrificed by cervical dislocation before the hearts were quickly excised, rinsed with phosphate-buffered saline, and analyzed as indicated.
Histological Analysis
Serial 8-μm cryosections throughout the ventricles were prepared and collected on poly-d-lysine-coated slides. Immunostaining was performed with a polyclonal anti-mouse Opn antibody at 5 μg/ml (R&D Systems, Wiesbaden, Germany) and a macrophage-specific anti-rat monoclonal antibody (MOMA2) at 5 μg/ml (BMA, Augst, Switzerland). Bound primary antibodies were detected using biotinylated secondary antibodies that were visualized using a streptavidin-horseradish peroxidase complex and diaminobenzidine as supplied with the ABC Vectra staining kit (Vector Laboratories, Santa Cruz, CA). Slides were then counterstained with hematoxylin. Controls were performed without primary antibodies. Nonspecific binding of antibodies to calcified tissue was excluded by treatment of some specimens with citric acid before immunohistological staining.31
Analysis of Opn Plasma Concentrations
Plasma Opn concentrations were determined using an anti-mouse Opn enzyme-linked immunosorbent assay from Immuno Biological Laboratories, Hamburg, Germany. Blood samples were obtained by retro-orbital bleeding into lithium-heparin plasma separator tubes (Becton Dickinson, Heidelberg, Germany). After centrifugation plasma samples were diluted with saline (1:50) and processed as directed by the manufacturer.
Quantitative Analysis of mRNA Induction
On harvesting, whole hearts were quickly dissected to obtain comparable specimens from necrotic and noninjured myocardium. Samples were homogenized on ice in RNAclean (Hybaid-AGS, Heidelberg, Germany) to isolate total RNA. After DNase treatment and electrophoretic quality control, samples were reverse-transcribed and subsequently amplified using a Tth polymerase-based one-step reverse transcriptase-polymerase chain reaction (RT-PCR) kit (RNA Master SYBR Green I) for the LightCycler thermocycler (Roche, Mannheim, Germany). PCR product formation was assessed in real-time using SYBR Green I fluorescence. Relative gene induction was calculated using the ΔΔCt method comparing gene induction at various time points after freeze-thaw injury to baseline after normalization to 18S rRNA.32 Ct was obtained by crossing point analysis of triplicate amplifications after applying the second derivative maximum method implemented in the LightCycler Software. Primer pairs used for amplification were: ACACTTTCACTCCAATCGTCC/TGCCCTTTCCGTTGTTGTCCfor Opn, CTGCTGACCCCCACTGATAC/GTTGGACAACTGCTCCACCT for Tgfb1, and TCAAGAACGAAAGTCGGAGG/GGACATCTAAGGGCATCACA for 18S rRNA.
Sequence Analysis
mRNA was isolated from necrotic lesions of C3H/He hearts 3 days after freeze-thaw injury, reverse-transcribed to cDNA, and then amplified using these primer pairs for Opn: GTGGGCCTTGCTTGGGTT/TTCTGTGGCGCAAGGAGATT (GenBank BC002113), CCCGGTGAAAGTGACTGATTC/CAGAGGGCATGCTCAGAAGC, and CACATGAAGAGCGGTGAGTCTAA/AAGCTTTTGGTTACAACGGTGTTT (GenBank NM_009263). The PCR amplification products overlapped and covered the entire coding sequence of the Opn gene. PCR was performed using the Expand Fidelity PCR kit containing a Taq/Pfu polymerase mix (Roche). The thermocycler program included an initial 94°C denaturing step followed by 45 cycles consisting of short denaturing at 94°C (15 seconds), annealing at 58°C for 30 seconds, and extension at 72°C for 45 seconds. Annealing temperature was lowered by 2°C every 15 cycles. A final extension step at 72°C for 5 minutes concluded the cycling program. PCR products were sequenced in both directions by a commercial sequencing service (Seqlab, Goettingen, Germany).
Results
Strong Opn Expression in Co-Localization with Calcified Wound Matrix
Necrotic lesions were usually detectable by gross inspection as early as 1 day after freeze-thaw injury. On light microscopy, lesions appeared circumscript and exhibited fibrosis and inflammatory cell infiltration (Figure 1). In the lesions of C3H/He hearts (Figure 1A) infiltrating cells were inhomogeneous with respect to distribution and shape ranging from small spindle-like cells with dense, basophilic nuclei to large, more globular cells with large nuclei that stained only faintly basophilic and were mostly positive for MOMA-2, a macrophage-specific antibody (data not shown). Focal agglomerations of macrophages surrounding calcified material were noticed throughout the lesions. Polynuclear giant cells were not seen. In comparison, necrotic lesions of C57BL/6 hearts exhibited a more homogenous distribution of predominantly spindle-like cells consistent with fibroblasts (Figure 1B). At day 3, necrotic tissue appeared completely phagocytosed and replaced by fibrotic tissue. In this regard, wound healing appeared more advanced compared to C3H/He mice exhibiting histomorphological changes that were suggestive for delayed debridement.
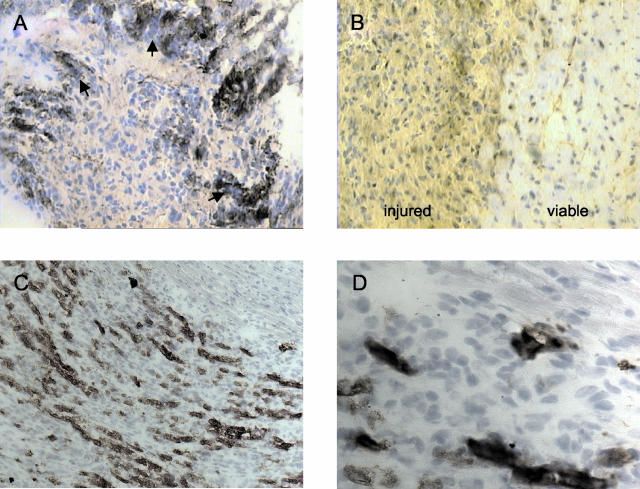
Figure 1.
Representative myocardial lesions 3 (A, B) and 5 (C, D) days after transdiaphragmal freeze-thaw injury in resistant strain C57BL/6 (B) and in DCC-affected C3H/He (A) and congenic B6.C3-(D7Mit56-D7Mit230) mice (C, D). A, C, and D depict mostly necrotic lesion area. Equal parts of injured and noninjured, viable myocardium are illustrated in B (see labels). Calcification is recognized by dark violet-blue staining (arrows in A) after the treatment of cryosections with hematoxylin. Opn protein is detected by brownish immunostaining (see Materials and Methods). Original magnifications: ×100 (C); ×200 (A, B); ×400 magnification (D).
Myocardial calcium deposits stained dark basophilic with hematoxylin, and were first noticed 2 days after freeze-thaw injury. In agreement with earlier observations, calcifications were absent in C56BL/6 mice (Figure 1B). This was also confirmed by calcium-specific Alizarin Red S staining (data not shown). In C3H/He strains calcification was seen only within the necrotic lesion area mostly close to noninjured, viable myocardium. Small foci of calcification developed into larger patches and streaks after 3 to 5 days (Figure 1A), and persisted at least up to 3 weeks (data not shown).
In C3H/He, intense immunohistological staining of Opn was found strictly co-localizing with calcium deposition beginning at day 2 (Figure 1A). In contrast, at day 3, C57BL/6 mice exhibited lesions staining for Opn rather diffusely and much less intensely compared to C3H/He mice (Figure 1B). Note that the extracellular matrix of the noninjured myocardium surrounding the lesion exhibited some faint Opn staining as well (Figure 1B).
Dramatic Transcriptional Induction of Opn in C3H/He
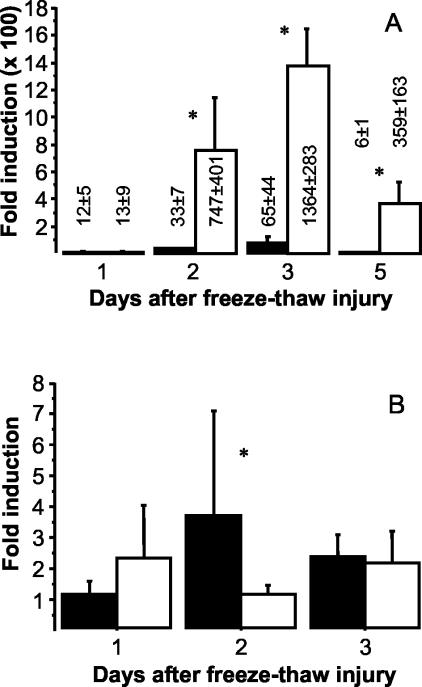
Opn gene induction was examined by quantitative real-time RT-PCR. Groups of four mice were sacrificed immediately after freeze-thaw injury (baseline) as well as 1, 2, 3, and 5 days after injury. Tissue samples were taken from lesions and from noninjured, healthy myocardium (only at baseline and days 1, 2, and 3). Necrotic and noninjured specimens were analyzed separately to exclude a significant contribution of Opn transcripts from myocardial remodeling in response to injury.33 Examination of necrotic tissue revealed comparable induction of Opn (∼12-fold) in both C57BL/6 and C3H/He 1 day after injury (Figure 2A). Coincident with increasing calcification of necrosis, up-regulation of Opn began at day 2 and peaked at day 3 in the C3H/He strain: compared to baseline, up-regulation reached ∼1300-fold, 20-times more than observed in C57BL/6 mice showing a maximal 65-fold increase relative to baseline. The significant strain-dependent difference (P < 0.05, Mann-Whitney U-test) persisted up to day 5 when Opn induction was declining in both strains. In contrast, in healthy myocardium of C57BL/6 mice Opn was up-regulated maximally ∼3.5-fold at day 2 compared to baseline, whereas Opn induction remained approximately twofold in strain C3H/He (Figure 2B).
Figure 2.
Opn gene induction in necrotic myocardium after transdiaphragmal freeze-thaw injury (A) and noninjured myocardium (B) of C3H/He (open bars) and C57BL/6 mice (filled bars). Results were obtained after triplicate analysis by real-time RT-PCR of total RNA (n = 5 in each group). Relative quantitation (see Materials and Methods) is expressed as mean fold-induction ± 1 SD compared to baseline. Asterisks indicate significant strain differences (P < 0.05, Mann-Whitney U-test).
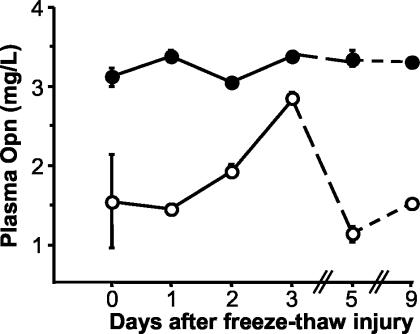
We examined Opn plasma concentrations to determine whether up-regulation of Opn was exclusively limited to necrotic tissue. Basal Opn plasma concentrations were approximately twofold higher in strain C57BL/6 than in C3H/He (Figure 3). In C3H/He, however, Opn plasma concentrations followed the time pattern of Opn up-regulation in necrotic tissue and reached nearly the plasma concentrations of C57BL/6 at day 3 after injury. We also analyzed separate groups of mice (n = 4 to 5) 3 days after myocardial freeze-thaw injury and after sham treatment. Consistent with a strain-dependent specific response to myocardial injury, Opn plasma concentrations were increased by 74% in C3H/He mice with myocardial injury compared to sham-operated mice. In C57BL/6 mice, myocardial injury increased levels observed in sham-treated animals by an additional 27%. Therefore, strong local Opn expression in the necrotic myocardium may have influenced systemic Opn levels as well.
Figure 3.
Opn plasma concentrations after myocardial freeze-thaw injury in C3H/He (open circles) and C57BL/6 mice (closed circles). Results of triplicate analyses of groups of two mice are expressed as mean ± 1 SD.
Opn Sequence Is Highly Polymorphic
A number of polymorphisms have been identified in Opn in various inbred mouse strains.34,35 We sequenced the entire Opn cDNA derived from calcified necrotic myocardium (day 3 after freeze-thaw injury) to examine strain differences and tissue-specific RNA splicing. Compared to C57BL/6, strain C3H/He exhibited one codon insertion (resulting in the insertion of K31) and 16 base differences, 9 of which translated into amino acid substitutions (Figure 4). A S101N and a D189N substitution affected two (S101-E112, S187-D189) of several phosphorylation sites, while a K208R substitution occurred in the middle of a calcium-binding domain (D202-S213). One of two putative heparin-binding sites (D276-I283) exhibited an R277H substitution. Additional V123F, N143D, D172Y, R225S, and Q233H amino acid substitutions did not occur in any known relevant protein motif including the signal peptide (M1-S16), the aspartic acid-rich hydroxyapatite-binding site (D86-D95), the RGD-motif (R145-D147) and additional integrin binding sites (S148-L153), the thrombin cleavage site (R154-S155), and the remaining phosphorylation and glycosylation sites.19,36–38 The D172Y substitution may yet be functionally important, because it created a shift from an acidic to a basic amino acid.
Figure 4.
Alignment of Opn nucleotide (A) and protein (B) sequences of resistant C57BL/6 and DCC-susceptible strain C3H/He after sequence analysis of Opn transcripts from necrotic myocardium. The start codon is underlined. Sequence identity is indicated by an asterisk.
A trans-Activator of Opn Transcription Maps on Chromosome 7
We used a novel congenic strain, B6.C3-(D7Mit56-D7Mit230), to determine whether any polymorphism at the Opn locus may be relevant for the differential regulation of Opn or the development of DCC. This congenic strain shared the genetic background with resistant C57BL/6 including the Opn locus residing on chromosome 5 and possessed an ∼24.5-cM segment of proximal chromosome 7 (extending from the acrocentric centromere to microsatellite marker D7Mit230). This segment was derived from C3H/He and rendered congenic mice susceptible to DCC as well. As illustrated in Figure 1, C and D, congenic mice exhibited macrophage infiltration and marked Opn expression in co-localization with calcification comparable to C3H/He mice. We concluded that the Opn locus on chromosome 5 is not necessary for DCC: the presumably functional calcium-binding and phosphorylation sites of the C57BL/6 Opn alleles could not prevent DCC. The strong expression of Opn in the necrotic lesions of DCC-susceptible strains C3H/He and B6.C3-(D7Mit56-D7Mit230) was determined by a locus on proximal chromosome 7.
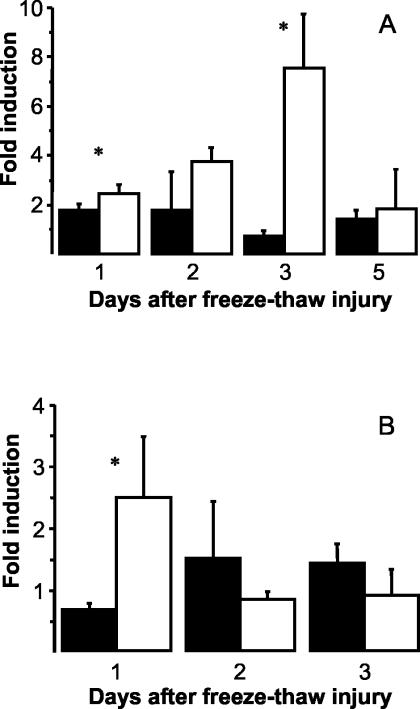
Tgfb1 has been identified as potent inducer of Opn12,39,40 and is also located on proximal chromosome 7. The induction of Tgfb1 was assessed by quantitative RT-PCR. In necrotic lesions we found a maximal eightfold induction at day 3 in C3H/He while induction ranged from ∼1.5-fold to twofold throughout days 1 to 5 in C57BL/6 (Figure 5A). In healthy myocardium, we noticed early induction (maximally 2.5-fold compared to baseline) 1 day after injury in C3H/He mice (Figure 5B). In contrast, there was no relevant induction of Tgfb1 mRNA in C57BL/6 mice during the first 3 days. Strain-specific regulation and time course of Tgfb1 induction in necrotic myocardium suggested that Tgfb1 may be the hypothesized trans-activator of Opn transcription.
Figure 5.
Tgfb1 gene induction in necrotic myocardium after transdiaphragmal freeze-thaw injury (A) and noninjured myocardium (B) of C3H/He (open bars) and C57BL/6 mice (filled bars). Results were obtained after triplicate analysis by real-time RT-PCR of total RNA (n = 5 in each group). Relative quantitation (see Materials and Methods) is expressed as mean fold-induction ± 1 SD compared to baseline. Asterisks indicate significant strain differences (P < 0.05, Mann-Whitney U-test).
Discussion
Dystrophic calcification is a well recognized hallmark of degenerative disease, chronic inflammation, atherosclerosis, and other conditions associated with tissue necrosis. Extensive mechanistic and experimental evidence led to several models explaining calcium deposition in vascular tissue and bone. Physiological and certain forms of pathological calcification may share common elements (eg, the formation of matrix vesicles) depending on the type of tissue and the etiology of tissue injury.41–43
The aim of the present study was to explore a putative role of Opn for the development of one type of pathological calcification, DCC, that occurs in the myocardium of genetically predisposed inbred strains. We used a model of severe myocardial freeze-thaw injury to produce reliably necrotic calcifications within a few days. Albeit not physiological, this model of nonischemic myocardial injury may be used to study basic elements of postinfarction healing and remodeling of the heart. Opn was first identified in myocardial necrosis in a rat model of freeze-thaw injury and was then confirmed in human myocardial infarction.22 Recently, Steitz and co-workers9 and Giachelli42 provided strong evidence that Opn inhibits in vivo ectopic calcification of glutaraldehyde-fixed bovine aortic valve leaflets and may also promote the phagocytotic regression of calcification through up-regulation of carbonic anhydrase II, which is necessary to allow calcium resorption in an acidic microenvironment. Interestingly, vascular calcification was previously demonstrated in mice deficient in carbonic anhydrase II.44 We hypothesized that Opn may be a key regulator of DCC as well, because it is relevant for macrophage chemotaxis, infiltration and adhesion, generation of cytotoxic nitric oxide, apoptosis, phagocytosis, and matrix organization.19,36–38,45
Histology of Myocardial Necrosis Suggested Impaired Opn Function
Studies of dermal wound healing in mice lacking functional Opn on a 129 × Black Swiss hybrid background provided histological evidence that was interpreted as compromised debridement. However, calcification of any histomorphological structures consistent with cell remnants was not seen.46 This may be explained by the genetic background of the mutants: neither strain 129 nor Black Swiss is known to be susceptible to DCC. Opn mutant and wild-type mice exhibited similar numbers of macrophages, yet more mannose receptor-positive and therefore inactive, resting macrophages were seen in the former group. The wound matrix of Opn-deficient mice was described as less organized and was characterized by significantly smaller collagen fibrils compared to wild-type controls.46
Our histological studies of myocardial freeze-thaw injury shared certain aspects of wound healing in Opn−/− mice. In DCC-susceptible C3H/He, we found focal accumulations of matrix structures that may have served as nucleator for early calcium deposition. The nature of these structures was not further analyzed, as was the composition of collagen fibrils. Light microscopy demonstrated predominantly macrophage-like cells, which surrounded calcifying foci suggesting functional Opn with respect to chemotaxis and adhesion. This macrophage population, however, apparently failed to phagocytose calcified matrix rapidly. Activation state and phagocytotic capacity of these macrophages remain to be elucidated. These functions may be compromised in C3H/He, which may also occur independent of Opn. In comparison, wound healing of C57BL/6 hearts exhibited a more physiological pattern: rapid and complete debridement in the absence of necrotic calcification and the formation of a homogenous fibroblast-rich fibrous scar.
Confirming previous reports we found early and significantly up-regulated Opn expression in response to myocardial injury. In DCC-susceptible strains, abundant Opn was strictly co-localized with calcified necrotic tissue.47 In resistant C57BL/6 mice, the matrix of the entire necrotic lesion as well as adjacent viable myocardium exhibited little and more homogeneously distributed Opn—probably because primary matrix calcification leading to induction and focal concentration of Opn was absent.
DCC Is Associated with Dramatic Induction of Opn Transcription
Up-regulation of Opn occurred early at the transcriptional level. The increase of Opn transcripts was dramatic in necrotic myocardium of strain C3H/He, comparable only to levels of induction seen in certain tumor cell lines or other cells in transformation.19 Time series analysis demonstrated that Opn gene induction peaked at day 3 and began to decline at day 5 in the necrotic tissue of both strains. We also analyzed steady-state and acute phase plasma concentrations of Opn. Compared to strain C3H/He, C57BL/6 exhibited twofold higher basal Opn plasma concentrations, which were hardly influenced by myocardial injury. In contrast, Opn plasma concentrations increased and nearly doubled in C3H/He mice consistent with the time course of Opn expression in the myocardial necrosis. This suggested that strong myocardial expression of Opn may have also influenced systemic concentrations. The genetics of Opn plasma concentrations may also affect pathological bone resorption: Opn-deficient mice had slightly higher bone density than wild-type controls and maintained a constant trabecular bone volume after ovariectomy in a model of postmenopausal osteoporosis.48
A trans-Activator of Opn Transcription Is Located on Chromosome 7
The strain-dependent differences in Opn regulation may be explained either by different activities of specific transcription factors or sequence variations in the transcribed and/or regulatory Opn sequence. Inhibition of mineralization requires a structurally functional calcium-binding domain and phosphorylation of the Opn protein.17 Sequence analysis of Opn transcripts isolated from necrotic myocardium of C3H/He mice revealed amino acid substitutions in both, the putative calcium-binding domain and two casein-kinase-II-mediated phosphorylation sites.36,37 Biological relevance of Opn polymorphisms was tested in a novel congenic mouse model. Congenic strains allow studying of a specific phenotype in inbred mice with defined, identical genetic background. This background is usually derived from an acceptor strain, but also contains a few percentages of the genome of a different donor strain. Phenotypic differences between congenic and acceptor strain can be attributed to the influence of genes residing within the congenic donor segment. We examined congenic B6.C3-(D7Mit56-D7Mit230) mice that shared the Opn locus with resistant strain C57BL/6 on chromosome 5. They exhibited DCC because of a homozygous chromosomal segment on proximal chromosome 7, which was derived from susceptible strain C3H/He. In mice, DCC occurs as a recessive trait, which is primarily influenced by several Dyscalc loci.49 The most important of these, Dyscalc1, was mapped on proximal chromosome 7,30,49–52 yet the identity of the underlying Dyscalc1 gene is still unknown.
It was notable that congenic mice exhibited macrophage-like cell infiltration and strong Opn expression in co-localization with calcification comparable to the C3H/He donor strain. Although Opn was derived from resistant strain C57BL/6, it was strongly up-regulated and could not prevent the mineralization of necrosis excluding causal mutations at the Opn locus. Strain-specific Opn up-regulation, however, may be determined by a transcriptional trans-activator located in the congenic segment on proximal chromosome 7. The regulation of Opn has been studied intensively and a number of transcription factors and cytokines have been proposed as potential regulators of Opn transcription including TGF-β.19,36 Tgfb1 is a particularly attractive candidate gene, because the locus maps at 6.5 cM on chromosome 7. Recently, the downstream TGF-β signaling molecules, Smad3 and Smad4, were found to bind as sequence-specific activators and to replace the transcription repressor Hox-9 demonstrating a unique TGF-β-induced transcription mechanism for Opn.53 Notably, Smad6 mutant mice also developed ossification of the aortic outflow tract54 emphasizing a potential implication of Smad signal transduction pathways for tissue calcification. Similar to the strain-dependent Opn gene induction, we found early and significantly higher Tgfb1 gene induction in C3H/He than in C57BL/6 mice in response to myocardial injury. However, we cannot exclude that both, Tgfb1 and Opn, were differentially regulated by a common mechanism of activation representing the true genetic basis of our findings. Other loci on proximal chromosome 7, including Dyscalc1, have not been excluded as the trans-activating locus of Opn transcription as well. Note that the loci of Tgfb1 and Dyscalc1 are not identical.30,49 Additional congenic strains are currently produced and analyzed to pinpoint the trans-activating locus of Opn transcription.
Is Opn Implicated in the Pathogenesis of DCC?
Although accumulated evidence attributed multiple functions to Opn, a consistent model of a role as “the good or the bad guy” has not been presented so far.37,45 Under physiological conditions Opn-deficient mice exhibited a surprisingly normal phenotype. Wound healing studies failed to identify calcification of wound matrix. This can be explained by the DCC-resistant strain background of these mutants or by other genes that may substitute for Opn in certain conditions.45 Only recently, however, examination of mice deficient in Opn and matrix GLA protein demonstrated convincingly that Opn is indeed an inducible inhibitor of pathological calcification in vivo.55 Transfer of the Opn mutant allele onto the C3H/He background may provide more conclusive evidence regarding the role of Opn for DCC. In this special form of pathological calcification initial calcium deposition occurs independently of Opn in decaying mitochondria very soon after irreversible tissue injury.29,56 Calcium deposition may continue to grow, if necrotic cellular components are not phagocytosed rapidly. This may promote particularly strong Opn up-regulation.22 Several alternatives must be discussed to explain the dramatic induction of Opn in DCC: 1) Opn transcripts were more stable in DCC-susceptible strains; 2) Opn was up-regulated by autocrine macrophage pathways or other positive-feedback mechanisms stimulated in response to incomplete phagocytosis; 3) transcription of Opn was activated through a differentially regulated pathway. To support this hypothesis, we examined Tgfb1, a known inducer of Opn.
We reported evidence implicating genetic differences of Opn regulation in the pathogenesis of dystrophic myocardial calcification. It remained unclear, however, why calcification of necrotic myocardium occurred despite dramatic up-regulation of Opn. The role of Opn for macrophage function, and in particular phagocytosis, deserves further investigation. Genetic mouse models of DCC are certainly helpful in this regard.
Acknowledgments
We thank Drs. Lusis and Drake for valuable discussions, Dr. Schulze-Bergkamen for assistance in histological imaging, and Dr. Noel for advice in matters of animal maintenance and welfare.
Footnotes
Address reprint requests to Dr. B. Ivandic, Medizinische Klinik III, Bergheimer Str. 58, Heidelberg, 69115 Germany. E-mail: boris.ivandic@med.uni-heidelberg.de.
Supported by the Deutsche Forschungsgemeinschaft (grants Iv10/3-1 and Iv10/3-2 to B.T.I.).
References
- Beadenkopf WG, Daoud AS, Love BM. Calcification in the coronary arteries and its relationship to arteriosclerosis and myocardial infarction. Am J Roentgenol. 1964;92:865–871. [PubMed] [Google Scholar]
- Niskanen LK, Suhonen M, Siitonen O, Lehtinen JM, Uusitupa MI. Aortic and lower limb artery calcification in type II (non-insulin-dependent) diabetic patients and non-diabetic control subjects: a five year follow-up study. Atherosclerosis. 1990;84:61–71. doi: 10.1016/0021-9150(90)90009-8. [DOI] [PubMed] [Google Scholar]
- Lehto S, Niskanen LK, Suhonen L, Ronnemaa T, Laakso M. Medial artery calcification: a neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- Anderson HC. Matrix vesicle calcification: review and update. Chicago: Elsevier Science Publishers; Bone and Mineral Research. 1985 [Google Scholar]
- Boskey AL. Matrix proteins and mineralization: an overview. Connect Tissue Res. 1996;35:357–363. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- Gorski JP. Acidic phosphoproteins from bone matrix: a structural rationalization of their role in biomineralization. Calcif Tissue Int. 1992;50:391–396. doi: 10.1007/BF00296767. [DOI] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer R, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix Gla protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan CM, Cary NRB, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini A, Mann KG, Kudryk BJ, Schoen FJ. Non-collagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1852–1861. doi: 10.1161/01.atv.19.8.1852. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler ER, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–552. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- Singh K, DeVouge MW, Mukherjee BB. Physiological properties and differential glycosylation of phosphorylated and nonphosphorylated forms of osteopontin secreted by normal rat kidney cells. J Biol Chem. 1990;265:18696–18701. [PubMed] [Google Scholar]
- Beninati S, Senger DR, Cordella-Miele E, Mukherjee AB, Chackalaparampil I, Shanmugam V, Singh K, Mukherjee BB. Osteopontin: its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J Biochem. 1994;115:675–682. doi: 10.1093/oxfordjournals.jbchem.a124395. [DOI] [PubMed] [Google Scholar]
- Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci USA. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt DT, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol. 2001;41:723–749. doi: 10.1146/annurev.pharmtox.41.1.723. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin—a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994;145:1450–1462. [PMC free article] [PubMed] [Google Scholar]
- McConnel BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, Turnbull DH, Georgakopoulos D, Kass D, Bond M, Niimura H, Schoen FJ, Conner D, Fischman DH, Seidman CE, Seidman JG. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest. 1999;104:1235–1244. doi: 10.1172/JCI7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatkin D, Christe ME, Aristizabal O, McConnel BK, Srinivasan S, Schoen FJ, Seidman CE, Turnbull DH, Seidman JG. Neonatal cardiomyopathy in mice homozygous for the Arg403Gln mutation in the α cardiac myosin heavy chain gene. J Clin Invest. 1999;103:147–153. doi: 10.1172/JCI4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroidis M, Capetanaki Y. Extensive induction of important mediators of fibrosis and dystrophic calcification in desmin-deficient cardiomyopathy. Am J Pathol. 2002;160:943–952. doi: 10.1016/S0002-9440(10)64916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton GJ, Custer RP, Johnson FN, Stabenow KT. Dystrophic cardiac calcinosis in mice: genetic, hormonal, and dietary influences. Am J Pathol. 1978;90:173–186. [PMC free article] [PubMed] [Google Scholar]
- Everitt JI, Olson LM, Mangum JB, Viskek WJ. High mortality with severe dystrophic cardiac calcinosis in C3H/OUJ mice fed high fat purified diets. Vet Pathol. 1988;25:113–118. doi: 10.1177/030098588802500202. [DOI] [PubMed] [Google Scholar]
- Gang DL, Barrett LV, Wilson EJ, Rubin RH, Medearis DN. Myopericarditis and enhanced dystrophic cardiac calcification in murine cytomegalovirus infection. Am J Pathol. 1986;124:207–215. [PMC free article] [PubMed] [Google Scholar]
- Brunnert SR. Morphologic response of myocardium to freeze-thaw injury in mouse strains with dystrophic cardiac calcification. Lab Anim Sci. 1997;47:11–18. [PubMed] [Google Scholar]
- Ivandic BT, Qiao JH, Machleder D, Liao F, Drake TA, Lusis AJ. A locus on chromosome 7 determines myocardial cell necrosis and calcification (dystrophic cardiac calcinosis) in mice. Proc Natl Acad Sci USA. 1996;93:5483–5488. doi: 10.1073/pnas.93.11.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeau A-P, Chaulet H, Daret D, Kockx M, Daniel-Lamaziere J-M, Desgranges C. Time course of osteopontin, osteocalcin, and osteonectin accumulation and calcification after acute vessel wall injury. J Histochem Cytochem. 2001;49:79–86. doi: 10.1177/002215540104900108. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CKS, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Graf K, Do YS, Ashizawa N, Meehan WP, Giachelli CM, Marboe CC, Fleck E, Hsueh WA. Myocardial osteopontin expression is associated with left ventricular hypertrophy. Circulation. 1997;96:3063–3071. doi: 10.1161/01.cir.96.9.3063. [DOI] [PubMed] [Google Scholar]
- Ono M, Yamamoto T, Nose M. Allelic difference in the nucleotide sequence of the Eta-1/Op gene transcript. Mol Immunol. 1995;32:447–448. doi: 10.1016/0161-5890(95)00053-h. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Setoguchi M, Yoshida S, Higuchi Y, Akizuki S, Yamamoto S. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J Biol Chem. 1990;265:14432–14438. [PubMed] [Google Scholar]
- Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. EMBO J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- O’Regan A, Berman JS. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol. 2000;81:373–390. doi: 10.1046/j.1365-2613.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–622. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Noda M, Yoon K, Prince CW, Butler WT, Rodan GA. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type β transforming growth factor. J Biol Chem. 1988;263:13916–13921. [PubMed] [Google Scholar]
- Noda M, Rodan GA. Type β transforming growth factor regulates expression of genes encoding bone matrix proteins. Connect Tissue Res. 1989;21:71–75. doi: 10.3109/03008208909049997. [DOI] [PubMed] [Google Scholar]
- Boström K, Demer LL. Regulatory mechanisms in vascular calcification. Crit Rev Eukaryot Gene Expr. 2000;12:151–158. [PubMed] [Google Scholar]
- Giachelli CM. Ectopic calcification. Gathering hard facts about soft tissue mineralization. Am J Pathol. 1999;154:671–675. doi: 10.1016/S0002-9440(10)65313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen J, Landis WJ. A contribution with review to the description of mineralization of bone and other calcified tissues in vivo. Anat Rec. 1991;230:435–450. doi: 10.1002/ar.1092300402. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Lewis SE, Tashian RE, Schulte BA. Mice carrying a CAR-2 null allele lack carbonic anhydrase II immunohistochemically and show vascular calcification. Am J Pathol. 1989;134:947–954. [PMC free article] [PubMed] [Google Scholar]
- Rittling SR, Denhardt DT. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp Nephrol. 1999;7:103–113. doi: 10.1159/000020591. [DOI] [PubMed] [Google Scholar]
- Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee MD, Nanci A. Secretion of osteopontin by macrophages and its accumulation at tissue surfaces during wound healing in mineralized tissues: a potential requirement for macrophage adhesion and phagocytosis. Anat Rec. 1996;245:394–409. doi: 10.1002/(SICI)1097-0185(199606)245:2<394::AID-AR19>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yoshitake H, Rittling SR, Denhardt DT, Noda M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc Natl Acad Sci USA. 1999;96:8160–8165. doi: 10.1073/pnas.96.14.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivandic BT, Utz HF, Kaczmarek PM, Aherrahrou Z, Axtner SB, Klepsch C, Lusis AJ, Katus HA. New dyscalc loci for myocardial cell necrosis and calcification (dystrophic cardiac calcinosis) in mice. Physiol Genomics. 2001;6:137–144. doi: 10.1152/physiolgenomics.2001.6.3.137. [DOI] [PubMed] [Google Scholar]
- Brunnert SR, Shi S, Chang B. Chromosomal localization of the loci responsible for dystrophic cardiac calcinosis in DBA/2 mice. Genomics. 1999;59:105–107. doi: 10.1006/geno.1999.5862. [DOI] [PubMed] [Google Scholar]
- van den Broek FA, Bakker R, den Bieman M, Bouwman-Fielmich AX, Lemmens AG, van Lith HA, Nissen I, Hoitinga-Ritskes JM, van Tintelen G, van Zutphen LF. Genetic analysis of dystrophic cardiac calcification in DBA/2 mice. Biochem Biophys Res Commun. 1998;253:204–208. doi: 10.1006/bbrc.1998.9776. [DOI] [PubMed] [Google Scholar]
- Colinayo VV, Qiao JH, Demant P, Krass K, Lusis AJ, Drake TA. Genetic characterization of the Dyscalc locus. Mamm Genome. 2002;13:283–288. doi: 10.1007/s00335-001-2148-1. [DOI] [PubMed] [Google Scholar]
- Shi X, Bai S, Li L, Cao X. Hox-9 represses transforming growth factor-β-induced osteopontin gene transcription. J Biol Chem. 2001;276:850–855. doi: 10.1074/jbc.M005955200. [DOI] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr, Falb D, Huszar D. A role for Smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Speer MY, McKee MD, Guldberg RE, Liaw L, Yang H-S, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla-protein deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Fleet JF, Ferrans VJ. Ultrastructural changes in inherited cardiac calcinosis of DBA/2 mice. Am J Vet Res. 1987;48:255–261. [PubMed] [Google Scholar]