Abstract
Activin A, a dimeric glycoprotein that belongs to the transforming growth factor-β superfamily, governs cellular differentiation in a wide variety of models and has been implicated in the regulation of angiogenesis. We examined the role of activin A and its downstream signaling pathway in a murine model of inflammatory corneal neovascularization induced by mechanical injury (debridement), and in vitro in corneal epithelial cells. Activin A expression increased steadily from day 2 until day 8 after mechanical debridement in vivo, paralleling vascular endothelial growth factor (VEGF) expression. Administration of recombinant activin A in mice increased the area of neovascularization, VEGF expression, and the kinase activities of p38 and p42/44 MAPKs after mechanical debridement. Systemic inhibition of activin A in vivo with a neutralizing antibody reduced the area of neovascularization, VEGF expression, and p38 and p42/44 MAPK activity, whereas administration of an isotype-matched control antibody had no effect. In vitro treatment with activin A increased VEGF secretion, as well as p38 and p42/44 MAPK activity in corneal epithelial cells, whereas concurrent administration of specific inhibitors of p38 or p42/44 MAPK abolished the stimulatory effect of activin A on VEGF production. We conclude that activin A stimulates inflammatory corneal angiogenesis by increasing VEGF levels through a p38 and p42/44 MAPK-dependent mechanism.
Corneal neovascularization is a disabling condition that results in loss of the immunological privilege of the cornea and ultimately in visual impairment. It is a common manifestation of inflammatory, infectious, and traumatic diseases of the cornea and the limbal stem cell barrier. Although laser treatment and surgical intervention offer potential therapeutic options, corneal neovascularization still remains a therapeutic puzzle because, in many occasions, cornea avascularity and transparency are not restored. The potential benefits of controlling angiogenesis have been demonstrated in experimental models for various untreatable ocular conditions that involve neovascularization.1–3 These studies have highlighted the pivotal role of vascular endothelial growth factor (VEGF) in regulating endothelial cell growth and neovascularization.
VEGF refers to a family of angiogenic and vascular permeability-enhancing peptides derived from alternatively spliced mRNAs.4 VEGF bioactivity is primarily mediated via two high-affinity cognate receptors, KDR/Flk-1 and Flt-1.5,6 Recently, it was suggested that VEGF plays an important role in corneal neovascularization because exogenous VEGF stimulates this process7 and a neutralizing anti-VEGF antibody inhibits it.8 We have previously reported that VEGF expression is up-regulated in scraped corneas during the course of corneal neovascularization. Understanding the molecular mechanisms regulating this forced expression will help identify potential therapeutic candidates for the treatment or even prevention of corneal neovascularization.
Activins represent a distinct group of the transforming growth factor (TGF)-β superfamily, that comprise one α and three β chains (βA, βB, and βC).9,10 The bioactive molecule activin A consists of two monomeric βA chains linked by disulfide bonds.10 The biological effects of activins are mediated via signaling through two families (type I and II) of transmembranous serine-threonine kinase receptors.11–15 After ligand binding, a heterodimeric complex is formed by a type I and a type II receptor that initiates phosphorylation of the type I receptor and activation of downstream signaling cascades involving the Sma and Mad (mothers against decapentaplegic)-related protein (Smad).16
One of the most important functions of activin A is the regulation of cell differentiation. Activin A controls several aspects of hematopoiesis17,18,19 and regulates cell differentiation in the ovary, placenta, prostate, and testis.10,20 During embryogenesis, it is instrumental for axis development and organogenesis in a variety of species.21 In adults, activins function as hormone-like feedback regulators in the reproductive system.22 Furthermore, the presence of activin has been related to wound healing. In the eye, members of the activin family have been discovered in retinal pigment epithelium.23 Activin A and its receptors were recently described in preretinal membranes from eyes with ischemic and nonischemic vitreoretinal proliferative diseases, a result that agrees with a role of activin A in neovascularization.24
We have recently found that VEGF and activin A expression is co-ordinated in fibrovascular membranes from patients with age-related macular degeneration (manuscript submitted). Because we have also shown that genes encoding for the βA chain as well as several activin receptors are transcribed in the cornea,25 we investigated the role of activin A in corneal angiogenesis and the regulation of VEGF expression.
Materials and Methods
Cell Culture
Human corneal epithelial cells (passage 2; Cascade Biologics, Portland, OR) were maintained in tissue culture media according to the manufacturer’s instructions. Cells were plated into six-well plastic dishes and used for experiments when they reached 80% confluence. Fresh serum-free media were placed on the cells 12 hours before experiments. The p38 kinase inhibitor SB 202190 or the MAPK inhibitor PD 098059 (Calbiochem, La Jolla, CA) or vehicle (dimethyl sulfoxide), were added to the cells at a concentration of 50 μmol/L, followed 1 hour later by activin A (100 nmol/L) for an additional 12 hours. Each experimental condition was prepared in triplicate, and the experiments were performed at least three times with reproducible results. Representative experiments are shown in the figures.
Animals
C57 BL/6 mice, weighting 20 to 25 g were purchased from Jackson Laboratories (Bar Harbor, ME). All animal experiments followed the guidelines of the Association for Research in Vision and Ophthalmology and were approved by the Animal Care and Use Committee of Cologne, Germany. All surgical procedures were performed under general anesthesia [xylazine hydrochloride (5 mg/kg) and ketamine hydrochloride (35 mg/kg) i.m.]. To monitor systemic side effects of the treatment, body weight and temperature were measured on every observation day. Animals were kept in groups of 10 and fed regular lab chow and water ad libitum. A 12-hour day and night cycle was maintained.
Induction of Corneal Neovascularization
Under intramuscular general anesthesia with xylazine (10 mg/kg; Bayer, Leverkusen, Germany) and ketamine hydrochloride (150 mg/kg; Phoenix, MO) and additional topical application of lidocaine (Alcon, CA), inflammatory neovascularization was induced by application of 2 μl of 0.15 mmol/L NaOH to the central cornea of each mouse. The mice were randomly divided into three groups that received treatment with vehicle, systemic treatment with a neutralizing polyclonal antibody against activin A, an isotype-matched control antibody, or with recombinant activin A (R&D Systems, Minneapolis, MN). Each group consisted of 10 animals unless otherwise specified (20 corneas per group in total). The corneal epithelium was subsequently scraped off with a blunt von Graefe’s knife. The limbal areas were gently massaged over 360° for 3 minutes. To prevent infection, eyes were treated with antibiotic ointment (neomycin sulfate, 3.5 I.E./mg; bacitracin, 0.3 I.E./mg; and polymyxine B sulfate, 7.5 I.E./mg, Polyspectran; Alcon, Germany) after surgery. Each set of experiments was repeated three times.
Visualization and Quantification of Corneal Neovascularization
For visualization of endothelial cells and pericytes, immunostaining for CD31 was performed. Corneas were carefully dissected and rinsed in phosphate-buffered saline (PBS). To allow penetration of the antibodies and flattening of the tissue, the corneal epithelium and endothelium were whipped off and four peripheral incisions were made. Corneas were then fixed in ice-cold acetone for 20 minutes and after subsequent washes in PBS transferred to the antibody solution and incubated overnight at 4°C. Phycoerythrin-coupled anti-mouse CD31 (BD Biosciences, Franklin Lakes, NJ) was used in a dilution of 1:500. After further washing on PBS, corneas were mounted with anti-fading agent and analyzed by fluorescence microscopy.
Quantification of Corneal Neovascularization
Images of the CD31-stained corneas were captured using a CD-330 charge-coupled device camera (Dage-MIT; Improvision, Inc., Heidelberg, Germany) attached to a Zeiss microscope (Zeiss, Oberkochem, Germany). The images were captured on an Apple G4 Computer (Apple, Cupertino, CA) and analyzed using Openlab software (Improvision Inc.). The images were resolved at 624 × 480 pixels and converted to tagged information file format (.tiff) files. The neovascularization was quantified by setting a threshold level of fluorescence, above which only vessels were captured. The entire mounted cornea was analyzed to minimize sampling bias. The total corneal surface was outlined using the innermost vessel of the limbal arcade as the border. The total vascularized area was then normalized to the total corneal area and the percentage of the cornea covered by vessels was calculated. All quantifications and calculations were performed in a masked manner.
Implantation of Osmotic Pumps
The delivery of the drug with osmotic pumps, instead of simple intraperitoneal injections, is necessary to achieve steady levels of each drug in the circulation, avoid peak levels caused by every day injections, and limit the chance of toxicities. One week after the scraping of their corneas, the mice were deeply anesthetized, and osmotic pumps (Alzet, Salt Lake City, UT) containing either the vehicle (PBS), or 20 μg of a neutralizing rat/human/mouse polyclonal antibody against activin A (R&D Systems, Minneapolis, MN), or 20 μg of isotype-matched control antibody (R&D Systems), or 250 μg of recombinant activin A (R&D Systems), each diluted in 200 μl of PBS, were inserted into the peritoneal space. In detail, the abdominal skin was shaved, scrubbed with betadine, and wiped with alcohol. A small incision of 15-mm in length was made within the midline, through the skin and muscles, to enter the peritoneal cavity. Then the pumps were inserted into the peritoneal space floating freely without attachment to a certain structure. Each pump was 1.3 cm long and 6 mm in diameter. The wound was closed with separate suturing of the muscle layer and the skin.
Enzyme-Linked Immunosorbent Assay for VEGF and Activin A
Mice were scraped as described above and treated with recombinant activin A, or the neutralizing antibody against activin A (seven animals in each group), or the isotype-matched control antibody, or vehicle (eight animals), and sacrificed on days 2, 4, or 8 after treatment. Corneas were dissected and placed in 60 μl of lysis buffer (20 mmol/L imidazole HCl, 10 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L EGTA, 1% Triton, 10 mmol/L NaF, 1 mmol/L Na molybdate, 1 mmol/L EDTA, pH 6.8) supplemented with a protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN) followed by mechanical homogenization. The lysate was cleared of debris by centrifugation at 14,000 rpm for 15 minutes (4°C), and the supernatant was collected. Total protein was determined using a commercial assay (bicinchoninic acid kit; Bio-Rad, Hercules, CA). VEGF and activin A levels were determined using enzyme-linked immunosorbent assay according to the manufacturer’s instructions (R&D Systems) and normalized to total protein.
Analysis of p38 MAPK Kinase Activity
P38 MAP kinase activity was analyzed in whole retinal tissue using a commercially available enzyme-linked immunosorbent assay based method (Assay Designs, Inc.). Briefly, retinal tissue was homogenized in lysis buffer containing 20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerolphosphate, 1 mmol/L Na3O4, 1 μg/ml leupeptin, 1 mmol/L phenylmethyl sulfonyl fluoride. The lysates were cleared by centrifugation and protein was quantified with the bicinchoninic acid assay (Biorad). Corneal lysates, or recombinant phopsho-p38 MAPK standards (provided by the manufacturer), were subsequently incubated with a monoclonal antibody against the phosphorylated (activated) form of p38 MAPK (Assay Designs), immobilized on a microtiter 96-well plate for 1 hour at room temperature on a plate shaker at 500 rpm. After washes with a Tris-buffered saline-based solution (provided by the manufacturer), the plate was incubated with a rabbit polyclonal antibody against phopsho-p38 for 1 hour at room temperature on a plate shaker at 500 rpm. This antibody binds to the phopsho-p38 bound on the plate. After the incubation, the excess antibody was removed with repetitive washes with the Tris-buffered saline-based solution and the plate was incubated with a donkey anti-rabbit IgG conjugated with horseradish peroxidase that binds to the polyclonal phospho-p38 antibody. After a short incubation for 1 hour at room temperature as above, and subsequent washes, the peroxidase reaction was developed and measured at 450 nmol/L with a reference wavelength at 570 nmol/L. The measured optical density is directly proportional to the concentration of phospho-p38 in either the standards or the samples. A standard curve was plotted for the standards and the concentration of phopsho-p38 of each of the samples was determined by interpolation.
Analysis of p42/44 MAPK Kinase Activity
P42/44 MAP kinase activity was analyzed in whole retinal tissue using a commercially available enzyme-linked immunosorbent assay based method (Assay Designs, Inc.). Briefly, retinal tissue was homogenized in lysis buffer containing 20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerolphosphate, 1 mmol/L Na3O4, 1 μg/ml leupeptin, 1 mmol/L phenylmethyl sulfonyl fluoride. The lysates were cleared by centrifugation and protein was quantified with the bicinchoninic acid assay (Biorad). Corneal lysates, or recombinant phopsho-p42/44 MAPK standards (provided by the manufacturer), were subsequently incubated with a monoclonal antibody against the phosphorylated (activated) form of p42/44 MAPK (Assay Designs), immobilized on a microtiter 96-well plate, for 1 hour at room temperature on a plate shaker at 500 rpm. After washes with a Tris-buffered saline-based solution (provided by the manufacturer), the plate was incubated with a rabbit polyclonal antibody against phopsho-p42/44 for 1 hour at room temperature on a plate shaker at 500 rpm. This antibody binds to the phopsho-p42/44 bound on the plate. After the incubation, the excess antibody was removed with repetitive washes with the Tris-buffered saline-based solution and the plate was incubated with a goat anti-rabbit IgG conjugated with horseradish peroxidase that binds to the polyclonal phospho-p42/44 antibody. After a short incubation for 1 hour at room temperature as above, and subsequent washes, the peroxidase reaction was developed and measured at 450 nmol/L with a reference wavelength at 570 nmol/L. The measured optical density is directly proportional to the concentration of phospho-p42/44 in either the standards or the samples. A standard curve was plotted for the standards and the concentration of phopsho-p42/44 of each of the samples was determined by interpolation.
Statistical Analysis
To analyze the differences between treated and control eyes, as well as within the treatment groups, an unpaired t-test with two-tailed P value or analysis of variance (for multiple comparisons) was used. Results are presented as mean ± SD.
Results
Recombinant Activin A Increases VEGF Expression on Corneal Epithelial Cells via p38 and MAPK in Vitro
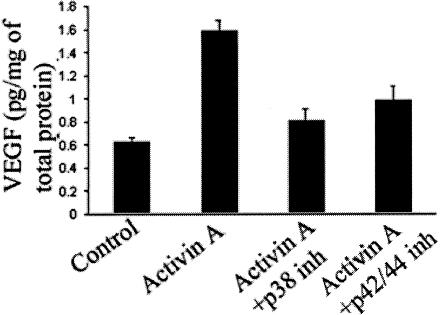
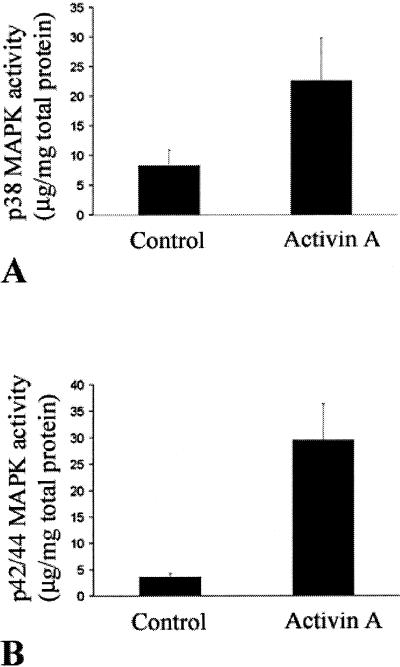
We have previously shown that activin A expression correlates with VEGF expression in fibrovascular epiretinal membranes from patients with age-related macular degeneration (AM Jaussen and V Poulaki, submitted). To investigate the role of activin A in the regulation of VEGF expression, we used corneal epithelial cells in vitro. Treatment of corneal epithelial cells with activin A up-regulated VEGF levels (1.58 ± 0.099 versus 0.62 ± 0.046 pg/mg of total protein in vehicle-treated cells, P < 0.001; Figure 1). As we have previously shown, mitogen-associated kinases such as p38 and p42/44MAPK can regulate VEGF expression.26 Therefore, we investigated the role of the above kinases in the activin-induced VEGF up-regulation. P38 and p42/44 MAPK inhibition reduced activin-induced VEGF up-regulation in corneal epithelial cells (0.803 ± 0.104 and 0.97 ± 0.12 pg/mg of total protein, respectively, versus 0.158 ± 0.099 pg/mg of total protein for cells treated with activin alone, P < 0.001; Figure 1). Activin A stimulated both p38 (22.5 ± 7.2 in activin A treated versus 8.26 ± 2.65 in vehicle-treated cells) and p42/44 activity (29.5 ± 6.89 in activin A treated versus 3.58 ± 0.68 in control-treated cells, P < 0.001 in both cases; Figure 2).
Figure 1.
Activin A up-regulates VEGF expression in corneal epithelial cells in vitro. Cells were treated overnight with recombinant activin A or the inhibitors as described in the Materials and Methods section and VEGF protein levels were measured in their supernatant. Bars represent the VEGF protein levels released in the supernatant after the various treatments (mean ± SD). Treatment with activin A, but not with the vehicle (control) up-regulated VEGF protein levels, whereas inhibition of p42/44 MAPK or p38 MAPK suppressed activin A-induced VEGF up-regulation.
Figure 2.
Activin A up-regulates both p38 and p42/44 MAPK activity in corneal epithelial cells. Cells were treated with activin A and p38 (A) and p42/44 (B) MAPK activity was measured as described in the Materials and Methods section. Bars represent the 450-nm reading that corresponds to the p38 or p42/44 MAPK activity (mean ± SD). Treatment with activin A up-regulated both p38 and p42/44 MAPK activity.
Levels of Activin A Increase During the Course of Corneal Neovascularization and the Increase Parallels that of VEGF
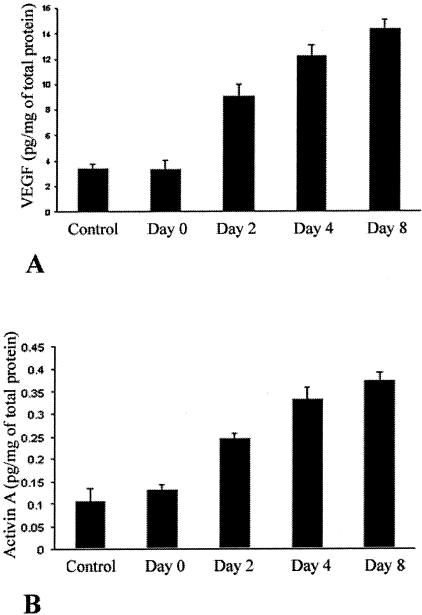
We have previously shown that VEGF levels increase during the course of neovascularization in the cornea scrape model. Because recombinant activin A increases production of VEGF in our in vitro model, we investigated whether systemic activin A levels increase during the course of neovascularization in vivo. In agreement with our previous findings, VEGF levels increased from 3.36 ± 0.74 pg/mg of total protein on day 0, to 9.05 ± 0.92 pg/mg of total protein on day 2 (P < 0.005), to 12.162 ± 0.93 pg/mg of total protein on day 4 (P < 0.005), to 14.34 ± 0.65 pg/mg of total protein on day 8 (P < 0.005) after the scraping (Figure 3A). Activin A levels paralleled the increases in VEGF levels from 0.13 ± 0.01 pg/mg of total protein on day 0, to 0.25 ± 0.01 μg/mg of total protein on day 2 (P < 0.001), to 0.33 ± 0.02 μg/mg of total protein on day 4 (P < 0.005), to 0.37 ± 0.01 μg/mg of total protein on day 8 (P < 0.001) (Figure 3B).
Figure 3.
Protein levels of activin A increase systemically during the course of corneal neovascularization in our murine model and correlate with the increases in the VEGF protein levels. Mice were treated as described in the Materials and Methods section, sacrificed at various days after the scraping, and the levels of activin A and VEGF were measured in corneal lysates. Bars represent the VEGF levels (A) and the activin A levels (B) of the corneal lysates at the indicated days after the corneal scraping (mean ± SD). As previously described, endogenous VEGF protein levels increase steadily after scraping (D0), on days 2 (D2), 4 (D4), and 8 (D8) (A). In parallel, endogenous levels of activin A increase also after the corneal scraping on days D2, D4, and D8, correlating with the increases in endogenous VEGF levels.
Activin A Modulates VEGF Levels in the Cornea Scrape Model
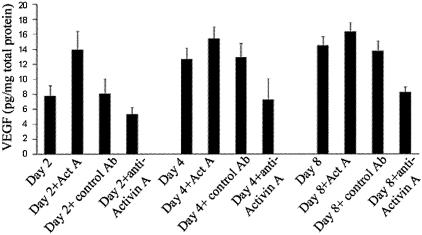
Administration of recombinant activin A increased VEGF levels (13.93 ± 2.46 versus 7.74 ± 1.39 pg/mg of total protein on day 2, P < 0.001, 15.36 ± 1.62 versus 12.67 ± 1.45 pg/mg of total protein on day 4, P < 0.05 and 16.31 ± 1.22 versus 14.44 ± 1.21 pg/mg of total protein on day 8, P < 0.02 in the animals treated with the recombinant activin A, versus the vehicle, respectively) in the cornea scrape model. Inhibition of endogenous activin A via the administration of a neutralizing antibody against activin A reduced VEGF levels (5.31 ± 0.92 versus 8.03 ± 1.99 pg/mg of total protein on day 2, P < 0.001; 7.2 ± 2.4 versus 12.87 ± 1.86 pg/mg of total protein, P < 0.05 on day 4; and 8.24 ± 0.67 versus 13.7 ± 1.37 pg/mg of total protein on day 8, P < 0.01) (Figure 4 in the animals treated with the neutralizing antibody against activin versus the isotype-matched control antibody, respectively).
Figure 4.
Activin A modulates VEGF expression in our murine model of corneal neovascularization. Mice were scraped as described in the Materials and Methods section and treated with either recombinant activin A, a neutralizing antibody against activin A, or the vehicle. The mice were sacrificed either on day 2 (D2), 4 (D4), or 8 (D8) after the scraping. The bars represent the VEGF levels in the corneal lysates (mean ± SD). Administration of recombinant activin A (ActA) increased even further the levels of VEGF, whereas neutralization of endogenous activin A with a neutralizing antibody (anti-ActA) reduced them. Administration of the isotype-matched control antibody did not have a significant effect on the amount of VEGF.
Activin A Modulates Corneal Neovascularization in the Cornea Scrape Model
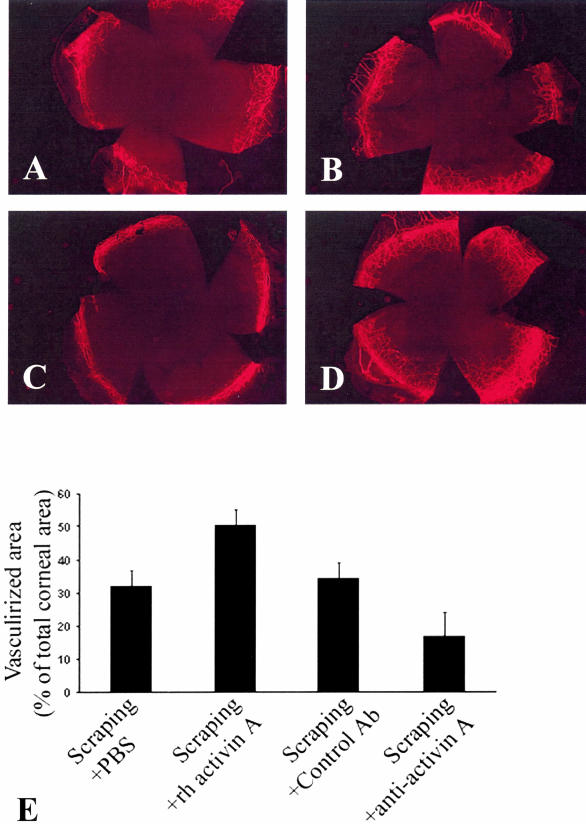
In agreement with our previous report,27 the percentage of vascularized corneal surface increased on day 12 after the scraping to 32.16 ± 6.79%, whereas unscraped corneas are not vascularized (therefore the percentage of vascularized corneal area is ∼0). Administration of recombinant activin A significantly enhanced neovascularization (increase to 50.22 ± 4.76%, versus 32.16 ± 6.79% in the vehicle-treated mice P < 0.0001) (Figure 5). Administration of a neutralizing antibody against activin A and not of an isotype-matched control antibody, suppressed the increase of neovascularization in the scrape murine model (increase by only 18.62 ± 9.47%, versus 35.5 ± 6.78% in the isotype-matched control antibody-treated animals; P < 0.000001) (Figure 5).
Figure 5.
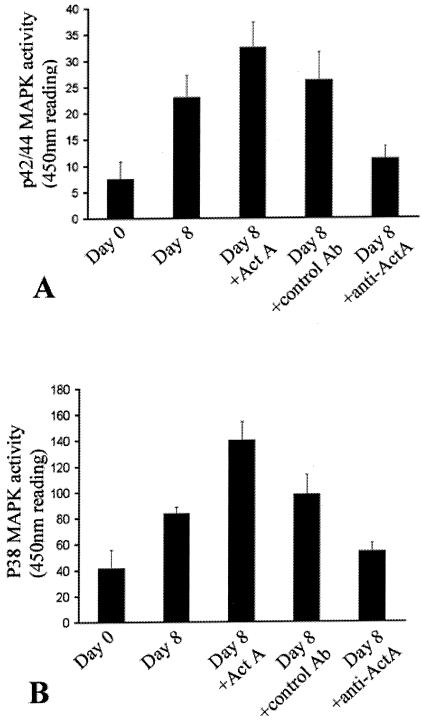
P42/44 (A) and p38 (B) kinase activity increases during the course of corneal neovascularization in our murine model. Animals were treated as described in the Materials and Methods section, sacrificed on day 0 (D0) or day 7 (D7) after the scraping, and the activities of p42/44 and p38 were measured in corneal lysates. Bars represent the 450-nm reading that corresponds to the p42/44 (A) or p38 (B) kinase activity, respectively (mean ± SD). P42/44 and p38 kinase activity increased during corneal neovascularization in the murine model paralleling the increases in both activin A and VEGF. Administration of recombinant activin A (ActA) increased even further the activity of p42/44 and p38 MAPK, whereas administration of a neutralizing antibody against activin A (AntiA) decreased the activation of both p42/44 and p38 MAPK. Administration of the isotype-matched control antibody did not have a significant effect on the amount of either p38 or p42/44 MAPK.
P42/44 and p38 MAPK Activity Increases During the Course of Corneal Neovascularization and Is Modulated by Activin A
We have previously demonstrated that p42/44 and p38 MAPK regulate VEGF expression during the course of diabetes.26 To investigate the role of the MAPK signaling pathway during corneal neovascularization and the effect of activin A on their enzymatic activity, we measured the activity of p38 and p42/44. P42/44 activity increased during neovascularization (22.96 ± 4.25 μg/mg of total protein on day 8 versus 7.53 ± 3.3 μg/mg of total protein on day 0, P < 0.005; Figure 5A), as did p38 MAPK activity (83.6 ± 13.86 μg/mg of total protein on day 8 versus 41.7 ± 4.87 on day 0, P < 0.005; Figure 5B).
Administration of recombinant activin A significantly increased both p42/44 MAPK activity (32.5 ± 4.8 μg/mg of total protein on day 7 in mice treated with recombinant activin A versus 22.96 ± 4.25 μg/mg of total protein, in the vehicle-treated mice, P < 0.001; Figure 5A) and p38 MAPK activity (140.2 ± 14.12 μg/mg of total protein on day 8 in mice treated with recombinant activin versus 83.6 ± 13.86 μg/mg of total protein in the vehicle-treated mice, P < 0.01; Figure 6B).
Figure 6.
Activin A regulates corneal neovascularization in the scrape murine model. A to D: Mice were sacrificed on day 12 (D12) after the scraping and corneal vasculature was labeled with FITC-labeled ConA as described in the Materials and Methods section. Representative microscopic images of scraped murine corneas are shown: A: Scraped and treated with vehicle; B: scraped and treated with the isotype-matched control antibody; C: scraped and treated with the neutralizing antibody against activin A; or D: scraped and treated with activin A. E: The vascularized corneal surface expressed as a percentage of the total corneal area was calculated as described in the Materials and Methods section for the various groups (mice scraped and received the vehicle, mice scraped and received activin A, the neutralizing antibody against activin A, or the isotype-matched control). Given that unscraped murine corneas are not vascularized (therefore, percentage of vascularized corneal area ∼0%), scraped murine corneas show increased vascularization and administration of recombinant activin A increases even further the amount of corneal neovascularization, whereas neutralization of endogenous activin A decreases the amount of corneal neovascularization.
Administration of activin A-neutralizing antibody and not the isotype-matched control antibody, reduced both p42/44 MAPK (11.22 ± 2.35 μg/mg of total protein on day 8 in mice treated with the neutralizing anti-activin A antibody versus 26.27 ± 5.38 in the isotype-matched control antibody-treated mice, P < 0.001, Figure 5A) and p38 MAPK activity (53.94 ± 6.43 μg/mg of total protein on day 8 in mice treated with the neutralizing anti-activin A antibody versus 98.06 ± 15.35 μg/mg of total protein isotype-matched control antibody-treated mice, P < 0.005; Figure 5B).
Discussion
Corneal neovascularization is a sight-threatening complication of severe insults to the cornea, such as chemical burns and corneal infections. It is characterized by an ingrowth of neovessels originating from the limbus and is often accompanied by an inflammatory response. VEGF is an important factor in this process because it stimulates corneal neovascularization7 and a neutralizing anti-VEGF antibody inhibits it.8 We have previously demonstrated that VEGF expression is up-regulated during corneal neovascularization.7,27 We now report that activin A up-regulates VEGF expression and activates MAPK family members p38 and p42/44 in vitro in corneal epithelial cells. Activin A expression parallels that of VEGF during the course of neovascularization and stimulates it through the MAPK signal transduction pathway. Inhibition of endogenous activin A down-regulates VEGF expression and neovascularization, whereas administration of recombinant activin A up-regulates p38 and p42/44 activity, VEGF expression, and neovascularization.
The activation of the kinases p38 and p42/44 during the course of neovascularization in our murine scrape model can be attributed to a variety of growth factors that are up-regulated during the course of inflammatory corneal angiogenesis, such as VEGF, tumor necrosis factor (TNF)-α, and basic fibroblast growth factor.28,29 We have previously shown that VEGF is up-regulated early in this model with a peak on day 2 after scraping.7 TNF-α is produced by various cells in the inflammatory site30 and induces angiogenic cytokines, including interleukin-8, VEGF, and basic fibroblast growth factor, which are involved in neovascularization.31 These cytokines have been shown to up-regulate p38 and MAPK activity in various settings32,33 and can contribute to the observed activation in our model. Interestingly, activation of the p38 and p42/44 MAPK is shown to up-regulate VEGF, TNF-α, and various proinflammatory cytokines,26 thus creating a positive autoregulatory loop for the sustained increase of these cytokines.
In our murine scrape model, activin A is up-regulated during the course of corneal neovascularization. In support, enhanced corneal expression of activin A is observed in various models of corneal wound healing both in vivo and in vitro.34,35 In the cornea, three cell types may produce activin A: resident cells, such as epithelial or stromal cells; infiltrating inflammatory cells; and endothelial cells of newly formed blood vessels. We have recently shown that mRNA for activin A and its receptors (ActR-IA, ActR-IB, and ActR-II) is transcribed in human corneal fibroblasts and regulates the expression of various markers for myofibroblastic differentiation.25 Our in vitro experiments suggest that murine corneal epithelial and endothelial cells are capable of producing activin A (data not shown). Alternatively, activated circulating monocytes and tissue macrophages, as well as bone marrow stromal cells are capable of synthesizing and releasing activin A in response to inflammatory stimuli.36,37 A variety of proinflammatory stimuli, including TNF-α, interleukin-1, or interleukin-6, up-regulate activin A production by endothelial cells, that may be an important source of activin A during systemic inflammation.36 These cytokines are operative in the murine scrape model and can contribute to the increased levels of activin A.
Our findings of increased VEGF expression in activin A-treated corneal epithelial cells in vitro, and reduced VEGF expression in scraped murine corneas on inhibition of activin A, support a direct stimulatory effect of activin A on VEGF expression, probably via a MAPK signaling pathway. The type I activin receptors phosphorylate Smad 2 and Smad3, the same signal transduction proteins involved in TGF-β signaling.38 The observation that both activins and inhibins as well as TGF-βs elicit a whole pleotropia of effects but signal through the same signal transduction proteins has been explained by both the existence of co-factors modulating Smads and interactions with other signal transduction pathways. In this respect, there is growing evidence for interaction of the Smad pathway with the p38 kinase pathways, a notion that is supported by the present study.38
Recently, activin A was demonstrated to activate p38 MAPK in T47D breast cancer cells,39 although, contrary to our model, inhibition of p44/42 did not inhibit the anti-proliferative effect of activin A. On the other hand, activin A modulates osteoclast differentiation through both p44/42 and p38 kinases via the Smad2 signaling pathway.40 Some of the downstream events of activin-induced MAPK signal transduction cascade have also been characterized, such as the activation of the transcription factor ATF-241 that creates a positive feedback loop by activating p38.39,42,43 A link between receptors and MEK kinases has been established with the discovery of the MAPK kinase kinase homolog TGF-β activated kinase 1 (TAK-1) that leads to activation of p38 by phosphorylating MEK enzymes.44,45 In TGF-β signaling, TAK-1 interacts with a binding protein (TAK-binding protein 1) and is activated by the hematopoietic progenitor kinase-1 that functions as MAPKKKK.45 Finally, inhibition of activin A binding to its receptor by the inhibitor SB431542 blocks activation of ERK and p38 MAPK in various models.46
Recent studies have suggested a crosstalk between tyrosine kinase receptors, such as basic fibroblast growth factor receptor, and the serine threonine kinases such as activin A receptors, an effect in which MAPK and Smad 2 activation plays a role at an upstream and downstream level, respectively.47,48 Basic fibroblast growth factor is known to be up-regulated in the murine scrape cornea model and can collaborate with activin A on the observed activation of p38 and MAPK and, through them, on VEGF up-regulation. Alternatively, activin A has been shown to stimulate prostaglandin E2 and thromboxane B2, as well as activate the nitric oxide pathway,49 which, as we have previously shown, is central in the adhesion of the leukocytes to the endothelium27,50 and the up-regulation of VEGF.
The role of activin A in the regulation of neovascularization is controversial. Many investigators argue in favor of an anti-angiogenic role for activin A, by demonstrating that it can inhibit endothelial cell proliferation51,52 in vitro and angiogenesis in vivo.53 In contrast, activin A and its receptors were detected in the vascular endothelial cells, fibroblast-like cells, and round-shaped macrophage-like cells in preretinal proliferative membranes and seem to be involved in the proliferative membrane formation in both ischemic and nonischemic vitreoretinal proliferative diseases.24 In addition, other TGF-β family members, such as growth and differentiation factor-5, have been shown to induce angiogenesis in the rabbit corneal pocket model.54
We found that inhibition of endogenous activin A decreases VEGF expression and neovascularization in the murine scrape model. This leads to the conclusion that activin A regulates neovascularization by increasing VEGF levels during inflammatory angiogenesis. The angiogenic role of activin A in our model correlates with its established proinflammatory role in several settings. Activin A has been implicated in several immune process.55 Activin A increases on lipopolysaccharide treatment, showing a biphasic response that correlates closely with the biphasic fever response, as well as TNF-α levels in the animals. (KL Jones, JN Brauman, DJ Phillips: personal communication). Activin A has also been implicated in the pathogenesis of localized inflammatory syndromes, specifically inflammatory bowel diseases, rheumatoid arthritis, and gout.7 Activin A was also detected in the mucosa and the submucosa of inflamed intestine, especially fibroblasts and inflammatory cells in ulcerative colitis and Crohn’s disease and its expression is up-regulated by interleukin-1.56 Activin A is also detected in the synovial fluid from patients with rheumatoid arthritis but not osteoarthritis, a noninflammatory degenerative condition.57 An intriguing aspect of activin’s role in inflammatory processes is the dichotomy between pro- and anti-inflammatory actions. This behavior is reminiscent of that of TGF-β, which although mainly anti-inflammatory, does exhibit some proinflammatory aspects.58 It has been suggested that the local tissue concentration of activin A may dictate whether it has a pro- or anti-inflammatory effect.47 This can explain the dichotomy between the pro- and anti-angiogenic role of activin A in different models.
In conclusion, we have established an angiogenic role for activin A in our model of inflammatory angiogenesis. We have found that activin A levels increase in our murine scrape model and up-regulate VEGF through p38 and p42/44 MAPK. Inhibition of endogenous activin A decreases VEGF expression and neovascularization. Agents that inhibit activin A may, therefore, become clinically useful for a variety of ocular diseases involving neovascularization, such as wound- and inflammation-related corneal angiogenesis with limbal insufficiency, which is still pharmacologically untreatable.
Footnotes
Address reprint requests to Antonia M. Joussen, Department of Vitreoretinal Surgery, Center of Ophthalmology, University of Cologne, 50931 Köln, Germany. E-mail: joussena@aol.com.
Supported by the Deutsche Forschungsgemeinschaft (grants DFG Jo-324/4-1 to A.M.J., V.P., and B.K., and DFG Jo-324 6-1 to A.M.J.), the Kämpgen Stiftung Köln (to A.M.J. and B.K.), and the Foundation Fighting Blindness (to V.P.).
References
- Danesi R, Agen C, Benelli U, Paolo AD, Nardini D, Bocci G, Basolo F, Campagni A, Tacca MD. Inhibition of experimental angiogenesis by the somatostatin analogue octreotide acetate (SMS 201-995). Clin Cancer Res. 1997;3:265–272. [Google Scholar]
- Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Kruse FE, Volcker HE, Kirchhof B. Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1999;237:920–927. doi: 10.1007/s004170050387. [DOI] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Moromizato Y, Stechschulte S, Miyamoto K, Murata T, Tsujikawa A, Joussen AM, Adamis AP. CD18 and ICAM-1-dependent corneal neovascularization and inflammation after limbal injury. Am J Pathol. 2000;157:1277–1281. doi: 10.1016/S0002-9440(10)64643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P. Chemical and biological characterization of the inhibin family of protein hormones. Recent Prog Horm Res. 1988;44:1–34. doi: 10.1016/b978-0-12-571144-9.50005-3. [DOI] [PubMed] [Google Scholar]
- Mather JP, Moore A, Li RH. Activins, inhibins, and follistatins: further thoughts on a growing family of regulators. Proc Soc Exp Biol Med. 1997;215:209–222. doi: 10.3181/00379727-215-44130. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Cheifetz S, Massague J. Novel activin receptors: distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992;68:97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- Massague J, Andres J, Attisano L, Cheifetz S, Lopez-Casillas F, Ohtsuki M, Wrana JL. TGF-beta receptors. Mol Reprod Dev. 1992;32:99–104. doi: 10.1002/mrd.1080320204. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Montalvo E, Massague J. Activation of signalling by the activin receptor complex. Mol Cell Biol. 1996;16:1066–1073. doi: 10.1128/mcb.16.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews LS, Vale WW. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991;65:973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin CH. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Massague J. SMADs: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- Eto Y, Tsuji T, Takezawa M, Takano S, Yokogawa Y, Shibai H. Purification and characterization of erythroid differentiation factor (EDF) isolated from human leukemia cell line THP-1. Biochem Biophys Res Commun. 1987;142:1095–1103. doi: 10.1016/0006-291x(87)91528-2. [DOI] [PubMed] [Google Scholar]
- Murata M, Onomichi K, Eto Y, Shibai H, Muramatsu M. Expression of erythroid differentiation factor (EDF) in Chinese hamster ovary cells. Biochem Biophys Res Commun. 1988;151:230–235. doi: 10.1016/0006-291x(88)90583-9. [DOI] [PubMed] [Google Scholar]
- Shiozaki M, Kosaka M, Eto Y. Activin A: a commitment factor in erythroid differentiation. Biochem Biophys Res Commun. 1998;242:631–635. doi: 10.1006/bbrc.1997.8020. [DOI] [PubMed] [Google Scholar]
- Mather JP, Woodruff TK, Krummen LA. Paracrine regulation of reproductive function by inhibin and activin. Proc Soc Exp Biol Med. 1992;201:1–15. doi: 10.3181/00379727-201-43473. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Harger P, Mitchell A, Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994;371:487–492. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D, Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986;321:776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Harrison CE, Lui GM, Roberts WL, Goldsmith PC, Mesiano S, Jaffe RB. Activin expression by cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:2924–2931. [PubMed] [Google Scholar]
- Yamamoto T, Takeuchi S, Suzuki K, Yamashita H. Expression and possible roles of activin A in proliferative vitreoretinal diseases. Jpn J Ophthalmol. 2000;44:221–226. doi: 10.1016/s0021-5155(99)00216-6. [DOI] [PubMed] [Google Scholar]
- You L, Kruse FE. Differential effect of activin A and BMP-7 on myofibroblast differentiation and the role of the Smad signaling pathway. Invest Ophthalmol Vis Sci. 2002;43:72–81. [PubMed] [Google Scholar]
- Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002;109:805–815. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Beecken WD, Moromizato Y, Schwartz A, Kirchhof B, Poulaki V. Inhibition of inflammatory corneal angiogenesis by TNP-470. Invest Ophthalmol Vis Sci. 2001;42:2510–2516. [PubMed] [Google Scholar]
- Saita N, Fujiwara N, Yano I, Soejima K, Kobayashi K. Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infect Immun. 2000;68:5991–5997. doi: 10.1128/iai.68.10.5991-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Bohlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci USA. 1987;84:5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricson BE, Benjamin WR, Vogel SN. Differential cytokine induction by doses of lipopolysaccharide and monophosphoryl lipid A that result in equivalent early endotoxin tolerance. Infect Immun. 1990;58:2429–2437. doi: 10.1128/iai.58.8.2429-2437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WL, Guo X, Chen QQ, Guo ZG. VEGF protects bovine aortic endothelial cells from TNF-alpha- and H(2)O(2)-induced apoptosis via co-modulatory effects on p38-and p42/p44-CCDPK signaling. Acta Pharmacol Sin. 2002;23:45–49. [PubMed] [Google Scholar]
- Donnahoo KK, Shames BD, Harken AH, Meldrum DR. The role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- Hubner G, Hu Q, Smola H, Werner S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev Biol. 1996;173:490–498. doi: 10.1006/dbio.1996.0042. [DOI] [PubMed] [Google Scholar]
- Seishima M, Nojiri M, Akiyama T, Noma A, Etoh Y, Kitajima Y. Expression of activin A in human keratinocytes at early stages of cultivation. FEBS Lett. 1996;398:120–124. doi: 10.1016/s0014-5793(96)01221-5. [DOI] [PubMed] [Google Scholar]
- Jones KL, Brauman JN, Groome NP, de Kretser DM, Phillips DJ. Activin A release into the circulation is an early event in systemic inflammation and precedes the release of follistatin. Endocrinology. 2000;141:1905–1908. doi: 10.1210/endo.141.5.7531. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, Jones KL, Scheerlinck JY, Hedger MP, de Kretser DM. Evidence for activin A and follistatin involvement in the systemic inflammatory response. Mol Cell Endocrinol. 2001;180:155–162. doi: 10.1016/s0303-7207(01)00516-0. [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Cocolakis E, Lemay S, Ali S, Lebrun JJ. The p38 MAPK pathway is required for cell growth inhibition of human breast cancer cells in response to activin. J Biol Chem. 2001;276:18430–18436. doi: 10.1074/jbc.M010768200. [DOI] [PubMed] [Google Scholar]
- Fuller K, Bayley KE, Chambers TJ. Activin A is an essential cofactor for osteoclast induction. Biochem Biophys Res Commun. 2000;268:2–7. doi: 10.1006/bbrc.2000.2075. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Tretter YP, Hertel M, Munz B, ten Bruggencate G, Werner S, Alzheimer C. Induction of activin A is essential for the neuroprotective action of basic fibroblast growth factor in vivo. Nat Med. 2000;6:812–815. doi: 10.1038/77548. [DOI] [PubMed] [Google Scholar]
- de Caestecker MP, Parks WT, Frank CJ, Castagnino P, Bottaro DP, Roberts AB, Lechleider RJ. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998;12:1587–1592. doi: 10.1101/gad.12.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusing RM, Barsig J. Induction of prostanoid, nitric oxide, and cytokine formation in rat bone marrow derived macrophages by activin A. Br J Pharmacol. 1999;127:919–926. doi: 10.1038/sj.bjp.0702626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy SA, Bicknell R. Inhibition of vascular endothelial cell growth by activin-A. J Biol Chem. 1993;268:23066–23071. [PubMed] [Google Scholar]
- Breit S, Ashman K, Wilting J, Rossler J, Hatzi E, Fotsis T, Schweigerer L. The N-myc oncogene in human neuroblastoma cells: down-regulation of an angiogenesis inhibitor identified as activin A. Cancer Res. 2000;60:4596–4601. [PubMed] [Google Scholar]
- Plendl J. Angiogenesis and vascular regression in the ovary. Anat Histol Embryol. 2000;29:257–266. doi: 10.1046/j.1439-0264.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Shimizu A, Kato M, Nishitoh H, Ichijo H, Hanyu A, Morita I, Kimura M, Makishima F, Miyazono K. Growth/differentiation factor-5 induces angiogenesis in vivo. Exp Cell Res. 1997;235:218–226. doi: 10.1006/excr.1997.3664. [DOI] [PubMed] [Google Scholar]
- Woodruff TK. Regulation of cellular and system function by activin. Biochem Pharmacol. 1998;55:953–963. doi: 10.1016/s0006-2952(97)00477-2. [DOI] [PubMed] [Google Scholar]
- Hubner G, Brauchle M, Gregor M, Werner S. Activin A: a novel player and inflammatory marker in inflammatory bowel disease? Lab Invest. 1997;77:311–318. [PubMed] [Google Scholar]
- Yu EW, Dolter KE, Shao LE, Yu J. Suppression of IL-6 biological activities by activin A and implications for inflammatory arthropathies. Clin Exp Immunol. 1998;112:126–132. doi: 10.1046/j.1365-2249.1998.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]