Abstract
Organ-specific injury after transplantation presents with a variety of clinical and pathological phenotypes, yet the factors influencing development of each outcome are poorly understood. Because primed T lymphocytes must re-encounter their antigen within the target organ to engage effector functions, we postulated that the cellular location of antigen within that organ could significantly impact the induced pathology. We challenged female Marilyn CD4 T-cell receptor transgenic mice, in which all T cells are specific for the male minor transplantation antigen, with male heart transplants expressing the relevant peptide: major histocompatibility complex on either graft parenchymal/vascular cells or alternatively, on graft-infiltrating mononuclear cells. The two different graft donors led to equivalent activation of recipient T cells as assessed by frequency, cell surface marker expression, cytokine production, and the ability to traffic to the graft. Nonetheless, if the target antigen was expressed on graft vascular and/or parenchymal cells, the outcome was acute graft destruction. In contrast, if the antigen was expressed only on graft-infiltrating mononuclear cells the same effector T-cell repertoire caused chronic rejection and vasculopathy. This unique result, that target antigen location can influence pathological outcome, has significant implications for understanding the pathogenesis of chronic allograft injury in humans.
Naïve T lymphocytes are activated in secondary lymphoid organs in response to their specific antigens when such antigens are expressed on professional antigen-presenting cells (APCs) and in the context of appropriate co-stimulatory signals.1–5 On full activation, the primed T cells develop the ability to circulate to peripheral tissues6–9 and are subject to chemoattractant signals that facilitate diapedesis across endothelial barriers and draw them into sites of inflammation.10–12 If the primed T lymphocytes then re-encounter their specific ligand in the periphery, this interaction rapidly triggers the T cells’ effector machinery, resulting in cytotoxicity, and the release of cytokines and chemokines.2,6,8,9 The end result is local inflammation, parenchymal cell destruction and, ultimately, tissue injury. For most viral infections the induced inflammation is local and short-lived, and results in destruction of the infected parenchymal cells followed by rapid resolution of the response and full healing of the target organ. Persistent viral infections can result in continued expression of foreign antigen in the target organ and prolonged inflammation that can lead to chronic injury.13–15
T-cell immunity directed at transplanted tissues can additionally persist for prolonged periods and the pattern of injury to a given graft can differ significantly from one instance to another. Some anti-graft immune responses result in severe, diffuse inflammation and acute organ loss16–20 whereas other anti-transplant immune responses culminate in subacute or chronic injury, manifested by intragraft fibrosis, transplant vasculopathy, and a slow deterioration of organ function.21–24
The features of a given immune-mediated disease process that determine the type of injury produced are only partially understood but may include the induced frequency of responding T cells,18 the individual effector functions used by the effector cells (including cytotoxic lymphocyte (CTL) activity and which cytokines are produced),25–32 the size of the target organ,18,33 and the amount of antigen expressed on the graft.18,33,34 Because activated T cells must re-encounter their specific ligand at the target site to engage effector functions, the cellular location of the target antigen in the end organ could theoretically influence the induced pattern of injury, although this issue has not been previously addressed.
After transplantation, high frequencies of recipient T cells are activated through both the direct pathway [donor major histocompatibility complex (MHC): peptide complexes on donor APCs] and through the indirect pathway (donor antigen processed and presented by recipient APCs).19,20,31,35–37 The complexity of such transplant-reactive immune repertoires has limited our ability to independently test whether antigen location in the target organ affects the downstream pathological consequences. In an effort to circumvent this limitation, we performed a series of experiments using a CD4 T cell receptor (TCR) transgenic mouse as transplant recipients such that we could control the specificity of the induced effector T cells. The results provide evidence that the pathological characteristics of the injured target organ can be specifically attributed to where the target antigen is expressed in the graft at the effector stage.
Materials and Methods
Animals
Male and female C57BL/6 (H-2b) and C3H (H-2k) mice, age 6 to 8 weeks, were purchased from The Jackson Laboratory (Bar Harbor, ME). Male and female C57BL/10NA;-(Tg)TCR Marilyn-(KO) Rag2 N11, N2 mice (H-2b, Marilyn), age 6 to 8 weeks, were obtained as a generous gift from Polly Matzinger (National Institutes of Health, Bethesda, MD) and Olivier Lantz (INSERM, Paris, France). All animals were maintained and bred in the pathogen-free animal facility at the Cleveland Clinic Foundation.
Flow Cytometry
Fluorescein isothiocyanate-conjugated anti-mouse CD44, CD62L, biotin-conjugated anti-mouse CD44, streptavidin-phycoerythrin, and streptavidin-PerCP were purchased from BD PharMingen (San Diego, CA). Spleen cells from the heart graft recipients or naïve mice were labeled and incubated on ice for 30 minutes with appropriate antibody followed by three washes in phosphate-buffered saline (PBS) 0.1% bovine serum albumin. When biotin-conjugated antibodies were used, cells were additionally incubated with streptavidin-phycoerythrin or streptavidin-PerCP followed by three more washes in PBS. The labeled cells were analyzed on a Becton-Dickinson FACScan using CellQuest software (Becton Dickinson, Mountain View, CA). Events (n = 10,000 to 100,000) were acquired and analyzed for each experiment.
Peptides
HYDbyp (NAGFNSNRANSSRSS) and chicken ovalbumin 323-339 (OVA323--339, KISQAVHAAHAEINEAG) were synthesized by Research Genetics, Huntsville, AL) at >90% purity.
Placement and Evaluation of Heart and Skin Grafts
Full thickness skin grafts were placed as performed by our laboratory,20,31,35 bandages were removed on day 7 and the grafts were inspected daily. Rejection was defined as >90% necrosis. Vascularized heterotopic cardiac allografts were placed in the abdomen as described20,38 and palpated daily for evidence of a heartbeat. Rejection was defined as a loss of palpable heartbeat. All grafts were harvested at the time of rejection or at predetermined time points after transplant. Graft survival was compared using Kaplan-Meier analysis.
Histology and Immunohistochemistry
Formalin-fixed paraffin sections of graft tissues were stained with hematoxylin and eosin (H&E) and for elastin as described.20,38 For single-color immunohistochemistry, frozen sections of cardiac tissue were acetone-fixed, hydrated in PBS for 10 minutes, blocked with the avidin-biotin blocking system (DAKO, Carpinteria, CA), washed with PBS three times, treated with 3% H2O2 (Sigma, St. Louis, MO) for 8 to 10 minutes, washed three times in PBS, and incubated for 60 minutes at room temperature with biotinylated anti-CD4 (1:50 dilution in PBS-1% bovine serum albumin; BD Pharmingen). After three additional PBS washes, the sections were incubated with peroxidase-conjugated streptavidin (stock concentration, DAKO) and developed using the diaminobenzidine substrate kit (Vector Laboratories, Burlingame, CA), counterstained, dehydrated with ethanol, and mounted.
For two-color immunohistochemistry, frozen sections of cardiac tissue were acetone-fixed, hydrated in PBS for 10 minutes, blocked with the biotin blocking system (DAKO), washed with PBS three times, and incubated for 60 minutes at room temperature with biotinylated anti-H-2/I-Ab (1:50 dilution in PBS-1% bovine serum albumin; BD Pharmingen). After three additional PBS washes, the sections were incubated with peroxidase-conjugated streptavidin (stock concentration, DAKO) and developed using the Novared substrate kit (Vector Laboratories). Sections were then washed three times in PBS, and incubated for 90 minutes at room temperature with fluorescein isothiocyanate-conjugated anti-CD4 antibody (BD Pharmingen). After three washes with PBS, slides were incubated with rabbit F(ab′) alkaline-phosphatase-conjugated anti-fluorescein isothiocyanate (DAKO). After three final washes in PBS the slides were developed using the Vector Blue alkaline-phosphatase substrate kit (Vector Laboratories). Sections were dehydrated with ethanol and mounted for analysis.
ELISPOT Assays
Assays were performed as outlined previously in detail.20,31,35,38 Briefly, MultiScreen ELISPOT plates (Millipore, Bedford, MA) were coated overnight with the capture antibodies (BD Pharmingen) in sterile PBS, blocked with sterile 1% bovine serum albumin in PBS, and washed three times with sterile PBS. Spleen cells (0.2 to 1 × 106 per well) were plated in HL-1 medium (BioWhittaker, Walkersville, MD) with or without mitomycin C-treated stimulator cells (400,000 per well) and/or soluble antigens (HY2pb and OVAp at 0.1 to 10 μmol/L) and then incubated at 37°C, 5% CO2 for 24 hours. After washing with PBS followed by PBS 0.025% Tween (PBST), detection antibodies (BD PharMingen) were added overnight. After washing with PBST, alkaline phosphatase-conjugated anti-biotin antibody (Vector Laboratories) diluted 1:1000 in PBST was added for 2 hours at room temperature. The plates were developed as previously described.20,31,35,38 The resulting spots were counted on an ImmunoSpot Series 1 Analyzer (Cellular Technology, Cleveland, OH).20,31,35,38
Results
Experimental Rationale
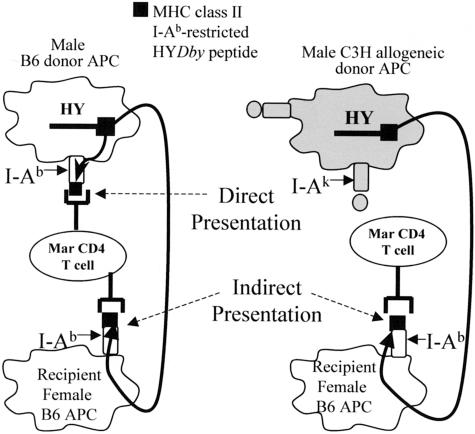
We sought to design a model system in which CD4+ T cells of defined specificity could be permitted to respond either directly to donor antigens expressed on donor graft parenchymal/vascular cells or alternatively, to donor antigens that were processed and presented only by recipient APCs infiltrating the donor organ. To this end, we studied the male H-Y transplant antigen using mouse models of skin and heart graft rejection. Female C57BL/6 (B6) mice reject syngeneic male skin through T-cell-dependent mechanisms and the peptide specificities of the responding CD4+ and CD8+ anti-H-Y T cells are known.34,39–41 The predominant MHC class II-binding male peptide is I-Ab-restricted, derives from the Dby gene, and is heretofore called HYDbyp.34,42 We used female Marilyn (Mar) TCR transgenic mice, which are on a B6 RAG−/− background and only contain CD4+ T cells that are specific for I-Ab + HYDbyp complexes,42 as recipients for our studies. Female Mar T cells do not cross-react with any antigen expressed on male or female allogeneic C3H (H-2k) tissues.42
Female Mar Mice Acutely Reject Syngeneic but Not Allogeneic Male Heart Grafts
In the first set of experiments we determined the functional capacity of the Mar T cells by challenging Mar females with cardiac allografts. We reasoned that transplantation of male B6 heart grafts would present donor male antigen on donor heart-derived male I-Ab+ APCs, permitting direct priming of recipient female Mar T cells (Figure 1). In this strain combination, the donor-derived male antigen could also theoretically be processed and presented by recipient APCs (I-Ab+ female APCs) so as to also activate recipient Mar T cells through the indirect pathway (Figure 1). In contrast, when female Mar mice are transplanted with male C3H heart grafts, the male antigen would be expressed on endogenous graft cells in the context of I-Ak or I-Ek and thus could not directly interact with Mar T cells. Nonetheless, the allogeneic C3H hearts should still prime the recipient T cells through the indirect pathway (Figure 1) because the donor male antigen can be processed and presented by recipient female I-Ab+ B6 APCs. Thus, by altering the donor strain, we can use this experimental design to permit T cells of single specificity to become activated through either the direct plus indirect pathways (male B6 grafts) or through the indirect pathway alone (male C3H grafts), and determine how the recognition pathway affects the subsequent induced effector T-cell frequency and function, and ultimately, graft outcome.
Figure 1.
Theoretical antigen presentation pathways for MHC class II-(I-Ab)-restricted HYDbyp after transplantation. Schematic representation of HYDbyp antigen presentation to Mar T cells after transplantation with B6 male (left) or C3H male (right) heart grafts.
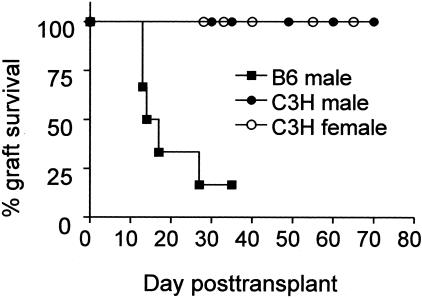
Female Mar mice acutely rejected male B6 heart grafts, as all grafts ceased beating by day 35 (Figure 2). In contrast, Mar females rejected neither male nor female allogeneic C3H heart grafts—graft heartbeats were palpable for >60 days.
Figure 2.
Mar females reject B6 male but not C3H male heart grafts. Survival of heart grafts transplanted in Mar female recipients is shown (n = 4 to 6 per group). There was a statistically significant difference in graft survival between the recipients of B6 male hearts and both of the other two groups. The one recipient of a B6 male heart graft that continued to beat was sacrificed on day 35. Histological examination revealed diffuse mononuclear cell infiltration consistent with acute rejection.
Syngeneic and Allogeneic Male Heart Grafts Equally Activate Recipient T Cells
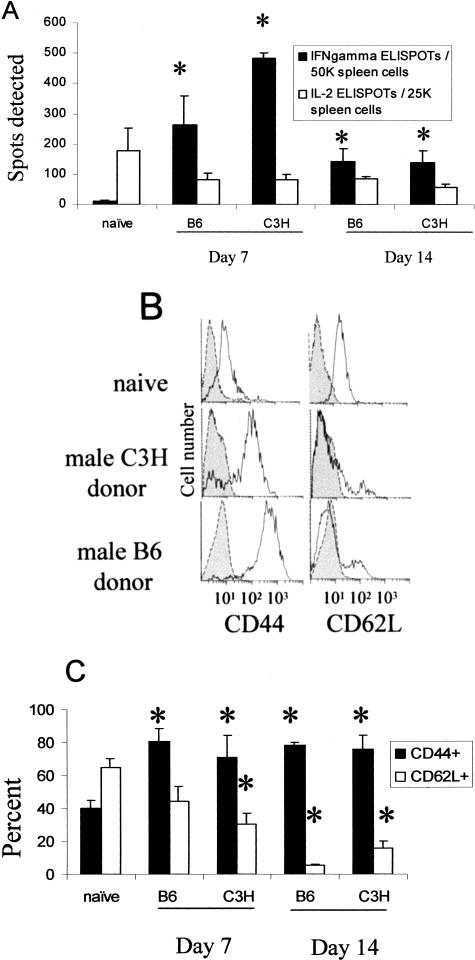
To evaluate Mar T-cell activation after transplant, we next performed anti-HYDbyp-specific cytokine ELISPOT assays using recipient spleen cells. We have previously shown that interferon (IFN)-γ production in this short-term ex vivo assay is one marker of an activated versus a naïve T cell.20,32,35,38 To our initial surprise, the frequency of primed, IFN-γ-producing HYDbyp-specific T cells was somewhat higher in recipients of male C3H (indirect pathway only) versus male B6 (direct plus indirect) heart grafts on day 7 after transplant. The frequencies of HYDbyp-specific IFN-γ-producers in both sets of recipients were significantly higher than in naïve controls (<10 of 50,000 spleen cells, Figure 3A). On day 14 after transplant the frequency of IFN-γ-producing HYDbyp-specific spleen cells remained significantly elevated over that found in naïve Mar females but did not differ between recipients of male C3H and B6 grafts. Specificity controls further revealed no response to control OVA peptide in the heart graft recipients (<10 of 50,000 spleen cells, not shown). Spleen cells isolated from recipients of female C3H hearts produced essentially no IFN-γ in response to male antigen (n = 3, <10 of 50,000 spleen cells, data not shown), similar to the response in naïve recipients, and confirming that the male antigen was required to be present in the graft to activate the recipient Mar T cells.
Figure 3.
Transplantation of B6 male and C3H male heart grafts equally activate recipient Mar T cells. A: Spleen cells from naïve mice or mice transplanted with heart grafts at the time points noted were tested in IFN-γ and interleukin-2 ELISPOT assays for reactivity to HYDbyp. Results represent mean plus standard errors for three to four animals per group. Responses to HYDbyp in recipients of female C3H hearts were similar to controls (<10 of 50,000 IFN-γ ELISPOTs, not shown). Reactivity to a control antigen OVA323-339 was <10 of 100,000 spleen cells, not shown. B: Representative flow cytometry plots for CD44 and CD62L expression gated on splenic CD4 T cells in naïve Mar females or in Mar females 7 days after transplantation with heart grafts. Shaded regions represent isotype controls. C: The percentage of CD44hi and CD62Lhi Mar T cells in each group is shown as an average of two to four animals per group. Cell surface expression on CD4 T cells from recipients of female C3H hearts was similar to that found in naïve mice (not shown). *, P < 0.05 versus naïve controls.
Flow cytometry experiments confirmed that Mar T cells in recipients of either male C3H hearts or male B6 hearts expressed an activated cell surface phenotype (CD44hi, CD62Llo; Figure 3, B and C). Splenic Mar T cells isolated from naïve mice (Figure 3B) and from recipients of female allografts (not shown) expressed a naïve cell surface phenotype (CD44lo, CD62Lhi). These results suggest that transplantation of B6 male and C3H male heart grafts led to equivalent activation of recipient Mar T cells. Nonetheless, female Mar mice rejected male B6 heart grafts but did not reject male C3H grafts (Figure 2).
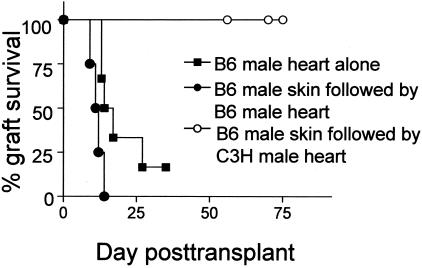
It remained possible that transplantation of a male B6 heart versus a male C3H heart preferentially induced additional/alternate T-cell effector functions that we did not measure through our cytokine ELISPOT and flow cytometry assays (eg, cytotoxicity). Such alternate effector functions could theoretically influence the recipient T cells’ ability to mediate organ destruction. To account for this possibility we first transplanted female Mar mice with male B6 skin grafts so as to activate recipient effector T cells capable of mediating tissue destruction. Consistent with published reports,42 the Mar females rejected B6 skin grafts by day 16 (n = 3) associated with induction of donor-reactive IFN-γ-producing effector T cells (>120 HYDbyp-specific IFN-γ-producing cells of 100,000 spleen cells versus <10 of 100,000 spleen cells in naïve mice, n = 3, data not shown). At the time of the male skin graft rejection (>90% necrosis, days 14 to 16 after transplant) the recipients were then challenged with either male B6 or male C3H heart allografts. As shown in Figure 4, female Mar mice primed with B6 male skin rejected B6 male heart grafts with accelerated kinetics compared to those mice that did not receive a previous skin graft (all grafts rejected by day 15). Rejection of male B6 skin was therefore effective at inducing effector T cells capable of mediating efficient cardiac allograft rejection. In contrast, although female Mar mice primed with B6 male skin contained effector T cells, these animals did not reject allogeneic male heart transplants—C3H male heartbeats were palpable for >60 days (Figure 4). These results demonstrate that the differential outcomes of male B6 versus male C3H heart grafts cannot be attributed to differences in T-cell specificity, frequency, or effector function of the T cells, nor to the priming pathway (direct versus indirect) per se.
Figure 4.
Mar females do not reject C3H male heart grafts even in the presence of effector T cells. Marilyn females were transplanted with B6 male skin grafts. At the time of rejection (days 14 to 16) these animals were transplanted with B6 male (•) or C3H male (○) heart grafts (n = 4 to 5 per group). Graft survival of B6 male hearts transplanted into naïve Mar females (▪, same data as shown in Figure 2) was added for comparison. B6 male heart grafts transplanted into recipients primed with B6 male skin rejected faster than the other two groups (P < 0.05, Kaplan-Meier analysis).
Transplant Vasculopathy Specifically Develops in Transplanted C3H Male Heart Grafts
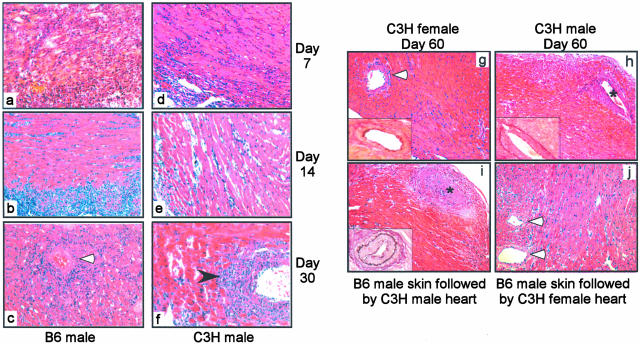
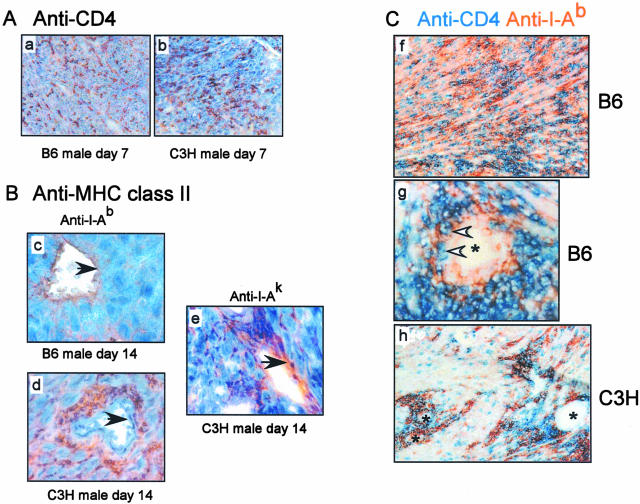
To provide further insight into the mechanisms resulting in the disparate outcome in these two groups of transplant recipients we next performed a kinetic analysis of graft histology (Figure 5). Male B6 heart grafts were rapidly infiltrated with large numbers of mononuclear cells that progressed throughout 30 to 35 days to diffuse and severe intraparenchymal infiltration. In contrast, the C3H male hearts exhibited mononuclear cell infiltration on days 7 to 14, but this infiltrate primarily resolved by days 21 to 30. Interestingly, the residual mononuclear cells were found in the perivascular regions of the C3H male hearts, particularly surrounding the larger arteries (Figure 5f). Immunohistochemical staining revealed that the infiltrates in both the donor C3H and B6 heart grafts contained CD4 T cells (Figure 6A, the recipients are Mar RAG−/− females, so all detected CD4 T cells are Mar T cells). Quantitative image analysis further showed that the amount of CD4 staining did not differ in the C3H versus the B6 male heart grafts on day 7 after transplantation. Thirty-six percent versus 39%, respectively, of the surface areas of the grafts stained positive for CD4 (mean of five high-power fields examined per animal, n = 3 per group, no statistical difference, data not shown). This latter finding suggests that the effector Mar T cells can initially traffic to both B6 male and C3H male grafts with equivalent efficiency.
Figure 5.
B6 male heart grafts and C3H male heart grafts transplanted in Mar females exhibit different pathological features. a to f: H&E-stained sections of cardiac allografts obtained 7 (a, d), 14 (b, e), and 30 (c, f) days after transplantation of B6 male (a–c) or C3H male (d–f) hearts. Note the diffuse mononuclear infiltrate in the B6 male hearts and the perivascular infiltrate particularly prominent in C3H male hearts on day 30 (black arrowhead). The photographs are fully representative of three to four animals evaluated per group. g to i: H&E-stained sections with insets showing elastin staining of C3H male or female heart grafts 60 to 70 days after transplantation in Mar females. C3H female hearts transplanted in Mar females (g) exhibited normal histology without evidence of vasculopathy. C3H male hearts transplanted into naïve Mar females (h) or into Mar females that previously rejected B6 male skin (i) developed classic transplant vasculopathy. j: H&E-stained female C3H heart graft in a Mar female harvested 40 days after rejection of a B6 male skin graft. Note the normal histological appearance and absence of infiltrates or vasculopathy. The photographs are fully representative of three to four animals evaluated per group. White arrowhead, blood vessels; *, blood vessels with vasculopathy. Original magnifications: ×40 (a–f and insets); ×20 (g–i).
Figure 6.
Antigen expression patterns differ in B6 male versus C3H male heart grafts transplanted into Mar female recipients. A: Recipient CD4 T cells traffic to both B6 male (a) and C3H male (b) heart grafts. Frozen graft sections were obtained on day 7 after transplant and stained with anti-CD4 mAb. The results are fully representative of three to four animals studied per group. Quantitative image analysis using ImagePro software revealed no difference in the amount of CD4 detected between the two groups (P < 0.05, data not shown). B: Single-color immunohistochemical staining for I-Ab (c and d) or I-Ak (e) in heart grafts obtained on day 14 after transplant. Note that I-Ab is expressed on the vascular endothelium (black arrowheads) of the B6 grafts, but in the perivascular area (and not on the endothelium) of the C3H grafts. A representative vessel from a C3H graft stained with anti-I-Ak is shown in e as a specificity control. Each photograph is fully representative of three individual animals studied per group. Similar expression patterns were detected at 3 weeks after transplant (not shown). C: Two-color immunohistochemistry for I-Ab (orange) and CD4 (blue) of a B6 male heart graft (f and g) and a C3H male heart graft (h) obtained on day 21 after transplant. Note that in the B6 heart grafts there is diffuse staining for both I-Ab and CD4 (f) and a close association between CD4 T cells and I-Ab-expressing endothelial cells of a blood vessel (white arrowheads, g). In contrast, C3H heart grafts show co-localization of CD4 and I-Ab only in the perivascular area (h). The findings are fully representative of three to four animals studied per group. *, Blood vessel. Original magnifications: ×20 (a–b, f, h); ×40 (c–e, g).
Histological examination of C3H male heart grafts on days 60 to 70 after transplant revealed transplant vasculopathy consistent with chronic immune-mediated injury (Figure 5h, Table 1). In contrast, the female C3H hearts examined on days 60 to 70 after transplant were essentially normal and were indistinguishable from syngeneic control grafts (Figure 5g, Table 1). Minimal epicardial inflammation and little evidence of vasculopathy were detected in the female hearts. Quantitative analysis of the transplant vasculopathy revealed a statistically significant difference in the extent of vascular injury between the male and the female heart grafts (Table 1). Both the number of involved vessels and the amount of lumenal narrowing per vessel (as assessed by the neointimal index23,43) were greater in the male compared to the female C3H cardiac allografts. C3H heart grafts placed into Mar female recipients that previously rejected B6 male skin also exhibited vasculopathy and fibrosis on day 60 (Figure 5i). The extent of vasculopathy did not differ from that found in C3H male grafts transplanted into naïve Mar females (not shown). Of note, the Mar recipients are bred onto a RAG−/− background precluding any possibility that the resultant vasculopathy could be attributed to donor-reactive antibodies.
Table 1.
Transplant Vasculopathy Develops in Male but Not Female C3H Hearts Transplanted into Female Marilyn Recipients
| Transplanted heart | Day harvested after transplant | Number of vessels with NI > 10% | Cumulative NI |
|---|---|---|---|
| C3H male | 59 | 8/10 | 46% |
| C3H male | 61 | 8/12 | 39% |
| C3H male | 69 | 14/21 | 28% |
| C3H male | 62 | 18/20 | 55% |
| C3H male | 61 | 5/16 | 12.5% |
| C3H female | 65 | 1/7 | 6% |
| C3H female | 76 | 1/8 | 6% |
| C3H female | 56 | 0/12 | < 5% |
Two to three elastin-stained sections of each heart graft were analyzed by computer-assisted image analysis for both the number of vessels with neointimal index (NI) of > 10% and the cumulative score for all vessels computed as outlined in Materials and Methods. Both the numbers of involved vessels and the overall NI were significantly greater in the male versus the female heart grafts (P < 0.05).
The Pattern of I-Ab Expression Differs in Male C3H Versus B6 Grafts and Correlates with the Differential Outcome
The striking difference in pathology between male B6 and male C3H hearts (Figure 2) despite activation of similar numbers of recipient antigen-specific T cells (Figure 3) is consistent with the notion that the location of the antigen in the target organ may contribute to determining the pathological features of injury, independent of T-cell frequency and initial priming pathway. To compare the patterns of potential antigen expression in the B6 versus C3H male grafts we next performed immunohistochemical staining for the relevant restriction element capable of cognate interactions with the Mar TCR, I-Ab (and for I-Ak as a control). B6 male heart grafts stained positive for I-Ab-expressing cells in the graft (Figure 6, B and C). In particular, the vasculature of the B6 male hearts stained positive for I-Ab by day 14 after transplant (Figure 6Bc) consistent with up-regulation of endothelial cell expression of endothelial MHC class II, and providing a potential target with which the circulating primed recipient Mar T cells could specifically interact. I-Ab-positive cells were also detected in C3H heart grafts infiltrating the graft parenchyma (Figure 6Bd) on day 14 after transplant consistent with graft-infiltrating recipient-derived monocytes/macrophages. Importantly however, the vasculature of the C3H grafts did not stain for recipient I-Ab. C3H graft endothelium did stain positive for donor MHC class II, I-Ak, acting as a positive control (Figure 6Be).
Two-color immunohistochemical staining (I-Ab in orange and CD4 in blue) performed on day 21 after transplant revealed that graft-infiltrating Mar T cells were predominantly detectable in the male C3H heart grafts in the perivascular regions and in close proximity to infiltrating I-Ab+ cells (Figure 6Ch). This pattern was distinctly different from that found in the B6 male heart grafts in which both the Mar T cells and the I-Ab-expressing cells were detected diffusely through the graft (Figure 6Cf). In addition, Mar cells were detected in close proximity to the I-Ab+ endothelium in the B6 male hearts (Figure 6Cg). In conjunction with our previous data these findings provide strong correlative evidence that different patterns of antigen encounter in the graft, at the effector stage, lead to different pathological outcomes.
Effector T Cells Do Not Induce Pathology in the Absence of Antigen in the Target Organ
As a final test of how antigen expression patterns contribute to outcome (and as an additional specificity control), we reasoned that fully activated effector T cells require their specific antigen to be expressed in the graft to mediate pathology. We therefore engrafted female Mar recipients with male B6 skin so as to prime recipient Mar T cells. After skin graft destruction, we transplanted the primed recipients with C3H female heart grafts. Despite the activation of Mar T cells and the rapid rejection of the donor B6 male skin (all rejected by day 14, n = 5), the subsequently placed female heart grafts were not rejected (MST >40 days, n = 5, not shown). Histology (Figure 5j) on day 40 after transplant showed no evidence of acute or chronic pathology and there were few infiltrating mononuclear cells.
Discussion
The factors that influence the clinical and pathological outcome of a transplanted organ include the frequencies and specificities of the responding T cells, the priming pathway through which these T cells are activated, and the induced effector functions of the responding T cells.18,25–31,33 In the present report we highlight another factor in which the activated T cells re-encounter their specific ligand in the target organ at the effector stage. Our data show that heart grafts from two different donor strains can equally activate recipient T cells of singular specificity at similar responder frequencies and yet the outcome of the transplanted organ differs vastly. In the case of B6 male heart transplants, diffuse mononuclear cell infiltration followed by graft destruction occurred within 3 to 4 weeks after transplant. In contrast, C3H male hearts were not acutely rejected but instead developed evidence of chronic injury as manifested by transplant vasculopathy. The difference in outcome can primarily be attributed to the pattern of antigen expression in the graft with which the effector T cell interacts at the effector stage.
Multiple laboratories have shown that in the context of a proinflammatory stimulus, cardiac myocytes, interstitial cardiac DCs, and cardiac vascular endothelial cells can up-regulate MHC class II expression on their surface to present antigen to activated CD4+ T cells.44–46 Thus, donor B6 hearts can theoretically express I-Ab plus male antigen on each of these cell types within the graft providing multiple targets with which to have cognate interactions with the TCR expressed on the graft-infiltrating, primed Mar T cells. If such interactions trigger T-cell effector functions, the result would be direct injury to the donor graft cells. In addition, recipient female monocytes/macrophages infiltrate the male B6 graft and can theoretically process and present donor antigen in the context of recipient MHC class II to the primed Mar T cells. This latter re-encounter event should not result in direct injury to the donor cells, but would result in local cytokine release that would amplify inflammation and could contribute to local graft injury.
In contrast, Mar T cells entering the C3H male grafts could only have cognate interactions via their TCRs, with graft-infiltrating recipient APCs expressing reprocessed male antigen. The donor C3H parenchymal cells only express the H-2k MHC restriction elements on their cell surface, precluding the Mar T cells (specific for I-Ab + HYDbyp) from recognizing any antigen on the donor graft cells at this effector stage. Our data suggest that this inability to recognize any donor cells at the effector stage accounts for the different pathology, and ultimately the difference in graft survival, between the B6 male and C3H male grafts after transplantation into Mar females. It is interesting to note that the mononuclear cell infiltrate preferentially accumulated around the C3H male donor vasculature throughout time (Figure 5) and that Mar T cells co-localized with infiltrating I-Ab+ APCs within that region (Figure 6). Resultant local cytokine production, in particular IFN-γ production, may then function to amplify the immune response and contribute to the vascular injury. IFN-γ can induce vasculopathy in other models,47 and thus this proinflammatory cytokine could contribute to the development of vasculopathy seen in the male C3H grafts in these studies.
This proposed difference in antigen expression pattern in the graft is consistent with our immunohistochemical staining results (Figure 6). The expression of a potential ligand on the donor graft endothelium in B6 male hearts may provide a relevant target for the primed T-cell immune response. The induced damage to the endothelium could precipitate thrombosis, vascular inflammation, and ultimately ischemia to the end organ, leading to graft loss. In addition, endothelial expression of antigen may be one important signal mediating arrest of primed T cells at the site of inflammation.48 In the case of the B6 male heart graft, this mechanism could facilitate diapedesis into the graft where the primed T cells would then interact with other cells expressing male antigen to induce graft injury. The extensive infiltrates in the B6 versus C3H grafts may therefore be partially mediated by endothelial cell initiated signals that preferentially draw the primed Mar T cells into the male B6 heart.
We fully understand that although we can assess expression of I-Ab on donor and recipient cells in the graft, we cannot actually determine how many I-Ab molecules are bound to HYDbyp—no reagents specific for I-Ab/HYDbyp complexes are available. Thus, it remains possible that differences in the quantity of I-Ab/HYDbyp complexes found in the graft as well as the specific location of these complexes, exist between the male C3H and male B6 heart, and that this feature could contribute to the final pathology. Whether or not the amount of antigen expressed in the two grafts differ, our overall conclusion remains unchanged—antigen expression patterns in the target organ at the effector stage can contribute to the pathology independent of priming pathway and T-cell frequency.
It has long been hypothesized that T cells responding through the indirect allorecognition pathway preferentially mediate chronic allograft injury,49,50 and several correlative studies in human recipients of renal, cardiac, and lung transplants with transplant vasculopathy support this contention.51–54 Nonetheless, a gap exists between our understanding of how T cells are activated through the indirect pathway and how such activated T cells predominantly induce chronic pathology. The results presented in this report provide a potential explanation to bridge this gap. Our findings suggest that the nature of the re-encounter event in the graft can be crucial to determining the subsequent pathological characteristics of the transplanted organ. Indirectly primed T cells can solely re-encounter self-APCs expressing cross-presented donor antigen in the graft, and cannot directly interact with donor graft cells, a scenario that we have now shown can preferentially lead to chronic (rather than acute) graft injury. Thus, the results of our studies in this highly controlled TCR transgenic mouse model have implications potentially relevant to the pathogenesis of chronic allograft injury in humans.
Acknowledgments
We thank Earla Biekert and Alla Gomer for their superb technical assistance.
Footnotes
Address reprint requests to Peter S. Heeger, M.D., Associate Staff and Co-Director of the Transplantation Research Program, Department of Immunology, The Cleveland Clinic Foundation, NB30, 9500 Euclid Ave., Cleveland, OH 44195. E-mail: heegerp@ccf.org.
Supported by the American Heart Association (scientist development grant to A.V.), the American Society of Transplantation (women’s and minority faculty grant to A.V.), and the National Institutes of Health (grant R01AI-43578-02 to P.S.H.).
References
- Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- Bluestone JA. Costimulation and its role in organ transplantation. Clin Transplant. 1996;10:104–109. [PubMed] [Google Scholar]
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- el-Sawy T, Fahmy NM, Fairchild RL. Chemokines: directing leukocyte infiltration into allografts. Curr Opin Immunol. 2002;14:562–568. doi: 10.1016/s0952-7915(02)00382-5. [DOI] [PubMed] [Google Scholar]
- Rehermann B. Interaction between the hepatitis C virus and the immune system. Semin Liver Dis. 2000;20:127–141. doi: 10.1055/s-2000-9946. [DOI] [PubMed] [Google Scholar]
- Thomsen AR, Nansen A, Andreasen SO, Wodarz D, Christensen JP. Host factors influencing viral persistence. Philos Trans R Soc Lond B Biol Sci. 2000;355:1031–1041. doi: 10.1098/rstb.2000.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk G. Mechanisms of chronic enteroviral persistence in tissue. Curr Opin Infect Dis. 2001;14:251–256. doi: 10.1097/00001432-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Bolton EM, Armstrong HE, Briggs JD, Bradley JA. Cellular requirements for first-set renal allograft rejection. Transplant Proc. 1987;19:321–323. [PubMed] [Google Scholar]
- Bolton EM, Gracie JA, Briggs JD, Kampinga J, Bradley JA. Cellular requirements for renal allograft rejection in the athymic nude rat. J Exp Med. 1989;169:1931–1946. doi: 10.1084/jem.169.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ND, Turvey SE, Van Maurik A, Hara M, Kingsley CI, Smith CH, Mellor AL, Morris PJ, Wood KJ. Differential susceptibility of heart, skin, and islet allografts to T cell-mediated rejection. J Immunol. 2001;166:2824–2830. doi: 10.4049/jimmunol.166.4.2824. [DOI] [PubMed] [Google Scholar]
- Vella JP, Magee C, Vos L, Womer K, Rennke H, Carpenter CB, Hancock W, Sayegh MH. Cellular and humoral mechanisms of vascularized allograft rejection induced by indirect recognition of donor MHC allopeptides. Transplantation. 1999;67:1523–1532. doi: 10.1097/00007890-199906270-00005. [DOI] [PubMed] [Google Scholar]
- Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- Valujskikh A, Fedoseyeva E, Benichou G, Heeger PS. Development of autoimmunity after skin graft rejection via an indirect alloresponse. Transplantation. 2002;73:1130–1137. doi: 10.1097/00007890-200204150-00021. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Orosz CG, Strauch AR. Vascular smooth muscle alpha-actin expression as an indicator of parenchymal cell reprogramming in cardiac allografts. Transplantation. 1998;65:1652–1656. doi: 10.1097/00007890-199806270-00020. [DOI] [PubMed] [Google Scholar]
- Armstrong AT, Strauch AR, Starling RC, Sedmak DD, Orosz CG. Morphometric analysis of neointimal formation in murine cardiac grafts: III. Dissociation of interstitial fibrosis from neointimal formation. Transplantation. 1997;64:1198–1202. doi: 10.1097/00007890-199710270-00020. [DOI] [PubMed] [Google Scholar]
- Ardehali A, Fischbein MP, Yun J, Irie Y, Fishbein MC, Laks H. Indirect alloreactivity and chronic rejection. Transplantation. 2002;73:1805–1807. doi: 10.1097/00007890-200206150-00018. [DOI] [PubMed] [Google Scholar]
- Kist-van Holthe JE, Gasser M, Womer K, Najafian N, Dong V, Samsonov DV, Geehan CS, Chandraker A, Sayegh MH, Waaga AM. Regulatory functions of alloreactive Th2 clones in human renal transplant recipients. Kidney Int. 2002;62:627–631. doi: 10.1046/j.1523-1755.2002.00469.x. [DOI] [PubMed] [Google Scholar]
- Hancock WW, Sayegh MH, Carpenter CB. In vivo evidence for the Th1/Th2 paradigm: oral alloantigen-induced modulation of accelerated allograft rejection is associated with dense intragraft IL-4. Transplant Proc. 1992;24:2313–2314. [PubMed] [Google Scholar]
- Onodera K, Hancock WW, Graser E, Lehmann M, Sayegh MH, Strom TB, Volk HD, Kupiec-Weglinski JW. Type 2 helper T cell-type cytokines and the development of “infectious” tolerance in rat cardiac allograft recipients. J Immunol. 1997;158:1572–1581. [PubMed] [Google Scholar]
- VanBuskirk AM, Wakely ME, Orosz CG. Transfusion of polarized TH2-like cell populations into SCID mouse cardiac allograft recipients results in acute allograft rejection. Transplantation. 1996;62:229–238. doi: 10.1097/00007890-199607270-00014. [DOI] [PubMed] [Google Scholar]
- VanBuskirk AM, Wakely ME, Orosz CG. Acute rejection of cardiac allografts by noncytolytic CD4(+) T cell populations. Transplantation. 1996;62:300–302. doi: 10.1097/00007890-199607270-00026. [DOI] [PubMed] [Google Scholar]
- VanBuskirk AM, Wakely ME, Sirak JH, Orosz CG. Patterns of allosensitization in allograft recipients: long-term cardiac allograft acceptance is associated with active alloantibody production in conjunction with active inhibition of alloreactive delayed-type hypersensitivity. Transplantation. 1998;65:1115–1123. doi: 10.1097/00007890-199804270-00017. [DOI] [PubMed] [Google Scholar]
- Matesic D, Lehmann PV, Heeger PS. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation. 1998;65:906–914. doi: 10.1097/00007890-199804150-00008. [DOI] [PubMed] [Google Scholar]
- Matesic D, Valujskikh A, Pearlman E, Higgins AW, Gilliam AC, Heeger PS. Type 2 immune deviation has differential effects on alloreactive CD4+ and CD8+ T cells. J Immunol. 1998;161:5236–5244. [PubMed] [Google Scholar]
- Lappe MA, Graff RG, Snell GD. The importance of target size in the destruction of skin grafts with non-H-2 incompatibility. Transplantation. 1969;7:372–377. doi: 10.1097/00007890-196905000-00006. [DOI] [PubMed] [Google Scholar]
- Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- Lee RS, Yamada K, Houser SL, Womer KL, Maloney ME, Rose HS, Sayegh MH, Madsen JC. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci USA. 2001;98:3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Laufer TM, Gerth AJ, Chase CM, Colvin RB, Russell PS, Sayegh MH, Auchincloss H., Jr Further analysis of the T-cell subsets and pathways of murine cardiac allograft rejection. Am J Transplant. 2003;3:23–27. doi: 10.1034/j.1600-6143.2003.30105.x. [DOI] [PubMed] [Google Scholar]
- Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3:844–851. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- Silvers WK, Billingham RE, Sanford BH. The H-Y transplantation antigen: a Y-linked or sex-influenced factor? Nature. 1968;220:401–403. doi: 10.1038/220401a0. [DOI] [PubMed] [Google Scholar]
- Silvers WK, Collins NH. The behavior of H-Y-incompatible neonatal skin grafts in rats. Transplantation. 1979;28:57–59. doi: 10.1097/00007890-197907000-00013. [DOI] [PubMed] [Google Scholar]
- Wang W, Meadows LR, den Haan JM, Sherman NE, Chen Y, Blokland E, Shabanowitz J, Agulnik AI, Hendrickson RC, Bishop CE, Hunt DF, Goulmy E, Engelhard VH. Human H-Y: a male-specific histocompatibility antigen derived from the SMCY protein. Science. 1995;269:1588–1590. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- Braun MY, Grandjean I, Feunou P, Duban L, Kiss R, Goldman M, Lantz O. Acute rejection in the absence of cognate recognition of allograft by T cells. J Immunol. 2001;166:4879–4883. doi: 10.4049/jimmunol.166.8.4879. [DOI] [PubMed] [Google Scholar]
- Armstrong A, Strauch A, Starling R, Sedmak D, Orosz C. Morphometric analysis of neointimal proliferation in murine cardiac allografts. Transplantation. 1997;63:941–947. doi: 10.1097/00007890-199704150-00006. [DOI] [PubMed] [Google Scholar]
- Herskowitz A, Tamura F, Ueda K, Neumann DA, Slepian M, Rose NR, Beschorner WE, Baumgartner WA, Reitz BR, Sell KW, Ahmed-Ansari A. Induction of donor major histocompatibility complex antigens in coronary arterial vessels: mechanism of arterial vasculitis in rat allografts treated with cyclosporine. J Heart Transplant. 1989;8:11–19. [PubMed] [Google Scholar]
- Isobe M, Narula J, Southern JF, Strauss HW, Khaw BA, Haber E. Imaging the rejecting heart. In vivo detection of major histocompatibility complex class II antigen induction. Circulation. 1992;85:738–746. doi: 10.1161/01.cir.85.2.738. [DOI] [PubMed] [Google Scholar]
- Wang YC, Herskowitz A, Gu LB, Kanter K, Lattouf O, Sell KW, Ahmed-Ansari A. Influence of cytokines and immunosuppressive drugs on major histocompatibility complex class I/II expression by human cardiac myocytes in vitro. Hum Immunol. 1991;31:123–133. doi: 10.1016/0198-8859(91)90015-2. [DOI] [PubMed] [Google Scholar]
- Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med. 2003;197:643–656. doi: 10.1084/jem.20021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992;175:305–308. doi: 10.1084/jem.175.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella JP, Spadafora-Ferreira M, Murphy B, Alexander SI, Harmon W, Carpenter CB, Sayegh MH. Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation. 1997;64:795–800. doi: 10.1097/00007890-199709270-00001. [DOI] [PubMed] [Google Scholar]
- Suciu-Foca N, Ciubotariu R, Itescu S, Rose EA, Cortesini R. Indirect allorecognition of donor HLA-DR peptides in chronic rejection of heart allografts. Transplant Proc. 1998;30:3999–4000. doi: 10.1016/s0041-1345(98)01318-9. [DOI] [PubMed] [Google Scholar]
- Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001;167:7199–7206. doi: 10.4049/jimmunol.167.12.7199. [DOI] [PubMed] [Google Scholar]
- Reznik SI, Jaramillo A, SivaSai KS, Womer KL, Sayegh MH, Trulock EP, Patterson GA, Mohanakumar T. Indirect allorecognition of mismatched donor HLA class II peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Am J Transplant. 2001;1:228–235. doi: 10.1034/j.1600-6143.2001.001003228.x. [DOI] [PubMed] [Google Scholar]