Abstract
In multiple sclerosis (MS) the structural damage to axons determines the persistent clinical deficit patients acquire during the course of the disease. It is therefore important to test therapeutic strategies that can prevent or reverse this structural damage. The conventional animal model of MS, experimental autoimmune encephalomyelitis (EAE), typically shows disseminated inflammation in the central nervous system, which leads to a clinical deficit that cannot be directly attributed to a defined tract system. For this reason we have developed a localized EAE model, in which large inflammatory lesions are targeted to the dorsal columns of the spinal cord, an area including the corticospinal tract. These lesions show the pathological hallmarks of MS plaques and lead to reproducible and pronounced deficits in hindlimb locomotion. Because of the anatomical specificity of this technique we can now use highly sensitive behavioral tests that assess the functional integrity of specific axonal tracts. We show that these tests are predictive of the site and extent of a given lesion and are more sensitive for assessing the clinical course than the scales commonly used for disseminated EAE models. We believe that this targeted EAE model will become a helpful new tool for the evaluation of therapeutic approaches for MS that attempt to protect axons or support their repair.
Multiple sclerosis (MS) is the most common inflammatory demyelinating disease of the central nervous system (CNS).1 Our understanding of the mechanisms that underlie MS has progressed significantly throughout the last years, yet our means for therapeutic intervention are still very limited. It is believed that in MS an autoimmune dysregulation leads to an inflammatory attack on the resident cells of the CNS.1 Recent studies have emphasized that the target of this inflammatory attack is not the myelin sheath alone but rather the entire myelin-axonal unit.2,3 Neuropathological studies have provided evidence that acute structural damage to axons is a prominent feature of MS lesions starting from the very early stages of the disease.4–6 The clinical importance of the structural axon damage is further underlined by the close correlation between neuroradiological markers of axon damage and the persistent neurological deficit in a given MS patient.7,8 It is thus of central importance to develop therapeutic strategies that can prevent or repair axonal damage in MS. The basis for the development and evaluation of such strategies is the availability of suitable animal models, which should both reflect the pathological hallmarks of MS and allow for the quantification of therapeutic effects on axonal damage and repair.
Behavioral tests used to monitor rodent models of CNS trauma allow for the sensitive quantification of functional deficits of specific tract systems in the spinal cord.9,10 However, this kind of testing could thus far not be applied in experimental autoimmune encephalomyelitis (EAE), the most commonly used animal model of MS. In this model an induced immune reaction against myelin proteins causes disseminated inflammatory CNS lesions, which share important aspects of the pathology and pathomechanisms of MS lesions.11,12 As much as the dissemination and variability of the disease process in EAE reflects the characteristics of MS in humans, these properties make a correlation of functional and structural deficits very complex and often impossible. Therefore the application and interpretation of behavioral tests such as those used to stage traumatic spinal cord injury is problematic in disseminated EAE. These limitations could be overcome with a localized version of the EAE model, which would reflect a single prototypic MS lesion rather than the entire disease process. Targeting the EAE lesion to functionally important axonal tract systems, eg, in the spinal cord, would cause deficits that could be evaluated by using refined behavioral testing.10 At the same time, axonal damage and repair could be quantified much in the same way as usually done for localized noninflammatory lesions to the spinal cord.
Previously, focal inflammatory lesions have been induced, for example, by the local application of mycobacterial components, demyelinating toxins, or antibodies.13–15 Although these models allow for the induction of focal lesions in a predetermined location, they clearly differ with regard to pathomechanism and histopathological appearance from MS lesions. As a consequence, they are not optimally suited for the evaluation of therapeutic strategies aiming at MS.
As mentioned above, EAE resembles MS in many ways5,16 and would thus provide an ideal basis for the development of a localized model of MS. In the past, attempts to induce local EAE lesions used thermal or electrolytic injury to target EAE lesions to a specific location.17,18 These models are of very limited use for the study of structural damage, which in these models is a mixture of the pre-existing tissue damage and the ensuing EAE lesion. In addition, the animals usually develop signs of systemic EAE that further interfere with behavioral testing.
In the present study, to generate a new localized animal model of MS, we took advantage of the limited susceptibility of the Lewis rat to a specific form of EAE (MOG-EAE), which is induced by immunization with myelin-oligodendrocyte glycoprotein (MOG).19 We combined subthreshold immunization with MOG with local injections of proinflammatory cytokines into the spinal cord, which induce local immune cell infiltration.20–22 This approach led to the formation of focal EAE lesions with high reproducibility and produced the corresponding signs of clinical disease. Histopathological analysis revealed that the targeted EAE lesions share important pathological hallmarks with the human disease MS with respect to demyelination, inflammation, and axonal damage. We then targeted these lesions to the dorsal columns of the mid-thoracic spinal cord, an area including the corticospinal tract (CST), and used defined behavioral tests for functional analysis of the new targeted EAE model in comparison to a standard disseminated EAE model. Only the targeted EAE model allowed us to identify parameters of both acute and chronic behavioral deficits that are closely correlated to the total amount of structural damage and especially to damage to the CST. This MS model provides for the first time the opportunity to clinically monitor the structural tissue damage with high precision and sensitivity.
Materials and Methods
Animals
Adult (140 to 220 g) female Lewis rats were used for these experiments. The rats were obtained from Harlan (Horst, The Netherlands) and housed in groups under a 12:12-hour light/dark cycle with food and water ad libitum. All experiments were approved by the veterinary department of the Canton of Zurich.
Sensitization Procedure
For the induction of the targeted EAE model, recombinant protein (rMOG) corresponding to the N-terminal sequence of rat MOG (amino acids 1 to 125) was expressed in Escherichia coli and purified to homogeneity as previously described.23 The purified protein was dissolved in 6 mol/L of urea and dialyzed against 20 mmol/L of sodium acetate buffer (pH 3.0) to obtain a soluble preparation and stored at − 20°C.
Rats were anesthetized by inhalation anesthesia and injected subcutaneously at the base of the tail with a total volume of 100 μl of rMOG (5 to 100 μg diluted in saline) emulsified in incomplete Freund’s adjuvant (IFA; Sigma-Aldrich, Buchs, Switzerland). For control experiments rats were injected subcutaneously at the base of the tail with a total volume of 100 μl of saline emulsified in IFA. This sensitization protocol leads to the induction of an anti-MOG immune response without overt signs of clinical disease (Table 1). For the induction of a targeted EAE lesion MOG-sensitized rats were kept for 18 to 22 days and then subjected to a stereotactic injection of cytokines into a predetermined location of the spinal cord.
Table 1.
Influence of Antigen Dosage on the Disease Course of Targeted MOG EAE
| Antigen dosage | Number of animals | Disease incidence, % | Mean EAE score | Relapse, % | Disseminated EAE, % |
|---|---|---|---|---|---|
| IFA alone | 19 | 5.3 | 0.16 | 0 | 0 |
| MOG/IFA, 5 μg | 10 | 50 | 1.00 | 0 | 0 |
| MOG/IFA, 25 μg | 63 | 100 | 2.36 | 15.0 | 7.9 |
| MOG/IFA, 50 μg | 34 | 100 | 2.66 | 50 | 38.2 |
| MOG/IFA, 100 μg | 21 | 83.3 | 2.42 | 42.9 | 42.9 |
Disease incidence, percentage of animals with an EAE score ≥1.5 after local cytokine injection; mean EAE score, mean of the maximal EAE scores after local cytokine injection; relapse, percentage of animals with an increase of the EAE score ≥1 for at least 2 days after the initial disease phase; disseminated EAE, percentage of animals with an EAE score >0.5 before the local injection of cytokines.
A conventional disseminated EAE model was induced by active immunization with purified guinea pig myelin basic protein (MBP; Sigma-Aldrich) as previously described.24,25 Rats were anesthetized by inhalation anesthesia and injected subcutaneously at the base of the tail with a total volume of 100 μl of MBP (10 to 50 μg per rat diluted in saline) emulsified in complete Freund’s adjuvant (CFA) containing 5 mg/ml of mycobacterium tuberculosis H37RA (250 μg per rat; Difco, Detroit, MI). For control experiments rats were injected subcutaneously at the base of tail with a total volume of 100 μl of saline emulsified in CFA.
Stereotactic Spinal Cord Injections
Sensitized rats were anesthetized with a combination of Dormicum (midazolam, 0.6 mg per 100 g body weight; i.p.; Roche, Basel, Switzerland) and Hypnorm (fentanyl, 0.02 mg per 100 g body weight; i.p.; Janssen-Cilag, Berchem, Belgium). Minimal invasive stereotactic injections into the thoracic spinal cord were performed as previously described.21 Briefly, a dorsal laminectomy was performed at the thoracic level 8 (T8) and the spinal cord was exposed. The animals were then suspended in a stereotactic frame by clamping the vertebra T7. A finely drawn calibrated glass capillary tube was stereotactically inserted through a small opening of the dura into the spinal cord. To target the CST area the glass capillary was inserted to a depth of 0.7 mm in the spinal cord midline. The injection of 2 μl of a cytokine solution containing 250 ng of recombinant rat tumor necrosis factor-α (TNF-α; R&D Systems, Abingdon, UK) and 150 U of recombinant rat interferon-γ (IFN-γ; PeproTech, London, UK) dissolved in phosphate-buffered saline (PBS) was performed slowly throughout a 5-minute period. For control experiments rats were injected with 2 μl of PBS without cytokines. A trace of Monastral Blue (Sigma-Aldrich) was added to all solutions to enhance the visibility. After the injection the glass capillary was carefully withdrawn, the muscles were reapposed with sutures and the skin was closed with wound clips. In all cases the postsurgical recovery was uneventful with no overt clinical signs.
EAE Evaluation
For the evaluation of the disease course the rats were examined using an adapted EAE scoring scale as follows: grade 0, no clinical disease; grade 0.5, partial tail weakness or slight loss of muscle tone; grade 1, tail weakness; grade 1.5, slightly clumsy gait; grade 2.0, hindlimb paresis; grade 2.5, marked hindlimb paresis (partial dragging); grade 3.0, hindlimb paralysis; grade 3.5, hindlimb paralysis and forelimb paresis; grade 4.0, complete paralysis (tetraplegy); grade 5, moribund or dead.26,27 Because of the thoracic localization of the targeted EAE lesions animals usually scored between grade 0 and 3 on this scale.
For the targeted EAE model the disease incidence was determined as the percentage of animals with an EAE score ≥1.5 after local cytokine injection (Table 1). Relapses were defined as an increase in the EAE score ≥1 point lasting for at least 2 days. Only animals with a minimal survival time of 14 days after lesion induction were included in the analysis of relapse frequency. Animals that developed signs of disseminated EAE (score >0.5) before the local cytokine injection were excluded from further analysis.
Enzyme-Linked Immunosorbent Assay
For the determination of the serum titer of anti-MOG antibodies blood samples were taken by puncture of the sublingual veins. After centrifugation of the blood samples the serum was separated and the titer of MOG-specific antibodies was determined by enzyme-linked immunosorbent assay. Briefly, recombinant purified and subsequently gel-eluted MOG (rMOG) corresponding to the N-terminal sequence of rat MOG was coated on a 96-well plate (Greiner Bio-One, Soligen, Germany) at a concentration of 8 μg/ml. After incubation overnight at 4°C the plates were blocked with 5% (w/v) bovine serum albumin (Calbiochem, La Jolla, CA) in PBS for 4 hours at room temperature. Sera were prediluted 40-fold and then titrated in threefold dilutions throughout 12 steps in 5% (w/v) Top Block (Juro Supply, Luzern, Switzerland) in PBS. Detection was done with IgG-specific horseradish peroxidase-conjugated goat anti-rat antibodies (1:5000; Pierce, Rockford, IL) in PBS with 0.1% (v/v) Nonidet P-40 (Fluka, Buchs, Switzerland), 0.05% (w/v) bovine serum albumin, and 3,3′-5,5′-tetramethylbenzidine (BM-Blue, POD substrate; Rôche) was added as a substrate. Between each incubation step, the plates were washed five times with PBS containing 0.05% (v/v) Tween-20. Optical density was measured at 450 nm. Antibody titers were defined as the serum dilutions yielding absorption of twice background levels.
Histopathology
After various survival times animals were perfused transcardially with 4% paraformaldehyde. The spinal cords were dissected and embedded in paraffin. Histological evaluation was performed on 3-μm-thick sections of spinal cords as previously described.28 Sections were stained with hematoxylin and eosin, Luxol fast blue, and Bielschowsky silver impregnation to assess inflammation, demyelination, and axonal pathology, respectively. In adjacent serial sections, immunohistochemistry was performed with antibodies against macrophages/activated microglia (clone ED1; Serotec, Oxford, UK), T-cells (CD3, clone 1F4; Serotec), astrocytes (GFAP; DAKO, Hamburg, Germany), myelin basic protein (MBP; Boehringer Mannheim, Mannheim, Germany), neurofilament (NF200, clone N52; Sigma-Aldrich), and amyloid precursor protein (clone 22C11; Chemicon, Temecula, CA) as a marker for acute axonal damage. Bound antibody was visualized using an avidin-biotin technique with 3,3′-diaminobenzidine as chromogen. Control sections were incubated with isotype-matched control antibodies or with nonimmune sera.
Lesion Analysis
In the targeted EAE model we determined the entire lesion volume, the maximal lesion extension, and the minimal amount of spared white matter by a quantitative histological analysis of the animals at the chronic stage of the disease, 28 days after lesion induction. Serial cross-sections of the entire midthoracic spinal cord containing the EAE lesion were cut on a vibratome. The sections were air-dried, counterstained with cresyl-violet, and coverslipped with Eukitt (Kindler, Freiburg, Germany). Every fourth section was then analyzed by light microscopy (Zeiss, Jena, Germany) and the lesion area as well as the area of spared white matter for each of these sections were determined using the NIH Image Quant Software. For further analysis we calculated the total lesion volume. In addition the maximal lesion extension and the minimal amount of spared white matter on a spinal cord cross-section were determined by comparing all evaluated spinal cord cross-sections. The correlation between these parameters of structural damage and the parameters obtained by behavioral analysis was then analyzed using the Prism Software (Graphpad, San Diego, CA) as detailed below.
In the disseminated EAE model we determined the entire lesion volume by quantitative histological analysis of the animals at the chronic stage of the disease, 28 days after disease onset. Serial longitudinal sections of the entire spinal cord were cut on a vibratome and counterstained with cresyl-violet as described above. Every section was then analyzed and all lesion areas were measured. For further analysis we calculated the total lesion volume for each animal, and the correlation with the behavioral parameters was determined using the Prism Software (Graphpad) as detailed below.
Analysis of Corticospinal Tract Damage
To determine the extent of damage to the CST, the fibers of the hindlimb part of the CST were labeled by anterograde tracing. Briefly, 14 days after lesion induction in the targeted EAE animals and 14 days after disease onset in the disseminated EAE animals, we performed pressure injections of 900 nl of a 10% solution of biotinylated dextran amine (BDA 10,000, in 0.01 mol/L phosphate buffer, pH 7.4; Molecular Probes, Eugene, OR) into the hindlimb area of the left and right motor cortex (coordinates: 2 mm posterior to bregma, 2 mm lateral to bregma, 1.5 mm depth). Two weeks after BDA injections, animals were perfused transcardially with 4% paraformaldehyde and the spinal cords were dissected and postfixed overnight at 4°C. Cross-sections of the spinal cord were cut on a vibratome, serially mounted on Superfrost slides (Menzel-Gläser, Braunschweig, Germany), and processed for BDA detection as previously described.29 The sections were air-dried, counterstained with cresyl-violet, and coverslipped with Eukitt (Kindler). In the targeted EAE model we analyzed the extent of CST damage by determining the number of labeled CST fibers on 10 sections above (high cervical level) and below (low thoracic level) the midthoracic EAE lesion for each CST tract in each animal. In the disseminated EAE model we analyzed the extent of CST damage by determining the number of labeled CST fibers on 10 sections above (high cervical level) and below (low lumbar level) the majority of EAE lesions for each CST tract in each animal. The reduction of CST fibers was calculated and corrected for the reduction of CST fibers between the respective levels in unlesioned animals (n = 4). The CST damage for an individual animal was then determined as the mean of the corrected CST reduction of both CST tracts. The correlation between the CST damage and the parameters obtained by behavioral analysis was analyzed using Prism Software (Graphpad) as detailed below.
Behavioral Analysis
To assess hindlimb motor function in animals with targeted or disseminated EAE the following behavioral tests were used: the open field locomotion test and the Grid walk task. The open field locomotion score (BBB score) was designed to measure the recovery of hindlimb movements after spinal cord injury in rats during free open field locomotion.30 For this purpose the rats were placed in an open field (80 × 130 × 30 cm) with a pasteboard-covered nonslippery floor. In each testing session, the animals were observed individually for 3 to 5 minutes. Hindlimb locomotion was then scored from 0 to 21 points. A score of 0 points defines no movement of the hindlimb, and the maximum of 21 points defines normal locomotion as observed in unlesioned rats. Points are distributed according to criteria such as joint movement, weight support, forelimb-hindlimb coordination, and tail position. We used a modified version of the BBB score, if the sequence of recovering motor functions was not the same as described in the original score.10 The open field locomotion of the rats was evaluated at baseline and at 3, 7, 10, 14, 21, and 28 days after lesion induction in the targeted EAE model and 3, 7, 10, 14, 21, and 28 days after disease onset in the disseminated EAE model.
Deficits in descending motor control were examined by the Grid walk task. The animals had to walk on a 1-m-long horizontal runway of metal bars elevated 1 m above the ground as previously described.10,31 A defined 10 bar sector with irregular placed bars (1 to 4 cm) was chosen for analysis. Animals were trained for 3 days before baseline evaluation. For the analysis of the Grid walk performance the rats were videotaped while crossing the grid (twice). The total number of footfalls as well as the total number of steps were counted and the percentage of footfalls per step was calculated. A footfall was defined as a drop of the foot below the plane of the grid. Animals that could not support their weight on their hindlimbs (BBB <10) would make errors with every step and were thus assigned 100% of footfalls. The percentage of footfalls is then converted into a Grid score ranging from score 1 (0 to 10% of footfalls) to 10 (91 to 100% of footfalls).31 The Grid walk was evaluated at baseline and at 3, 7, 10, 14, 21, and 28 days after lesion induction in the targeted EAE model and 3, 7, 10, 14, 21, and 28 days after disease onset in the disseminated EAE model.
Statistical Analysis
Statistical analysis was performed using Prism Software (Graphpad). The behavioral scores were analyzed over time with the nonparametric Friedman test for repeated measurements. The correlation between the behavioral scores and the parameters of lesion size and CST damage were calculated with the Spearman rank test (Spearman r). P values of p<0.05 were considered statistically significant; values of p<0.01 were considered highly statistically significant.
Results
Injection of Proinflammatory Cytokines Targets EAE Lesions to the Spinal Cord
The formation of EAE lesions in a predetermined location was achieved by stereotactic injections of the proinflammatory cytokines IFN-γ and TNF-α, which facilitate the influx of mononuclear cells into the CNS.20–22 To target the main component of the CST we performed injections into the ventral part of the dorsal funiculus at midthoracic levels. Using a refined procedure the injection of a volume of up to 2 μl can be performed with minimal structural damage and minimal immune cell infiltration as determined by cresyl-violet staining (n = 12; Figure 1, c and d).
Figure 1.
Injection of proinflammatory cytokines into the spinal cord of MOG- or IFA-immunized animals. Spinal cord cross-sections (day 7) at the midthoracic level T8 of animals immunized with either IFA alone (a, c, e) or 25 μg of MOG in IFA (b, d, f) counterstained with cresyl-violet. The injection site is marked in black by the co-injection of Monastral Blue. Animals without an injection into the spinal cord (a, b) showed no infiltration of immune cells regardless of the immunization protocol. Animals injected with 2 μl of PBS (c, d) showed only a very mild immune cell infiltration with no obvious differences between animals immunized with IFA (c) and MOG (d). After local injection of the proinflammatory cytokines IFN-γ and TNF-α, however (e, f), animals immunized with 25 μg of MOG in IFA (f) developed large inflammatory lesions spreading through the entire dorsal funiculus, whereas animals immunized with IFA alone (e) displayed only a mild influx of inflammatory cells around the injection site. Original magnifications, ×50.
In our experimental setup the injection of IFN-γ (150 U per animal) and TNF-α (250 ng per animal) led to a mild influx of inflammatory cells into the spinal cord of control animals immunized with IFA (n = 8; Figure 1e). This inflammatory influx was restricted to the area directly adjacent to the injection site and was accompanied by minimal tissue damage. However, if the same cytokine mixture was injected into animals that had previously been sensitized with MOG in IFA (n = 20) the formation of large confluent, inflammatory demyelinating lesions ensued (Figure 1f). The inflammatory infiltration was localized in the white matter of the dorsal funiculus generally including the area of the CST. In animals with particularly large lesions the cell infiltration expanded to the white matter of the lateral and ventral funiculi and into the gray matter.
An important prerequisite for the induction of targeted EAE is the development of an optimized protocol for the sensitization with the CNS autoantigen MOG. MOG has been chosen for EAE induction because of the well-established pathological similarities between MOG-induced EAE and MS.5,11,16 An optimized sensitization protocol for a targeted EAE model has to meet the following two requirements: an autoimmune T- and B-cell reaction against MOG should be elicited as it is necessary to ensure the development of an EAE-like lesion;19 and, the development of a generalized EAE with disseminated lesion formation should be avoided. To achieve these aims we made use of the relatively low sensitivity of the Lewis rat to the induction of EAE by MOG emulsified in IFA.19,32 We performed a series of experiments to determine the optimal immunization dose for the MOG antigen (Table 1). Immunization with relatively high doses of MOG (50 to 100 μg) in IFA led to robust disease induction. However, a significant proportion of the animals became sick before local cytokine injection or developed signs of generalized and relapsing disease, which argues against the use of high MOG doses for the induction of a localized monophasic disease model. Immunization with lower MOG doses (5 to 25 μg) in IFA circumvented these problems: Animals showed signs of EAE almost exclusively after local cytokine injection and little evidence of spatial and temporal dissemination. Animals immunized with IFA alone did not develop EAE. Taken together these results suggest that immunization with 25 μg of MOG provides an optimal balance between the necessary induction of an anti-MOG immune response and the undesired manifestation of disseminated EAE.
This notion is supported by results obtained from the determination of anti-MOG antibody titers. IgG titers of anti-MOG antibodies were measured over time in animals immunized with either MOG in IFA (5 to 25 μg) or IFA alone (Figure 2b). No anti-MOG antibody titers were detected at baseline (the day of immunization: 18 days before cytokine injection, see Figure 2b; n = 25). However, 17 days after immunization (and thus 1 day before the local cytokine injection), high anti-MOG IgG titers were measured in all animals immunized with 25 μg of MOG [n = 10; mean titer: 5.6 (−40 × log3)], whereas only low titers were measured in some of the animals immunized with 5 μg of MOG [n = 9; mean titer: 0.6 (−40 × log3)]. No anti-MOG antibody titers were detected in any of the animals immunized with IFA [n = 6; mean titer: 0 (−40 × log3)]. These differences persisted for the rest of the experiment, with a continuous slight increase in antibody titer in the MOG-immunized animals. In summary, immunization with 25 μg of MOG led to the stable induction of high titers of anti-MOG antibodies without causing signs of disseminated EAE.
Figure 2.
Time course of anti-MOG antibodies and clinical deficits in animals with targeted EAE. a: Schematic representation of the experimental approach for the induction of the targeted EAE model. Animals were immunized with IFA alone or 5 μg or 25 μg of MOG in IFA at the onset of the experiment (D-18). After 18 days a local minimal invasive injection of the cytokine IFN-γ and TNF-α was injected into the spinal cord (D0). The experiment was then terminated after 28 additional days with the perfusion of the animals (D28). b: To follow the induction of an anti-MOG immune response, anti-MOG antibody titers were determined throughout the course of the experiment. Animals immunized with IFA alone (○) did not develop detectable antibody titers during the entire course of the experiment. Animals immunized with 5 μg of MOG (▴) displayed low anti-MOG antibody titers and animals immunized with 25 μg of MOG (▪) produced high titers of anti-MOG antibodies that were first detectable at day 1 and persisted up to the end of the experiment (n = 6 to 10 animals per group; data are means + SD. c: In parallel, the clinical deficits of the animals were determined daily according to the EAE clinical scoring system. All animals irrespective of the immunization showed no signs of clinical disease before the cytokine injection at day 0. After cytokine injection animals immunized with 25 μg of MOG (▪) developed severe clinical signs whereas animals immunized with 5 μg of MOG (▴) and animals immunized with IFA alone (○) displayed only mild clinical signs (n = 6 to 10 animals per group; data are means + SEM).
In line with our data on anti-MOG antibody titers, the clinical course after cytokine injection differed considerably between animals immunized with either 25 μg or 5 μg of MOG or IFA alone (Figure 2c). Animals immunized with 25 μg of MOG developed massive clinical signs of hindlimb motor impairment starting from 24 hours (day 1) after cytokine injection (n = 10). Peak clinical symptoms were reached between day 3 and day 5 and remained relatively stable up to 10 days after cytokine injection. A slow and incomplete recovery process followed up to the end of the study period (day 28). In contrast, animals immunized with 5 μg of MOG (n = 9) and animals immunized with IFA (n = 6) showed remarkably less severe clinical symptoms. Thus, the combination of immunization with a optimal dose of MOG (25 μg) and minimal invasive injection of the proinflammatory cytokines IFN-γ and TNF-α led to the development of clinical signs of targeted EAE with high incidence and reproducibility.
Targeted EAE Lesions Show the Pathological Hallmarks of MS
Subcutaneous immunization with MOG (25 μg) in IFA followed by local injection of TNF-α and IFN-γ at the level of T8 resulted in large confluent inflammatory lesions in the targeted dorsal funiculus (Figure 3b). In general, the lesions encompassed most of the dorsal funiculus including the CST. Gray matter involvement and extension into the ventrolateral funiculus were rarely observed. The inflammatory lesions were characterized by a massive infiltration of foamy macrophages, few CD-3-positive T cells, and perilesional microglial activation (Figure 3, m and n). Within the lesion, myelin was lost (Figure 3, g and j). At early stages after lesion induction (day 7), myelin degradation products could be detected in macrophages (Figure 3g). Immunohistochemistry for amyloid precursor protein indicated acute axonal damage, which was more prominent at early (day 7) than at later stages of lesion formation (day 28; Figure 3, i and l). Although axonal density was markedly reduced in acute and chronic lesions (Figure 3, h and k), axons, in contrast to myelin, were not completely lost in the EAE lesion. Reactive astrocytes were prominent even in early lesions and formed a dense network of GFAP-positive processes at chronic disease stages (Figure 3o). Most animals at early (day 7), but not at late time points (day 28) showed few mild perivascular mononuclear infiltrates in spinal cord regions distant to the focal lesion without any signs of demyelination and axonal damage.
Figure 3.
Histopathology of targeted EAE lesions. a–c: Lesions induced by focal cytokine injection are exclusively targeted to the midthoracic spinal cord as demonstrated by H&E staining of the cervical (a), thoracic (b), and lumbar (c) spinal cord. No signs of disseminated EAE are discernable. Immunohistochemistry for MBP (d, g, j) shows normal myelin in noninvolved tissue (d), active demyelination with MBP immunoreactivity in macrophages at the early stage (D7: g; inset with higher magnification) indicating active myelin phagocytosis and nearly complete demyelination at the late stage of lesion formation (D28; j). Bielschowsky silver impregnation (e, h, k) shows marked axonal loss at early (D7; h) as well as late disease stage (D28; k) compared to the normal axon density in noninvolved tissue (e). Amyloid precursor protein immunostaining (f, i, l) further demonstrates that axons are predominantly injured at the early stage (D7; i) and to a lesser extent also at the late stage (D28; l) whereas no amyloid precursor protein-positive end bulbs are present in noninvolved tissue (f). The inflammatory infiltrate is primarily composed of macrophages (ED1 immunohistochemistry, D7: m; left inset with higher magnification; right inset with isotype control staining) and to a lesser extent of perivascular and parenchymal T cells (CD3 immunohistochemistry, D7: n; left inset with higher magnification; right inset with isotype control staining). Immunostaining for GFAP demonstrates reactive astrogliosis in the lesion area at the late stages of the disease (D28, o; right inset with isotype control staining). Original magnifications: ×40 (a–c); ×400 (d–o and inset in n); ×1000 (inset in g, m). Scale bars: 100 μm (l, o); 10 μm (insets in g and m); 20 μm (inset in n).
Refined Behavioral Tests Can Be Applied in the Targeted EAE Model
The targeted nature of our localized EAE model enabled us to perform a detailed behavioral analysis of disease development. A basic evaluation of the overall motor performance of the animals was performed using an adapted version of the commonly used EAE scoring scale.26,27 This clinical score, primarily developed for disseminated EAE models, classifies the grade of paralysis of tail, forelimbs, and hindlimbs on a relatively crude five-step scale.
As depicted in Figure 4a, all animals immunized with 25 μg of MOG (n = 10) developed signs of disease 3 days after cytokine injection (mean clinical score, 2.2), and their overall motor performance remained impaired between days 3 and 10 followed by slow and often incomplete recovery up to day 28 (mean clinical score, 0.8). In contrast, animals immunized with 5 μg of MOG or IFA reached only mild clinical scores (day 3: mean clinical score, 0.6 for 5 μg of MOG; day 3: mean clinical score,: 0.2 for IFA), which resolved by day 14 (Figure 4a).
Figure 4.
Behavioral analysis of targeted and disseminated EAE. The deficits in hindlimb locomotion induced by a local midthoracic EAE lesion (a–c) and disseminated EAE (d–f) were evaluated using different behavioral test paradigms over time. For the targeted EAE, animals immunized with 25 μg of MOG (▪), 5 μg of MOG (▴), and IFA alone (○) were evaluated using the classical EAE clinical scoring system (a), the BBB locomotor scale (b), and the Grid walk test (c). For the disseminated EAE, animals immunized with MBP in CFA (▪) or CFA alone (○) were evaluated using the classical EAE clinical scoring system (d), the BBB locomotor scale (e), and the Grid walk test (f). n = 5 to 6 animals per control group and n = 9 to 15 animals per EAE group; *, only four animals were measured at this time point; data are means ± SEM. Baseline evaluation was performed before disease induction.
To assess the general locomotion of the animals we used the BBB locomotor scale evaluated in an open field.30 Over-ground locomotion is known to be influenced by several ventral and dorsal spinal motor tract systems as well as the local spinal circuitry.33,34 After induction of the targeted EAE lesion, all animals immunized with 25 μg of MOG displayed a severe impairment of hindlimb locomotor function in the open field (Figure 4b). Three days after cytokine injection animals averaged a score of 8 ranging from 0 (no observable hindlimb movement) to a maximum of 14 (consistent weight supported plantar steps, consistent forelimb-hindlimb coordination, exorotation of the feet). Animals immunized with 25 μg of MOG then recovered slowly leading to a persistent deficit at day 28 (mean score of 17).
Animals immunized with 5 μg of MOG displayed only a moderate impairment of hindlimb locomotor function (mean score of 18, ranging from 13 to 20; Figure 4b) and animals immunized with IFA alone displayed no or only very mild impairments of hindlimb locomotor function (mean score of 20.5, ranging from 19 to 21; Figure 4b).
We analyzed more specific aspects of hindlimb motor function using the Grid walk task. Because of the irregular spacing of the grid bars, this task requires a controlled placement of the hindlimbs that depends on supraspinal input in part through the CST.10,35 Because our targeted EAE lesions are localized in the dorsal midthoracic spinal cord, they damage dorsal and dorso-lateral spinal tract systems including the CST. Animals immunized with 25 μg of MOG exhibited a high percentage of footfalls, reaching a mean score of 9 ranging from 7 (61 to 70% footfalls) to 10 (91 to 100% footfalls) 3 days after cytokine injection (Figure 4c). The majority of these animals showed only a partial recovery leading to a persistent deficit in skilled placing throughout the entire study period (day 28, mean score 3). At day 3, animals immunized with 5 μg of MOG displayed a mean score of 6 (ranging from 1 to 10) and IFA-immunized animals a mean score of 4 (ranging from 1 to 8). These animals recovered controlled placement of the hindlimb very quickly and reached their baseline performance by day 10.
In a parallel experiment, the behavioral tests results of the targeted EAE model were compared with a standard disseminated EAE model using Lewis rats immunized with MBP (Figure 4; d to f). The evaluation of the overall motor performance applying the EAE scoring scale showed the classical clinical course of monophasic EAE (Figure 4d). All animals immunized with MBP in CFA (n = 15) developed severe signs of disease 3 days after disease onset (mean clinical score, 2.6) but then recovered completely up to day 14 after disease onset (mean clinical score, 0.13). Control animals immunized with CFA alone (n = 5) developed no signs of disease at any time point. Comparable results were obtained for the open-field locomotion measured with the BBB locomotor scale (Figure 4e). Animals immunized with MBP in CFA displayed severe deficits in open-field locomotion at day 3 (mean BBB score, 4.6) and then recovered completely up to day 14 (mean BBB score, 20.2), whereas animals immunized with CFA alone showed no deficits in open-field locomotion at any time point. The Grid walk task is of limited use in disseminated EAE (Figure 4f). At the peak of disease (day 3) the animals immunized with MBP in CFA usually had severe paraparesis and were thus assigned a 100% of mistakes on the Grid walk (day 3: mean Grid score, 9.4). Once the animals started to recover their hindlimb function they quickly reached their baseline performance (day 10: mean Grid score, 1.43). In contrast to the targeted EAE model no animals with disseminated EAE showed persistent deficits at day 28 in any of the behavioral test paradigms.
Refined Behavioral Tests Enhance the Sensitivity of Functional Analysis
The specific evaluation of defined components of motor function allows for a sensitive analysis of behavioral deficits and recovery. When the standard clinical EAE scoring system was applied to animals immunized with 25 μg of MOG, no significant recovery was detected between days 3 and 10 (Figure 5a). Conversely, when the same time period was analyzed using the BBB and Grid walk, early recovery of some of the animals was observed. The mean BBB locomotor score rose from 8 at day 3 to 11 at day 7 and to 12 at day 10 (Figure 5b; Friedman Test, P < 0.01). Similarly, early recovery of descending motor control measured by the Grid walk test was observed. Although animals displayed a mean Grid score of 9 at 3 days after local cytokine injection they recover significantly over time and averaged a score of 7 at day 7 and 5.5 at day 10 (Figure 5c; Friedman test, P < 0.01).
Figure 5.
Sensitivity of behavioral tests for the recovery process. Evaluation of the early recovery process between days 3 and 10 in animals immunized with 25 μg of MOG (♦) in different behavioral test paradigms (a–c). Using the classical EAE clinical scoring system (a) no significant differences could be detected in the clinical scores of the animals between days 3 and 10 (Friedman test, P > 0.05). In contrast, open field locomotion (b) and the Grid walk (c) detected the early recovery process and highly significant differences between day 3 and day 10 were obtained (Friedman test, P < 0.01 for open field locomotion; Friedman test, P < 0.01 for Grid walk). n = 10 animals. Open bars represent the means. **, Statistically highly significant (P < 0.01); ns, not significant.
Deficits in Open Field Locomotion Correlate with Structural Damage in the Targeted EAE Model
To determine the extent of structural damage in our targeted EAE model, we evaluated the lesion size 28 days after local cytokine injection. Because the acute inflammatory reaction has subsided by this time, the area of structural damage can be accurately evaluated on cresyl-violet stained sections. For each animal (n = 19), several parameters such as lesion volume, maximal lesion area, and the minimal area of spared white matter were determined on cross-sections. Subsequent correlative analysis with the results of the behavioral analysis (maximal or persistent deficit in the EAE clinical score, BBB locomotor score, and Grid walk) was performed.
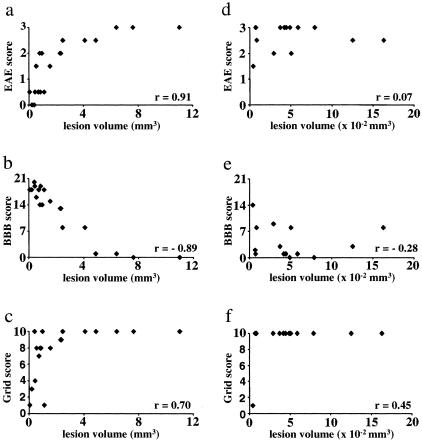
A highly significant and strong correlation was obtained when we compared the maximal EAE score with the lesion volume of each animal (Figure 6a; r = 0.91, P < 0.001). Correlations with the maximal lesion extension (r = 0.69, P < 0.01) and the amount of spared white matter (r = −0.69, P < 0.01) were lower but remained highly significant. These findings indicate that in the targeted EAE model the three-dimensional extent of structural damage critically determines the deficit measured with the EAE clinical score. Further analysis revealed that the persistent clinical deficits at day 28 also depended on the extent of structural damage (r = 0.79, P < 0.001). All animals with a lesion volume <2.5 mm3 (n = 13) recovered completely within the study period. On the other hand, all animals with a lesion volume above 2.5 mm3 (n = 5) developed persistent clinical deficits throughout the 28 days after lesion induction.
Figure 6.
Correlation of behavioral tests with the lesion volume in targeted and disseminated EAE. Analysis of the relation between lesion volume and behavioral test parameters in animals with targeted (a–c) and disseminated EAE (d–f). In the targeted EAE model, the lesion volume showed a strong and highly significant correlation with the maximal EAE clinical score (a: r = 0.91, P < 0.001) and the minimal BBB locomotor score (b: r = −0.89, P < 0.001) and a weaker correlation with the maximal score in the Grid walk task (c: r = 0. 70, P < 0.001). n = 19 animals. In contrast in the disseminated EAE model no significant correlation of the lesion volume is observed with either the maximal EAE score (d: r = 0.07, P > 0.05), the minimal BBB locomotor score (e: r = −0.28, P > 0.05), or maximal Grid score (f: r = 0.45, P > 0.05). n = 14 animals. r, correlation coefficient (Spearman rank test).
Similar conclusions arose from the analysis of the open field locomotion. A strong and highly significant correlation was observed between the minimal BBB score and the lesion volume (Figure 6b; r = −0.89, P < 0.001). Correlations with the maximal lesion extension (r = −0.69, P < 0.01) and the amount of spared white matter (r = 0.72, P < 0.001) were lower but remained highly significant. Similar to the findings with the EAE clinical score, the lesion volume correlates with persistent deficits (day 28) in the open field locomotion (r = 0.86, P ≤ 0.001). At day 28, 7 of 11 animals with a lesion volume <1.6 mm3 had recovered completely, whereas all animals with a lesion volume >1.6 mm3 (n = 7) had persistent clinical deficits.
The analysis of the Grid walk test revealed a weaker correlation with the parameters describing structural damage (Figure 6c; for lesion volume: r = 0.70, P < 0.001; for the maximal lesion extension: r = 0.75, P < 0.001; and for the amount of spared white matter: r = −0.46, P < 0.05).
To compare these results with a standard disseminated EAE model, we determined the total lesion volume in animals immunized with MBP in CFA (n = 14) 28 days after the disease onset (Figure 6; d to f). No significant correlations between the maximal behavioral deficits and the lesion volume were detected for either the EAE score (Figure 6d; r = 0.07, P > 0.05), the BBB score (Figure 6e; r = −0.28, P > 0.05), or the Grid walk (Figure 6f; r = 0.45, P > 0.05). As mentioned previously, no persistent deficits were observed in the animals with disseminated EAE and thus no correlations could be calculated for this later stage.
Deficits in Skilled Placing Correlate with the CST Damage in the Targeted EAE Model
To assess the extent of damage to the CST anterograde labeling with BDA was performed. Damage was measured by direct quantification of traced CST fibers above and below the midthoracic EAE lesion (Figure 7; a to d). CST damage in our animals (n = 17) ranged from 16 to 100% with a mean of 77%. Damage to the CST was correlated to the behavioral analysis (maximal or persistent deficit in EAE clinical score, BBB locomotor score, and the Grid walk score).
Figure 7.
CST damage and correlation with the Grid walk. a: Photomicrograph of a longitudinal section of the thoracic spinal cord with a targeted EAE lesion (*, outlined with gray arrows). Damage to the CST is visualized by anterograde tracing of the hindlimb CST fibers with BDA (black fibers, counterstained with cresyl-violet). b–d: Photomicrographs of the anterogradely labeled main CST (black fibers, outlined with gray line, arrows indicate the position of the midline) visualized on spinal cord cross-sections. b: Cervical spinal cord with undamaged main CST. c: Thoracic spinal cord with inflammatory infiltrate (cresyl-violet counterstaining) and damaged CST fibers (arrow). d: Lumbar spinal cord with remaining intact fibers of the main CST. Analysis of the relation of the Grid walk and CST damage indicates a strong correlation of the persistent deficits (day 28) in the Grid walk with the extent of CST damage (e: r = 0.80, P < 0.001). This correlation is even more pronounced when only animals with similarly large lesions (lesion volume >1.5 mm3) are analyzed (f: r = 0.91, P < 0.01). n = 16 animals (e), n = 8 animals (f). r, correlation coefficient (Spearman rank test). Original magnifications: ×50 (a); ×200 (b–d).
CST damage correlated best with the persistent deficits at day 28 in the Grid walk test (Figure 7e; r = 0.80, P < 0.001). All animals with CST damage greater than 95% (n = 4) developed a persistent deficit of at least score 3 (21 to 30% of footfalls) whereas all animals with a CST damage <95% (n = 12) recovered to a score of 1 to 2. The correlation between CST damage and Grid score was low at the maximum of the disease (r = 0.47, P > 0.05) but progressively increased over time (day 14; r = 0.75, P < 0.001). The Grid walk however does not solely reflect CST function, as the rhythmic stepping process is also influenced by the intraspinal circuitry and thus the extent of the EAE lesion. This is supported by the strengthening of the correlation (Grid walk versus CST damage) observed with animals harboring similarly large lesions (lesion volume >1.5 mm3; Figure 7f, r = 0.91, P < 0.01).
EAE and BBB locomotor score showed only a moderate correlation with CST damage throughout time (for the EAE score: maximal deficit, r = 0.71, P < 0.01 versus day 14, r = 0.50, P < 0.05 versus day 28, r = 0.71, P < 0.001 and for the BBB score: maximal deficit, r = −0.63, P < 0.01 versus day 14, r = −0.62, P < 0.01 versus day 28, r = −0.76, P < 0.001). In addition, these tests correlated substantially less with the CST damage than with the lesion volume (Figure 6).
In a standard disseminated EAE model the extent of CST damage was determined 28 days after the disease onset (n = 12). The CST damage ranged from 5 to 51% with a mean of 32%. No significant correlations between the maximal behavioral deficits and the CST damage were detected for either the EAE score (r = 0.16, P > 0.05), the BBB score (r = −0.25, P > 0.05), or the Grid walk (r = 0.22, P > 0.05). No persistent deficits remained in animals with disseminated EAE and thus no correlations could be calculated for this later stage.
Discussion
In the present study we demonstrate that targeting EAE lesions to a predetermined location in the rat spinal cord allows us to behaviorally assess the function of specific motor tract systems in an animal model of MS. Targeting of EAE lesions was achieved by a combination of low-dose immunization with the myelin antigen MOG and the stereotactic intraspinal injection of the proinflammatory cytokines IFN-γ and TNF-α. EAE lesions were targeted to the area of the CST in the dorsal part of the midthoracic spinal cord. The resulting deficits of hindlimb motor function were assessed by the commonly used EAE clinical score but also by the more refined and sensitive assessments of open field locomotion and grid walking. The comparison with a standard disseminated EAE model demonstrates several major advantages of the targeted EAE model because it leads to persistent and defined functional deficits that allow for specific and sensitive behavioral testing of functional components and yield quantitative behavioral endpoints that are directly correlated to the overall structural damage and specifically to the extent of CST damage.
The immunological rationale behind the development of this targeted EAE model is to combine the low susceptibility of the Lewis rat to MOG-induced autoimmune disease with the ability of the proinflammatory cytokines TNF-α and IFN-γ to induce a local influx of inflammatory cells into the CNS.20,26,36 Lewis rats immunized with an optimal MOG dose of 25 μg in IFA did not develop disseminated EAE, but still mounted a substantial anti-MOG IgG response, indicating that an efficient priming of an anti-MOG-specific T- and B-cell response had taken place. These primed autoreactive T cells and antibodies circulate through the blood and can therefore be attracted to a predetermined target region by injection of proinflammatory cytokines. The ability of TNF-α and IFN-γ to attract immune cells has been described previously.20–22 When injected into the adult CNS TNF-α primarily leads to a monocytic cell infiltration,21 whereas IFN-γ causes a predominantly lymphocytic influx.22 Although the stereotactic injection of TNF-α and IFN-γ is clearly an artificial way to induce the formation of inflammatory CNS lesions, both cytokines are supposed to play a crucial role in the process of lesion induction both in EAE and MS.3 They can activate brain microvessels inducing increased expression of adhesion molecules and MHC class II molecules on endothelial cells.37–39 Both proinflammatory cytokines lead to an increased adherence and transmigration of encephalitogenic T cells into the CNS.40,41 Once in the CNS, those T cells that recognize a CNS antigen, in our case MOG, will persist, recruit other inflammatory cells, and initiate the formation of an inflammatory, demyelinating CNS lesion.42
The combination of a subclinical immune response against MOG and the localized stereotactic injection of proinflammatory cytokines into the thoracic spinal cord led to the formation of a focal inflammatory demyelinating lesion. The inflammatory infiltrate in these lesions was composed of foamy macrophages with the interspersion of few perivascular and parenchymal CD3-positive T cells. Both the loss of myelin sheaths and active myelin degradation by macrophages were found in the lesions. Although the axonal density in acute and chronic lesions was markedly reduced, axons, in contrast to myelin, were not completely lost in the EAE lesion. Glial scar formation was detected already early in the disease course.
Focal inflammatory demyelinating spinal cord lesions reflected key features of MS pathology. Myelin loss, axonal damage, infiltration with foamy macrophages and T cells, microglia activation, and reactive gliosis are central characteristics of early MS lesions. Axonal damage and subsequent axonal loss are prominent in targeted spinal cord lesions indicating a destructive inflammatory process resembling MS cases with fulminant disease and antibody- and complement-mediated tissue damage.43–45
Studies throughout the last years have emphasized that structural damage to axons is a key determinant for the persistent clinical deficit in patients suffering from MS.2,3 Despite sustained efforts, the number of promising experimental studies to prevent or reverse structural damage in conventional EAE models is so far very low.46,47 One central problem of commonly used acute EAE models is that in many cases the structural damage is not reflected in the clinical deficit in a way that can be easily quantified and correlated to the damage.48,49
Our current data corroborate this view, as in a standard disseminated EAE model, neither the structural damage nor the damage to the CST showed any significant correlation with the results of our behavioral tests. In these models, animals classically develop a monophasic disease, which, if survived, is followed by a complete recovery over the course of a few days. The acute clinical deficit has been correlated to the extent of immune infiltration and is thus most likely a consequence of immune-mediated axon dysfunction rather than of persistent structural damage.48,50,51 Irreversible axonal damage does occur but may be limited to a small fraction of axons from a given axonal tract system, which if minor can be compensated by the remaining intact fibers. Only in models with a chronic disease phase is the accumulated structural damage translated into measurable clinical deficits.49,52
The dissemination of EAE lesions in conventional EAE models leads to a variable number of axonal tracts being adversely affected. This renders refined behavioral testing, which generally relies on the quantification of specific functional deficits, virtually impossible. A sensitive quantification of the functional deficit is, however, of central importance for the evaluation of protective and repair strategies as they, in contrast to immunological approaches, cannot be expected to alter immune-related parameters such as disease onset or incidence. Targeted EAE lesions give rise to defined functional deficits and can thus be evaluated using specific and sensitive behavioral tests.
In the targeted EAE model the maximal deficits in the EAE and BBB scores showed the best correlation with the total amount of structural damage. These findings can be at least partially explained by the fact that both the BBB and EAE scores evaluate general locomotion and are thus influenced by various tract systems of the dorsal, lateral, and ventral funiculi.33,34 Large lesions will inevitably affect several of these axonal tracts whose damage will be strongly reflected in the general locomotor analysis. The Grid walk on the other hand is strongly influenced by CST function.10,35 Because our cytokine injections were targeted to the region of the CST, even small lesions still showed a substantial destruction of this tract, resulting in a weaker correlation of the Grid walk with the overall amount of structural damage. This notion is further supported by our finding that the persistent but not the maximal deficits in the Grid walk showed the best correlation with the extent of CST damage. Initial deficits in the Grid walk are most likely linked to a mixture of structural CST damage and inflammation-induced CST dysfunction. Conversely later deficits are more likely to reflect predominantly structural CST damage. It should be noted however, that persistent deficits in the Grid walk were only observed in those animals with a very substantial damage to the CST limiting the direct behavioral assessment of CST damage to this group of animals. The influence of the descending motor input mediated by the CST on skilled placing is likely to explain the strong correlation between Grid walk and CST damage. The weaker correlation of EAE and BBB score with the extent of CST damage is further compatible with the assumption that over-ground locomotion measured in EAE and BBB scores is not primarily dependent on CST function but rather influenced by a combination of spinal tract systems.
Similar defined behavioral tests have been used for many years for the quantification of clinical deficits in various models of localized CNS injury35,53,54 and many of the test paradigms have been initially developed for spinal cord injury.30,55 However, our results also reveal marked differences between inflammatory and traumatic spinal cord lesions. Compared to animals with traumatic spinal cord lesions, those with inflammatory lesions showed a faster and more complete recovery. The early and relatively fast recovery process up to day 14 may be in part explained by the subsiding inflammatory reaction. The ongoing slower recovery up to the end of the study period may indicate a structural repair process that should be facilitated in an incomplete inflammatory tract lesion as compared to a more complete, traumatic tract lesion. Overall however, our present study demonstrates that both clinical and structural deficits induced by localized inflammatory reactions of the spinal cord can be analyzed in a very similar manner to those induced by spinal cord trauma.
The localized EAE model should thus open new possibilities for the evaluation of protective or repair-oriented therapeutic strategies for MS, which have so far been primarily investigated in models of traumatic CNS injury. Growth factors, eg, of the families of neurotrophic factors, have shown considerable potential as therapeutic agents for MS.47,56,57 Their particular appeal resides in the pleiotrophy inheritant to their actions, which comprise the protection of axons and myelin, stimulation of axonal regeneration, as well as immunomodulatory properties.58,59 The development of repair strategies aiming at both the replacement of damaged glial cells and the functional reconnection of interrupted axonal connections are further exciting perspectives for future research.9,60 Cell transplantation approaches are obviously facilitated in a localized EAE model with a predetermined localization of the lesion.61–63 Furthermore, therapeutic strategies increasing plastic reorganization can only be thoroughly evaluated if a defined axonal tract system and its reorganization can be followed.64
In conclusion, we have developed a reproducible model of MS in the spinal cord that has quantifiable histological and behavioral endpoints and can thus help to improve both our understanding of tissue damage as well as the evaluation of therapeutic strategies for MS.
Acknowledgments
We thank Jeanette Scholl for excellent technical support; Roland Schoeb for expert help with the figures; Dr. Olivier Raineteau for surgical assistance; and Drs. Lisa Schnell, Thomas Misgeld, and Leanne Godinho for valuable suggestions and critical reading of the manuscript.
Footnotes
Address reprint requests to Martin Kerschensteiner, M.D., Dept. of Anatomy and Neurobiology, Washington University School of Medicine, 660 S. Euclid, CB 8108, St. Louis, MO 63110. E-mail: martink@pcg.wustl.edu.
Supported by the Swiss National Science Foundation (Nr.: 31-63633 to M.E.S.), the National Center of Competence in Research (NCCR) Neural Plasticity and Repair, and the Deutsche Forschungsgemeinschaft (postdoctoral Emmy-Noether fellowship KE774/2-1 to M.K.).
This study is part of the thesis for B.S.B.
References
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Trapp BD. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr Opin Neurol. 2001;14:271–278. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121:1469–1477. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- van Waesberghe JH, Kamphorst W, De Groot CJ, van Walderveen MA, Castelijns JA, Ravid R, Lycklama a Nijeholt GJ, van der Valk P, Polman CH, Thompson AJ, Barkhof F. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–754. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- Johns TG, Kerlero DR, Menon KK, Abo S, Gonzales MF, Bernard CC. Myelin oligodendrocyte glycoprotein induces a demyelinating encephalomyelitis resembling multiple sclerosis. J Immunol. 1995;154:5536–5541. [PubMed] [Google Scholar]
- Wekerle H, Kojima K, Lannes-Vieira J, Lassmann H, Linington C. Animal models. Ann Neurol. 1994;36:S47–S53. doi: 10.1002/ana.410360714. [DOI] [PubMed] [Google Scholar]
- Newman TA, Woolley ST, Hughes PM, Sibson NR, Anthony DC, Perry VH. T-cell- and macrophage-mediated axon damage in the absence of a CNS-specific immune response: involvement of metalloproteinases. Brain. 2001;124:2203–2214. doi: 10.1093/brain/124.11.2203. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: a comparative study. Glia. 1999;25:216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Westland KW, Pollard JD, Sander S, Bonner JG, Linington C, McLeod JG. Activated non-neural specific T cells open the blood-brain barrier to circulating antibodies. Brain. 1999;122:1283–1291. doi: 10.1093/brain/122.7.1283. [DOI] [PubMed] [Google Scholar]
- Storch MK, Stefferl A, Brehm U, Weissert R, Wallstrom E, Kerschensteiner M, Olsson T, Linington C, Lassmann H. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–694. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Hoenig EM. Induced localization of allergic adrenalitis and encephalomyelitis at sites of thermal injury. J Immunol. 1968;100:1310–1318. [PubMed] [Google Scholar]
- Clark G, Bogdanove L. The induction of the lesions of allergic meningoencephalitis in a predetermined location. J Neuropathol Exp Neurol. 1955;14:433–437. [PubMed] [Google Scholar]
- Weissert R, Wallstrom E, Storch MK, Stefferl A, Lorentzen J, Lassmann H, Linington C, Olsson T. MHC haplotype-dependent regulation of MOG-induced EAE in rats. J Clin Invest. 1998;102:1265–1273. doi: 10.1172/JCI3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RD, Willenborg DO. Direct injection of cytokines into the spinal cord causes autoimmune encephalomyelitis-like inflammation. J Neurol Sci. 1990;104:112–113. doi: 10.1016/0022-510x(90)90010-k. [DOI] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Phillips LM, Lampson LA. Site-specific control of T cell traffic in the brain: T cell entry to brainstem vs. hippocampus after local injection of IFN-gamma. J Neuroimmunol. 1999;96:218–227. doi: 10.1016/s0165-5728(99)00034-x. [DOI] [PubMed] [Google Scholar]
- Adelmann M, Wood J, Benzel I, Fiori P, Lassmann H, Matthieu JM, Gardinier MV, Dornmair K, Linington C. The N-terminal domain of the myelin oligodendrocyte glycoprotein (MOG) induces acute demyelinating experimental autoimmune encephalomyelitis in the Lewis rat. J Neuroimmunol. 1995;63:17–27. doi: 10.1016/0165-5728(95)00124-7. [DOI] [PubMed] [Google Scholar]
- O’Brien NC, Charlton B, Cowden WB, Willenborg DO. Nitric oxide plays a critical role in the recovery of Lewis rats from autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol. 1999;163:6841–6847. [PubMed] [Google Scholar]
- Duplan V, Dutartre P, Mars LT, Liblau RS, Druet P, Saoudi A. LF 15-0195 inhibits the development of rat central nervous system autoimmunity by inducing long-lasting tolerance in autoreactive CD4 T cells. J Immunol. 2003;170:2179–2185. doi: 10.4049/jimmunol.170.4.2179. [DOI] [PubMed] [Google Scholar]
- Stefferl A, Brehm U, Storch M, Lambracht-Washington D, Bourquin C, Wonigeit K, Lassmann H, Linington C. Myelin oligodendrocyte glycoprotein induces experimental autoimmune encephalomyelitis in the “resistant” Brown Norway rat: disease susceptibility is determined by MHC and MHC-linked effects on the B cell response. J Immunol. 1999;163:40–49. [PubMed] [Google Scholar]
- Meyer R, Weissert R, Diem R, Storch MK, de Graaf KL, Kramer B, Bahr M. Acute neuronal apoptosis in a rat model of multiple sclerosis. J Neurosci. 2001;21:6214–6220. doi: 10.1523/JNEUROSCI.21-16-06214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass K, Lassmann H, Wekerle H, Wisniewski HM. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol. 1986;70:149–160. doi: 10.1007/BF00691433. [DOI] [PubMed] [Google Scholar]
- Herzog A, Brosamle C. ‘Semifree-floating’ treatment: a simple and fast method to process consecutive sections for immunohistochemistry and neuronal tracing. J Neurosci Methods. 1997;72:57–63. doi: 10.1016/s0165-0270(96)00156-2. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefferl A, Linington C, Holsboer F, Reul JM. Susceptibility and resistance to experimental allergic encephalomyelitis: relationship with hypothalamic-pituitary-adrenocortical axis responsiveness in the rat. Endocrinology. 1999;140:4932–4938. doi: 10.1210/endo.140.11.7109. [DOI] [PubMed] [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DS, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Kunkel-Bagden E, Dai HN, Bregman BS. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp Neurol. 1993;119:153–164. doi: 10.1006/exnr.1993.1017. [DOI] [PubMed] [Google Scholar]
- Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu HC, Lassmann H, Wekerle H. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol. 1993;23:1364–1372. doi: 10.1002/eji.1830230627. [DOI] [PubMed] [Google Scholar]
- Huynh HK, Dorovini-Zis K. Effects of interferon-gamma on primary cultures of human brain microvessel endothelial cells. Am J Pathol. 1993;142:1265–1278. [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, Washington RA, Balabanov R. Cytokine-mediated activation of cultured CNS microvessels: a system for examining antigenic modulation of CNS endothelial cells, and evidence for long-term expression of the adhesion protein E-selectin. J Cereb Blood Flow Metab. 1994;14:837–844. doi: 10.1038/jcbfm.1994.105. [DOI] [PubMed] [Google Scholar]
- Carvalho-Tavares J, Hickey MJ, Hutchison J, Michaud J, Sutcliffe IT, Kubes P. A role for platelets and endothelial selectins in tumor necrosis factor-alpha-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–1148. doi: 10.1161/01.res.87.12.1141. [DOI] [PubMed] [Google Scholar]
- Piccio L, Rossi B, Scarpini E, Laudanna C, Giagulli C, Issekutz AC, Vestweber D, Butcher EC, Constantin G. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- McCarron RM, Wang L, Racke MK, McFarlin DE, Spatz M. Cytokine-regulated adhesion between encephalitogenic T lymphocytes and cerebrovascular endothelial cells. J Neuroimmunol. 1993;43:23–30. doi: 10.1016/0165-5728(93)90071-6. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465–471. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, Lassmann H. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine CS, Cannella B, Hauser SL, Genain CP. Demyelination in primate autoimmune encephalomyelitis and acute multiple sclerosis lesions: a case for antigen-specific antibody mediation. Ann Neurol. 1999;46:144–160. doi: 10.1002/1531-8249(199908)46:2<144::aid-ana3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Zhang JG, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, Emery B, Turnley AM, Petratos S, Ernst M, Bartlett PF, Kilpatrick TJ. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- Berger T, Weerth S, Kojima K, Linington C, Wekerle H, Lassmann H. Experimental autoimmune encephalomyelitis: the antigen specificity of T lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab Invest. 1997;76:355–364. [PubMed] [Google Scholar]
- Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J Neuropathol Exp Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
- Sobel RA, van der Veen RC, Lees MB. The immunopathology of chronic experimental allergic encephalomyelitis induced in rabbits with bovine proteolipid protein. J Immunol. 1986;136:157–163. [PubMed] [Google Scholar]
- Matsumoto Y, Hara N, Tanaka R, Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986;136:3668–3676. [PubMed] [Google Scholar]
- McGavern DB, Murray PD, Rivera-Quinones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123:519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, Parsons AA. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Soblosky JS, Colgin LL, Chorney-Lane D, Davidson JF, Carey ME. Ladder beam and camera video recording system for evaluating forelimb and hindlimb deficits after sensorimotor cortex injury in rats. J Neurosci Methods. 1997;78:75–83. doi: 10.1016/s0165-0270(97)00131-3. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Villoslada P, Hauser SL, Bartke I, Unger J, Heald N, Rosenberg D, Cheung SW, Mobley WC, Fisher S, Genain CP. Human nerve growth factor protects common marmosets against autoimmune encephalomyelitis by switching the balance of T helper cell type 1 and 2 cytokines within the central nervous system. J Exp Med. 2000;191:1799–1806. doi: 10.1084/jem.191.10.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Howe CL, Rodriguez M. Growth factor treatment of demyelinating disease: at last, a leap into the light. Trends Immunol. 2002;23:512–516. doi: 10.1016/s1471-4906(02)02321-9. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Stadelmann C, Dechant G, Wekerle H, Hohlfeld R. Neurotrophic crosstalk between the nervous and immune systems: implications for neurological diseases. Ann Neurol. 2003;53:292–304. doi: 10.1002/ana.10446. [DOI] [PubMed] [Google Scholar]
- David S, Lacroix S. Molecular approaches to spinal cord repair. Annu Rev Neurosci. 2003;26:411–440. doi: 10.1146/annurev.neuro.26.043002.094946. [DOI] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Smith PM, Lakatos A, Barnett SC, Jeffery ND, Franklin RJ. Cryopreserved cells isolated from the adult canine olfactory bulb are capable of extensive remyelination following transplantation into the adult rat CNS. Exp Neurol. 2002;176:402–406. doi: 10.1006/exnr.2002.7936. [DOI] [PubMed] [Google Scholar]
- Kohama I, Lankford KL, Preiningerova J, White FA, Vollmer TL, Kocsis JD. Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. J Neurosci. 2001;21:944–950. doi: 10.1523/JNEUROSCI.21-03-00944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]