Abstract
Fluid shear stress greatly influences the biology of vascular endothelial cells and the pathogenesis of atherosclerosis. Endothelial cells undergo profound shape change and reorientation in response to physiological levels of fluid shear stress. These morphological changes influence cell function; however, the processes that produce them are poorly understood. We have examined how actin assembly is related to shear-induced endothelial cell shape change. To do so, we imposed physiological levels of shear stress on cultured endothelium for up to 96 hours and then permeabilized the cells and exposed them briefly to fluorescently labeled monomeric actin at various time points to assess actin assembly. Alternatively, monomeric actin was microinjected into cells to allow continuous monitoring of actin distribution. Actin assembly occurred primarily at the ends of stress fibers, which simultaneously reoriented to the shear axis, frequently fused with neighboring stress fibers, and ultimately drove the poles of the cells in the upstream and/or downstream directions. Actin polymerization occurred where stress fibers inserted into focal adhesion complexes, but usually only at one end of the stress fiber. Neither the upstream nor downstream focal adhesion complex was preferred. Changes in actin organization were accompanied by translocation and remodeling of cell-substrate adhesion complexes and transient formation of punctate cell-cell adherens junctions. These findings indicate that stress fiber assembly and realignment provide a novel mode by which cell morphology is altered by mechanical signals.
There is compelling evidence that early atherosclerotic lesions most often originate near arterial bends and branch sites, where complex blood flow patterns occur. This finding, together with observations that experimental perturbations in flow can initiate or redistribute lesions,1,2 strongly implicates local hemodynamics in atherogenesis. More specifically, exhaustive study of selected arterial sites, eg, the carotid bifurcation, has provided evidence that low and/or fluctuating shear stresses are proatherogenic.3
Shear stresses are very small forces that typically cause bulk deformations (strains) of artery walls that are much less than 1%;4 however, the endothelium of arteries is directly exposed to flowing blood and these cells have proven to be exquisitely sensitive to shear. This sensitivity may impact on atherogenesis through both modulation of classic functions of endothelium, ie, its role as a nonthrombogenic transport barrier that regulates inflammatory responses, and through regulation of growth and contractile functions of smooth muscle.5,6 Consequently, much effort has been expended to explore endothelial sensitivity to shear stress. Examination of candidate genes has revealed that many potentially atherosclerosis-related gene products display shear-sensitive expression by endothelium, including VCAM-1, MCP-1, ICAM-1, eNOS, platelet-derived growth factors, transforming growth factor-β1, and endothelin.7–11 More recently, microarray-based studies have demonstrated that expression of hundreds of genes display sensitivity to both the duration and nature (eg, laminar versus turbulent) of shear imposed on endothelial cells.12
The sensitivity of endothelium to shear forces is most obviously manifest through changes in cell morphology: the cells elongate, the cell outline and its cytoskeleton becomes highly oriented in the direction of shear stress, and the profile of the cells becomes substantially more flattened when they are exposed to shear stress.13 Morphological responses also include partial disassembly and then reassembly of adherens junctions, the primary sites of mechanical coupling between endothelial cells.13 Consequently, long-term alterations in secondary flow patterns near branch sites, eg, because of circadian fluctuations in tissue perfusion, may contribute to the high endothelial permeability and low-density lipoprotein uptake at atherosclerosis-prone regions near arterial bends and branch sites14 and thereby contribute to lesion formation. Finally, shear-stressed endothelial cells provide one of the few cases in which all cells in a monolayer undergo a common morphological response to a synchronized stimulus. Sheared endothelial cells therefore provide a unique model system with which to examine control of cell motility and morphology.
Given the importance of shear-induced morphological changes, it is surprising that little is known concerning how reorganization of cellular structures drives these changes. The actin cytoskeleton initiates most changes in cell morphology. Contractile function of actin-myosin complexes is responsible in some cases;15 however, actin polymerization is most commonly exploited.16 Usually, cell shape change follows from nucleation and assembly of thin parallel bundles of actin microfilaments at the cell edge that produce filopodia, ie, finger-like projections of the plasma membrane, or from assembly of dendritic arrays of actin that protrude foot-like processes referred to as lamellipodia. Coordinate remodeling of cell-cell and cell-substrate adhesion complexes then allows cell shape change to proceed. Actin also assembles into basal stress fibers but these are generally associated with adhesion to, or reorganization of, extracellular matrix.17
We have investigated the role of actin assembly, and associated changes in cell adhesion complexes, in endothelial morphological responses to shear stress. The onset and rate of morphological changes to the cells depended greatly on the state and duration of confluence of the cells before initiation of shear stress: much slower morphological responses were observed in cells that were long confluent. As cells changed shape, formation of filopodia or lamellipodia did not occur; instead, a novel mode of cell shape change was driven by coincident extension and reorientation of stress fibers at the basal aspect of the cell, fusion of adjacent stress fibers, and translocation of the focal adhesion complexes into which these stress fibers insert. Ultimately, stress fiber assembly drove protrusion of the cell membrane in the direction of the shear axis to elongate the cells. This process was independent of the zyxin/vasodilator-stimulated phosphoprotein (VASP) complex, which associates with focal adhesions and can assemble G-actin into microfilament bundles, because introduction of peptide sequences that disrupted this complex do not suppress shear-induced actin assembly. Focal adhesion complexes that were associated with stress fibers also translocated with stress fibers and elongated. Simultaneously, cell-cell adherens junctions transiently assumed a punctate pattern and then re-established a linear distribution, in association with sporadic junctional actin assembly.
Materials and Methods
In Vitro Shear Stress
Porcine aortic endothelial cells, at passages four to six, were maintained at 37°C in medium 199 plus 5% fetal bovine serum (Gibco, Invitrogen, Barlington, Canada), 1% amphotericin B (Fungizone), and 2% penicillin/streptomycin, and equilibrated with humidified 95% air and 5% CO2. At 2 days after confluence, cells were subjected to laminar shear stress of 15 dynes/cm2 in a parallel plate flow chamber.13,18 In some cases, subconfluent or just confluent cultures were exposed to shear stress to assess the effects of monolayer stabilization on responses to shear. Cells maintained in static culture served as controls (n ≥ 3 for each experiment).
Immunostaining
Cells were fixed with 3% paraformaldehyde as described,13 permeabilized with 0.2% Triton X-100 and washed 3 times for 5 minutes with phosphate-buffered saline (PBS). Cells were then incubated with primary antibody for 1 hour, and then washed with PBS followed by incubation with secondary antibody. Primary antibodies included mouse monoclonal antibodies to paxillin (1:200; Transduction Laboratories, Lexington, KY), vinculin (1:50; Sigma BioSciences, Oakville, Canada), tensin or VASP (1:50; Transduction Laboratories). Primary antibodies were detected using Alexa Fluor 488 goat anti-mouse antibody (1:50; Molecular Probes, Eugene, OR). Staining frequently was done in the presence of rhodamine phalloidin (1:20; Molecular Probes), which labels F-actin.
In Situ Staining of Adherens Junctions
Animal experiments were conducted in accord with the guidelines of the Canadian Council of Animal Care and were approved by the Animal Care Committee of the Toronto General Hospital. Rabbits were killed by intravenous infusion of 1.0 ml of euthanasia solution (T-61; Hoechst, Montreal, Canada).9 The carotid arteries were fixed and immunostained9 using a goat polyclonal antibody to β-catenin (1:800; Jackson ImmunoResearch Laboratories, West Grove, PA) and a fluorescein isothiocyanate-conjugated donkey anti-goat secondary antibody (1:50, Jackson ImmunoResearch Laboratories). The arteries were viewed en face by confocal microscopy.9
F-Actin Polymerization
The protocol for visualizing actin assembly was based on studies by Symons and Mitchison,19 with modifications.20 Cultures were gently washed with rinsing buffer (20 mmol/L Hepes, pH 7.5, 138 mmol/L KCl; 4 mmol/L MgCl2, 3 mmol/L EGTA) and then incubated for up to 10 minutes with fluorescently labeled G-actin in permeabilization buffer [20 mmol/L Hepes, pH 7.5, 138 mmol/L KCl, 4 mmol/L MgCl2, 3 mmol/L EGTA, 0.01% saponin, 0.5 μmol/L Alexa 488-conjugated G-actin from rabbit muscle (Molecular Probes, Eugene, OR)]. After washing with buffer, cells were fixed for 20 minutes with 3% paraformaldehyde followed by a 3 × 5-minute wash with PBS. To detect endogenous F-actin, cells were incubated with rhodamine-labeled phalloidin for 30 minutes.
In separate experiments, endothelial cells were triple stained for newly polymerized F-actin, endogenous F-actin, and with monoclonal anti-vinculin antibody (1:50; Sigma BioSciences) and a CY5-conjugated donkey anti-mouse secondary antibody, in the presence of rhodamine-labeled phalloidin.
Microinjection of Fluorescently Labeled G-Actin
To visualize the cellular actin pool in live cells, subconfluent cells grown on coverslips were microinjected with Alexa Fluor 488-conjugated actin (6 mg/ml, Molecular Probes) using an Eppendorf transjector 5246. Cells were exposed to shear stress 24 hours after injection. Time-lapse images were collected using a fluorescence microscope (TE300; Nikon, Melville, NY) equipped with a cooled charge-coupled device camera (ORCA ER; Hamamatsu).
Cell Membrane Labeling
To label cell membrane, cells were incubated for 10 minutes with 20 mmol/L DiI (1.1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Molecular Probes). Cells were fixed as described above.
Fluorescence Microscopy
Fixed endothelial cells were examined using a Bio-Rad MRC 1024 laser scanning confocal microscope (Nikon ×60 oil-immersion objective; NA = 1.4). The peak excitation/emission wavelengths for the fluorescent probes, and the detection filters used were: fluorescein isothiocyanate = 492/520 nm and detected at 522 nm; CY5 = 650/680 nm and detected at 680 nm; rhodamine = 554/573 nm and detected at 605 nm. For each experiment, at least10 fields per slide were collected.
Peptide Inhibitor of Zyxin-VASP Interaction
Peptide sequences comprising a domain of zyxin that mediates binding to VASP (PPEDFPLPPPPLAGD),21 or a scrambled version of this peptide (control, PPEDAPLPPPPLAGD), in tandem with a Trojan peptide sequence (a string of nine arginines)22 to facilitate cell entry, were synthesized by the Biotechnology Service Centre (Hospital for Sick Children, Toronto, Canada). The peptide (300 μmol/L) was introduced into the flow loop at 15.5 hours of shear stress and then incorporation of fluorescently labeled monomeric actin into microfilaments was assessed 30 minutes later, as described above. Four replicate experiments were performed.
Statistics
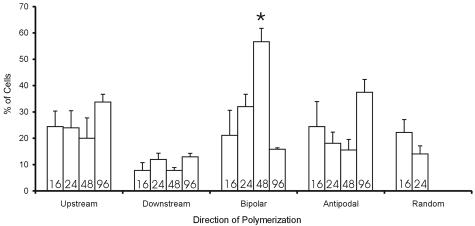
Actin most often assembled at one end of stress fibers. To assess bias in the direction of actin assembly, cells were classified according to whether actin assembly was predominantly in the upstream, downstream, or both ends of stress fibers (bipolar), or directed away from the nucleus (anti-podal). At least 30 cells per time point were scored by an unbiased (blinded) observer and the cells to be counted were defined from a predefined grid using the Vernier scales on the stage drives, so that cell selection was unbiased. Statistical analysis was analysis of variance followed by a Tukey’s post hoc test.
Results
Shear-Induced Changes to Endothelial Cell Shape and the Endothelial Cell-Cell Interface
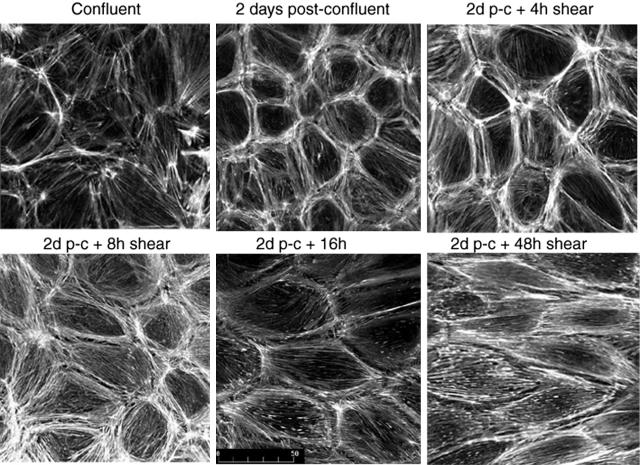
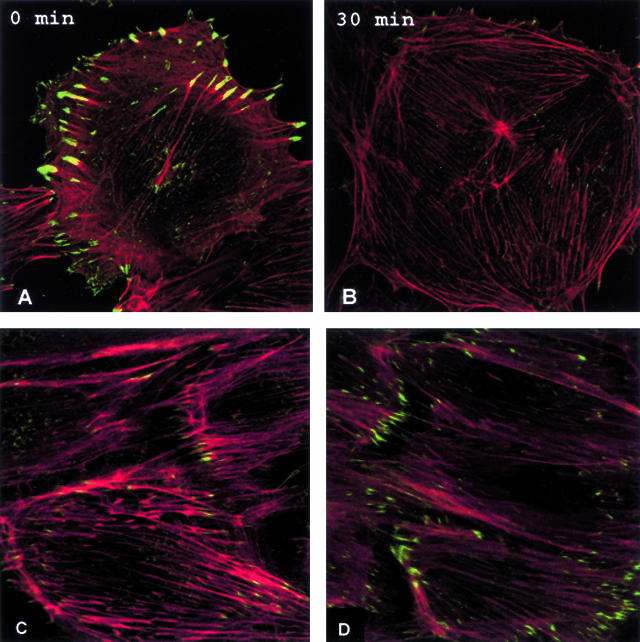
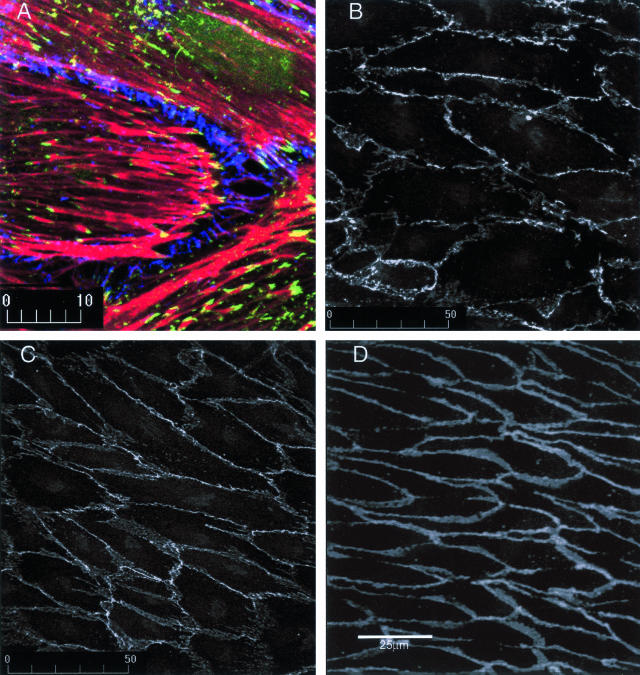
Endothelial cells in healthy arteries are permanently confluent; therefore, cells generally were not exposed to shear until 2 days after confluence was achieved. Throughout these 2 days, stress fibers became less prominent and a junctional dense peripheral band of F-actin was established (Figure 1), then no further changes in actin distribution were detectable. This stabilization proved important because subconfluent or just confluent cells initiate reorganization of actin very quickly after shear stress is imposed (not shown), whereas only very modest shear-related F-actin reorganization occurred in cells that were 2 days postconfluent until 8 hours of shear (Figure 1).13 Partial loss of the dense peripheral band of actin was then followed by elongation of cells and alignment of actin microfilaments with shear throughout 8 to 24 hours. No further changes in cell shape or actin distribution were observed.
Figure 1.
Postconfluency affects actin organization in endothelial cell responses to shear stress. Endothelial cells that have just achieved confluence (top left) display randomly oriented microfilament bundles and actin staining fails to delineate individual cells. At 2 days after confluence, a dense peripheral band of actin assembles at the cell-cell junctions and only sparse central stress fibers are seen. When shear is initiated at 2 days after confluence, no changes in cell shape or actin distribution are detectable at 4 hours and only modest and sporadic loss of the dense peripheral band of actin is detected at 8 hours. Cell elongation and actin alignment with shear stress becomes apparent during the next 8 hours and cells complete shape change after 24 hours of shear stress.
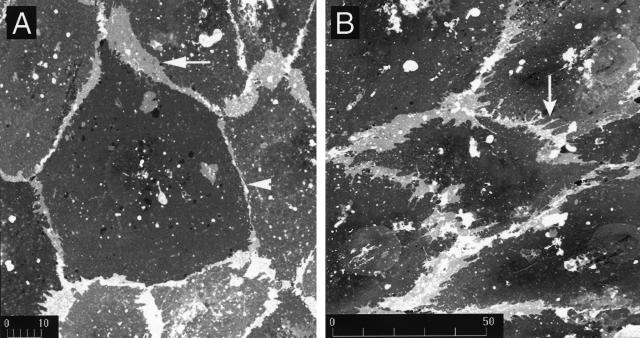
To define shear-induced changes to cell shape and cell junctions, we labeled membrane phospholipid with DiI.13 Under static conditions, the cells displayed a typical cobblestone morphology with multiple regions of membrane overlap that were manifest as areas of enhanced DiI fluorescence, because of fluorescence from multiple layers of membrane. These regions were separated by linear cell-cell junctions (Figure 2a). While elongating, cell margins did not display classical lamellipodia; instead, pointed projections were commonplace (Figure 2b). These structures that appeared to initiate shape change were often quite broadly based, but could not be definitely differentiated from filopodia, finger-like cellular projections that result from actin assembly at the cell edge.20
Figure 2.
Shear-induced changes to endothelium. Cell membranes of endothelial cells in static cultures (left) or after 16 hours of shear stress (right) were labeled with the DiI. Areas of cell-cell overlap are brighter because multiple layers of plasma membrane are detected. a: In static cultures, linear cell-cell junctions were interrupted by pad-like regions of cell overlap (arrow) that were separated by linear cell-cell junctions (arrowhead). b: Shear induced elongation of endothelial cells in the direction of shear vector (right to left) that occurred between 8 hours and 24 hours. During elongation, regions of cell-cell overlap that are neither filopodia-like or lamellipodia-like were observed; instead, triangle-shaped projections of cell membranes became oriented in the upstream and downstream directions (arrow). Each finding is representative of at least three replicate experiments.
Actin Assembly into Stress Fibers Coincides with Shear Stress-Induced Elongation and Alignment of Endothelial Cells
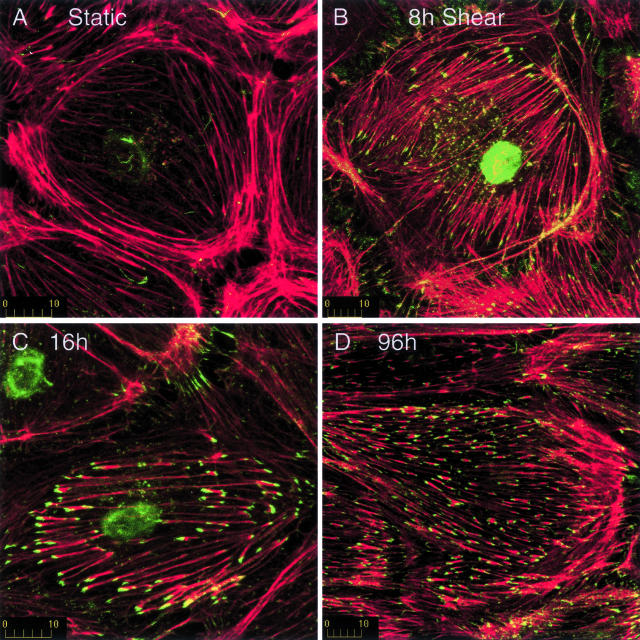
To assess actin assembly during changes in cell shape, we permeabilized endothelial cell membranes after varying periods of shear stress and then incubated the cells with Alexa 488-labeled monomeric actin (G-actin) briefly (up to 10 minutes) to define sites of actin assembly, then cells were fixed and stained for total F-actin. When it occurred, actin incorporation into microfilaments was detectable at 3 minutes of incubation, but optimal labeling was seen with 8 to 10 minutes of incubation. Uptake of G-actin into microfilaments was readily detected in subconfluent endothelial cells (not shown), which are highly motile, but little or no incorporation of labeled G-actin was detected in cultures 2 days after confluence that were not exposed to shear stress (Figure 3A). Approximately 50% of cells displayed incorporation of exogenous G-actin at ends of randomly oriented stress fibers after 8 hours of shear (Figure 3B, green). Most frequently, actin assembly occurred at only one end of the stress fibers (see below). Less prominent, punctate staining of exogenous actin was also observed along the lengths of microfilaments.
Figure 3.
Shear stress induces assembly of actin at the ends of stress fibers. Endothelial cells were incubated briefly with fluorescently labeled G-actin (green) after exposure to shear stress for 0 hours (A), 8 hours (B), 16 hours (C), or 96 hours (D). Cells were then fixed and filamentous actin was stained with rhodamine phalloidin (red). Little or no actin assembly occurred in postconfluent cells not exposed to shear stress (A). In contrast, exposure to shear stress induced actin assembly at the ends of stress fibers (B–D). Initially, actin assembly was randomly oriented, but assembly became aligned with shear stress by 16 to 24 hours. Shear stress is directed from right to left. Each finding is representative of at least three replicate experiments.
Most cells displayed actin assembly at the ends of stress fibers by 16 hours, when many cells were elongate and aligned with shear (Figure 3C). In shear-aligned cells, basal stress fibers and actin assembly were parallel to shear. Many other cells were elongated but not yet parallel to shear, in which case basal stress fibers tended to align with the long axis of the cell (not shown). Cell elongation and alignment with shear stress was completed at 24 hours; nonetheless, actin assembly continued for as long as we examined the cells (up to 96 hours, Figure 3D).
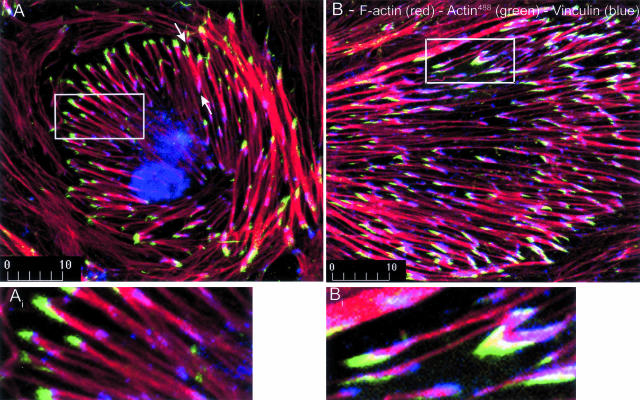
Optical sectioning with confocal microscopy and double staining for actin assembly and the focal adhesion proteins, vinculin and paxillin, demonstrated that actin assembly occurred at the basal aspect of the cells, where microfilament bundles insert into focal adhesion complexes (Figure 4). Notably, both ends of stress fibers terminated in adhesion complexes, but actin assembly most often occurred at only one of these sites (Figure 4, arrows). Importantly, actin assembly was constrained to subdomains of focal adhesion complexes, as indicated by only partial overlap of G-actin fluorescence with immunostaining for vinculin (Figure 4, insets). This finding probably reflects remodeling and translocation of the focal adhesion/stress fiber complex during adaptation to shear stress, as described below.
Figure 4.
Actin assembly occurs at focal adhesion complexes at one end of stress fibers and localizes to subdomains of these complexes. Endothelial cells were exposed to fluorescently labeled G-actin (green) after exposure to shear stress for 8 hours (A) or 16 hours (B), then stained for filamentous actin with rhodamine phalloidin (red) and immunostained for vinculin (blue). Actin assembly usually occurred at only one end of stress fibers (arrows in A). At these sites, newly assembled actin was excluded from subdomains of the focal adhesion complex, as indicated by overlap of vinculin and F-actin only (fuscia, insets). Shear stress is directed from right to left. Findings are representative of three replicate experiments.
Within individual cells, actin polymerized predominantly in the upstream direction, predominantly in the downstream direction, or predominantly directed away from the nucleus (anti-podal) with approximately equal frequencies (Figure 5). Assembly at both ends of the stress fiber (bipolar assembly) was also observed occasionally, but random distribution of actin polymerization to one or the other end of stress fibers within individual cells was seen in only 10 to 20% of cells and never at times later than 24 hours. The only other statistically significant time dependence in direction of actin assembly was the elevation in bipolar assembly at 48 hours. These findings indicate that shear stress determined the orientation but not direction of actin polymerization. Subtle intracellular or cell-cell signaling may provide cues that define the latter.
Figure 5.
Shear stress determines orientation but not direction of actin assembly. Graph displays percentage of cells in which actin assembly was predominantly at the upstream, downstream, or both ends (bipolar) of stress fibers, or when assembly was directed away from the nucleus at both ends of the cells (anti-podal). Numbers at the base of each bar indicate hours of shear stress. Also shown is the percentage of cells in which assembly was randomly distributed between the two ends of the stress fibers, which never occurred after 24 hours. The only other statistically significant temporal change was an increase in bipolar assembly at 48 hours.
Extension of Stress Fibers after Imposition of Shear Stress Is Independent of the Zyxin/VASP Complex
The scaffolding protein, zyxin, can link VASP to the actin-bundling protein, α-actinin, which concentrates at focal adhesions.23 VASP interaction with cytoplasmic G-actin-profilin complexes can then incorporate actin monomer into microfilaments. VASP localized to focal adhesions in endothelium (Figure 6, A and B) but it did not mediate shear-induced actin assembly at these sites. Accordingly, a peptide sequence that was designed to interfere with zyxin-VASP interaction successfully dislodged VASP from focal adhesions (Figure 6, A and B) but had no impact on shear-induced actin assembly (Figure 6, C and D). VASP was not dislodged from focal adhesions by control peptide (not shown).
Figure 6.
VASP does not mediate shear-induced actin assembly. Shear-induced stress fiber assembly was not VASP (green immunostaining) localized to focal adhesion complexes in endothelium (A) but was dislodged from these sites by a zyxin peptide sequence designed to interfere with zyxin-VASP interaction (B). VASP association with ends of stress fibers remained suppressed at 1 hour (not shown). Incorporation of Alexa 488-labeled monomeric actin (green) into the end of stress fibers (red) after 16 hours of shear stress (C) was not suppressed by peptides that disrupt association of VASP with focal adhesion complexes (D), or by control (scrambled) peptide (not shown).
Existing Stress Fibers Realign with the Shear Axis, Fuse, and Grow Unidirectionally to Drive Cell Shape Change
By 16 to 24 hours, actin assembly in all cells became oriented with the shear axis and frequently extended to the upstream and/or downstream poles of the cell (Figures 3 and 4). We therefore hypothesized that stress fiber reorientation and assembly protruded the cell membrane along the shear axis to achieve elongation. To test this hypothesis, we microinjected endothelial cells with fluorescently labeled G-actin and then exposed the cells to shear stress 24 hours later, when the labeled G-actin was distributed throughout the cellular pool, so that microfilaments in live cells could be visualized.
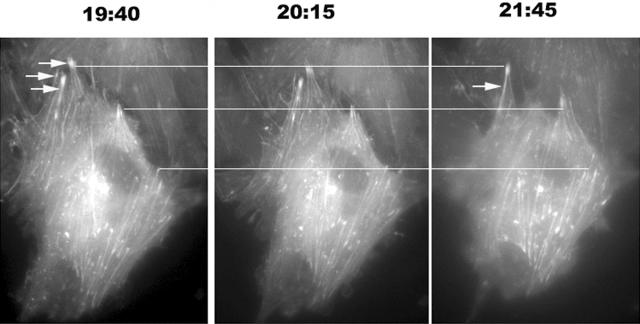
Individual stress fibers extended and changed their orientation, most often toward the shear axis, but excursion away from this direction also occurred. During and after alignment of stress fibers in the direction of shear, assembling stress fibers extended to the cell periphery and further actin assembly drove protrusion of the cell membrane in the direction of cell elongation (Figure 7). Stress fibers originated proximal to the cell periphery then extended to the cell-cell junction and advanced the adjacent cell membrane. In no cells did we see evidence of actin nucleation at the cell edge that would characterize filopodia. Interestingly, when assembling and reorienting stress fibers encountered adjacent stress fibers, they often merged to produce larger microfilament bundles (Figure 7, arrows).
Figure 7.
Stress fiber extension protrudes the endothelial cell membrane at the cell poles. Endothelial cells were microinjected with fluorescently labeled G-actin 24 hours before exposing the cells to shear stress for ∼20 hours. Top horizontal line joins the ends of stress fibers that do not advance during the observation time. The middle line joins stress fibers that advance between t = 20:15 hours and t = 21:45 hours. The bottom line is in the vicinity of three stress fibers that advance substantially throughout the period of observation. Note the advance of the cell membrane in the latter two cases. Frequently, two or more adjacent stress fibers fused throughout the period of observation (arrows). Shear stress is directed from top to bottom. Findings are representative of three replicate experiments.
Shear Induces Changes to Cell-Substrate Adhesion Complexes
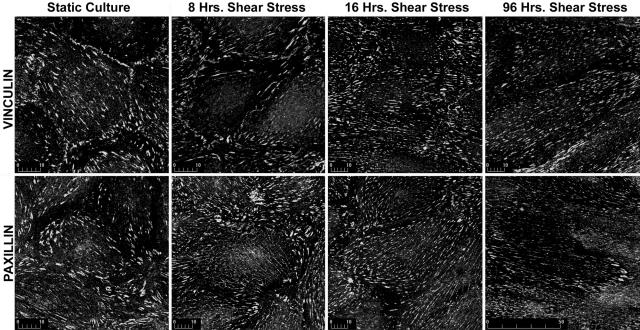
Reorganization of cell-substrate adhesion complexes accompanied realignment of stress fibers. In static cultures, focal adhesions were large, ovoid, or arrowhead complexes, whereas shear stress caused most of these complexes to assume an extended, linear morphology (Figures 8 and 9). These changes may reflect conversion from classical focal adhesions (focal contacts),24 seen in many stable cultures, to the fibrillar adhesions that characterize motile cells;24 however, not all of our data were consistent with this interpretation. We immunostained adhesion complexes with antibodies to vinculin, paxillin, and tensin, because the former two proteins preferentially localize to focal contacts whereas tensin preferentially concentrates at fibrillar adhesions.25,26 Some depletion of vinculin and paxillin at focal adhesions was seen with shear but there was also a dramatic loss of tensin that persisted until after shape change had gone to completion (Figure 9).
Figure 8.
Focal adhesion complexes become extended and linear under the influence of shear stress. The figure shows vinculin and paxillin immunostaining after 0 hours, 16 hours, and 96 hours of shear stress, which is directed from right to left. Shear stress induces the formation of long, linear adhesion complexes that are aligned with shear stress. Findings are representative of three replicate experiments.
Figure 9.
Focal adhesion complexes become depleted of tensin under the influence of shear stress. Endothelium in postconfluent cultures was immunostained for tensin and viewed by confocal microscopy. Tensin localized to focal adhesion complexes in static cultures (left) but dramatic decreases in tensin contents of focal adhesions were observed at 24 hours of shear (middle) as well as at 8 hours, 16 hours, and 48 hours (not shown). Recovery of tensin at focal adhesions was detected at 96 hours. Images were captured in the same microscopy session with identical settings on the microscope and identical conditions for figure production. Shear stress is directed from right to left. Findings are representative of three replicate experiments.
Vinculin also localizes to adherens junctions,27 but this association was permanently lost in endothelium when shear was imposed (Figure 8). Adherens junctions are primary mediators of cell-cell coupling; furthermore, reorganization of adherens junctions has been intimately linked to local actin assembly.20 We therefore further examined changes to adherens junctions that are induced by shear stress.
Chronic Shear Stress and Organization of Adherens Junctions
We previously reported13 that shear caused adherens junctions to convert from a generally linear distribution to extended junctional plaques that were often associated with peripheral actin (Figure 10A). This pattern persisted at 48 hours, ie, 24 hours after cell shape changes was completed.13 Nonetheless, adherens puncta proved to be transitory because these junctional complexes returned to a primarily linear distribution at 96 hours (Figure 10C). This latter distribution apparently reflects junctions in vivo, because immunostaining of adherens junctions (α-catenin) in rabbit carotid arteries was also linear (Figure 10D). Actin microfilaments were required for maintenance of puncta because treatment of cells after 48 hours of shear with the microfilament-disrupting agent, cytochalasin D (0.1 μmol/L), caused junctions to revert to a linear distribution (Figure 10B). Furthermore, actin assembly may participate in conversion of adherens puncta to linear adherens junctions because assembly sporadically localized to the junctional complexes at 48 hours (see white areas in Figure 9A) and 24 hours (not shown) of shear stress.
Figure 10.
A punctate distribution of adherens junctions in shear-stressed endothelium is transitory and dependent on actin microfilaments. A: Confocal micrograph shows F-actin (red), exogenous fluorescent actin (newly assembled actin, green), and the adherens junction protein, α-catenin, after 48 hours of shear stress. Note the discontinuous distribution of adherens junctions (adherens plaques). (The α-catenin signal alone is reproduced as online Figure 6 so that the discontinuous nature of adherens conjunctions can be easily visualized.) B: This distribution was dependent on filamentous actin because cytochalasin, which disperses microfilaments, induced the redistribution of adherens junctions into a linear array at 48 hours of shear stress. C: The punctate pattern that adherens junctions assumed under shear stress was transitory because these structures spontaneously resumed a linear distribution at the cell-cell junction at 96 hours, a distribution that mimicked that observed for β-catenin in unmanipulated rabbit carotid arteries in situ (D).
Discussion
Previous examinations of morphological adaptations of endothelial monolayers to shear stress have consistently reported cell elongation and alignment in the direction of shear. However, the time course of this adaptation in vitro has varied from minutes to many hours13,28–31 and the reason for this variability is unknown. We hypothesized that stabilization of endothelial monolayers after confluence is first achieved may strongly influence the time course of this response. For example, the endothelium must reorganize cell-cell junctional complexes to undergo shape change13 and it can take 48 hours after confluence is achieved for important constituents of these structures, eg, γ-catenin (plakoglobin) and desmoplakin, to accumulate at these sites.32,33 Also, endothelium gradually developed a dense peripheral band of actin34 time that was not apparent in subconfluent or just confluent cultures. Accordingly, we found that cells in subconfluent or just confluent monolayers very rapidly elongated in response to shear stress, often within 1 hour. In contrast, 48 hours postconfluent cultures showed no changes in cell shape or actin distribution at 4 hours and only modest changes at 8 hours. Shape change occurred throughout 12 to 24 hours and renormalization of cell-cell adherens junctions took between 48 and 96 hours to complete. It is noteworthy that changes in blood flow in arteries induce morphological changes to endothelium that also take 12 to 24 hours to go to completion; therefore, stabilized monolayer cultures are required to mimic morphological responses to shear.35,36
Previous work indicated a potential role for actin assembly in morphological responses of endothelial cells to shear stress. Modulation of the activities of the Rho, Rac, and cdc42 GTPase activities, which are primary regulators of actin assembly,37 have been implicated in these morphological responses;29 furthermore, inhibition of shear activation of p38 MAP kinase blocked downstream activation of the actin uncapping protein, HSP27, and abolished cell shape change.38 Usually cell shape change produced by actin assembly is secondary to formation of filopodia or lamellipodia; however, this was not the case for shear-stressed endothelium. Instead, assembly was concentrated at the ends of stress fibers that were randomly oriented. Subsequently, stress fibers aligned with the shear axis while polymerization continued and then further assembly drove the cell membrane centrifugally at the upstream and/or downstream poles of the cells. We are aware of no other physiological examples in which polymerization of stress fiber proceeds from the cell interior, extends to the cell surface, and then propels the cell membrane forward to elicit shape change.
Stress fibers aligned with the shear vector within 24 hours of the onset of shear. Nucleation and assembly of new stress fibers must occur because these structures are more numerous and larger in shear-adapted cells.35,39,40 However, stress fibers tended to display a common orientation within individual cells that were adapting to shear stress even before the cells aligned with shear. It is therefore likely that nascent stress fibers, like pre-existing stress fibers, participated in the elongation, alignment and fusion that we describe.
Microinjection of fluorescently labeled G-actin confirmed both extension and reorientation of stress fibers. Because cellular and stress fiber elongations were initially oblique to the shear vector, we infer that cell elongation is a primary response that is then accompanied and followed by reorientation. Reorientation of basal stress fibers inevitably led to direct contact of some of these structures, and then they fused into larger microfilament bundles. Presumably, actin-bundling proteins, eg, α-actinin, participate, but these were not identified in the current study.
Actin assembly continued at 96 hours of shear stress, long after gross morphological changes to the cells was completed. This chronic actin polymerization may be accompanied by treadmilling that preserves cell and stress fiber dimensions but its functional significance is uncertain. Possibly, actin assembly-disassembly is required to maintain an elongated cell morphology. Adaptation to shear involves a high degree of co-alignment of microfilaments, focal adhesion complexes, and microtubules.41 Because cytoskeletal and adhesive structures display considerable stochastic behavior,42,43 their continuous, active reorganization may offset an incessant, entropy-driven drift toward randomized orientation of cell structures and, ultimately, cell morphology.
Actin assembly usually localized to only one of the two focal adhesions into which stress fibers inserted; however the polarization of this assembly varied from cell to cell. Although our examination of constituents of focal adhesions was limited, we detected no differences in localization of vinculin, paxillin, or tensin to assembling versus nonassembling ends of stress fibers; therefore, the determinants of polarized actin assembly remain enigmatic.
Translocation of substrate adhesion complexes accompanies reorganization of the stress fibers that insert into them. Translocation of these structures in motile cells can involve the separation of fibronectin-associated α5β1 integrins from large, ovoid, focal adhesion complexes to form long, thin motile fibrillar adhesions that are rich in tensin.17 Accordingly, we found that adhesion complexes converted to a more extended, linear morphology during adaptation to shear; however, tensin content was dramatically decreased and it only returned to these sites at 96 hours. Thus, these focal adhesions in cells adapting to shear are hybrid complexes and we have not resolved whether they arise from classical focal adhesion complexes in a manner similar to that for the fibrillar adhesions that have been examined in other cells.25 We also observed actin assembly that localized to subdomains of vinculin-containing plaques and we propose that assembly of stress fibers at one subdomain of adhesion plaques, presumably in concert with aggregation of focal adhesion proteins, and disassembly of these structures at another, underlies their translocation toward the shear axis.
The machinery that assembles actin under the influence of shear stress remains unclear. The presence of VASP at focal adhesions, where shear induces assembly, was intriguing because the zyxin-VASP complexes assemble linear arrays of microfilaments at other sites.20 However, displacement of VASP from focal adhesions with synthetic peptides did not disrupt stress fiber assembly. Clearly other protein complexes mediate this process.
Elongation and alignment of endothelial cells also requires reorganization of cell junctional complexes. Adherens junctions are critical in this regard because of their primary role in mechanical coupling of cells. We previously reported that primarily linear adherens junctions become disrupted and discontinuous during adaptation to shear stress.13 This phenomenon persisted at 48 hours of shear, long after elongation and reorientation were completed and we inferred that it was a permanent manifestation of shear stress. We now report, however, a return to linear junctions at 96 hours, a distribution that replicates that seen in arteries in situ, thus morphological adaptations to shear stress take up to 4 days to go to completion. The transient puncta depended on an intact F-actin cytoskeleton because the junctions became linear when actin was dispersed; furthermore actin assembly sporadically co-localized to these sites. It is possible that actin assembly participates in conversion of adherens puncta to linear junctions, as it does in newly forming epithelial cell junctions,20 but this was not directly assessable because of the very slow reorganization of these structures under shear stress and the sporadic nature of actin assembly at puncta.
In conclusion, these studies revealed that endothelial cells become elongate and aligned in the direction of shear stress through a novel mechanism that involves growth, fusion, and reorientation of stress fibers that ultimately protrudes the upstream and/or downstream cell membrane. Actin assembly occurs at motile focal adhesion complexes that also elongate in the direction of shear stress and, sporadically, it occurs at cell-cell adherens junctions as these structures undergo the obligatory reorganization that occurs when cells in a confluent monolayer change shape.
Footnotes
Address reprint requests to B. Lowell Langille, Ph.D., Toronto General Research Institute, 200 Elizabeth St., NU 113d, Toronto, ON M5G 2C4, Canada. E-mail: langille@uhnres.utoronto.ca.
Supported by the Canadian Institutes for Health Research (grant 15044).
S.N. was a recipient of a Canadian Institutes for Health Research graduate studentship.
References
- Roach MR, Fletcher J. Effect of unilateral nephrectomy of the localization of aortic sudanophilic lesions in cholesterol-fed rabbits. Atherosclerosis. 1977;24:327–333. doi: 10.1016/0021-9150(76)90088-5. [DOI] [PubMed] [Google Scholar]
- Zand T, Hoffman AH, Savilonis BJ, Underwood JM, Nunnari JJ, Majno G, Joris IS. Lipid deposition in rat aortas with intraluminal hemispherical plug stenosis. Am J Pathol. 1999;155:85–92. doi: 10.1016/S0002-9440(10)65103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low and oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Ross R, Fuster V. The pathogenesis of atherosclerosis. Fuster V, Ross R, Topol EJ, editors. Philadelphia: Lippincott-Raven Publishers,; Atherosclerosis and Coronary Artery Disease. 1996:pp 441–460. [Google Scholar]
- Ross R. Mechanisms of disease—atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Ando J, Tsuboi H, Korenaga R, Takada Y, Toyama-Sorimachi N, Miyasaka M, Kamiya A. Shear stress inhibits adhesion of cultured mouse endothelial cells to lymphocytes by downregulating VCAM-1 expression. Am J Physiol. 1994;267:C679–C687. doi: 10.1152/ajpcell.1994.267.3.C679. [DOI] [PubMed] [Google Scholar]
- Ranjan V, Diamond SL. Fluid shear stress induces synthesis and nuclear localization of c-fos in cultured human endothelial cells. Biochem Biophys Res Commun. 1993;196:79–84. doi: 10.1006/bbrc.1993.2218. [DOI] [PubMed] [Google Scholar]
- Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995;15:2–10. doi: 10.1161/01.atv.15.1.2. [DOI] [PubMed] [Google Scholar]
- Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95:1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr, Gimbrone MA., Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85:504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- Caplan BA, Schwartz CJ. Increased endothelial cell turnover in areas of Evans blue uptake in the pig aorta. Atherosclerosis. 1973;17:401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy GG, Svitkina TM. Actin machinery: pushing the envelope. Curr Opin Cell Biol. 2000;12:104–112. doi: 10.1016/s0955-0674(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz B-Z, Matsumoto K, Lin DC, Lin S, Hahn CS, Yamada KM. Integrin dynamics and matrix assembly: tension-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Symons MH, Mitchison TJ. Control of actin polymerization in live and permeabilized fibroblasts. J Cell Biol. 1991;114:503–513. doi: 10.1083/jcb.114.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, Golysteyn RM. Characterization of the interaction between zyxin and members of the Ena/VASP (vasodilator stimulated phosphoprotein) family of proteins. J Biol Chem. 2000;275:22503–22511. doi: 10.1074/jbc.M001698200. [DOI] [PubMed] [Google Scholar]
- Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K, Krause M, Gimona M, Small JV, Wehland J. Zyxin is not colocalized with vasodilator-stimulated phosphoprotein (VASP) at lamellipodial tips and exhibits different dynamics to vinculin, paxillin, and VASP in focal adhesions. Mol Biol Cell. 2001;12:3103–3113. doi: 10.1091/mbc.12.10.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E, Katz B-Z, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz B-Z, Lin S, Lin DC, Bershadsky A, Kam Z, Geiger B. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- Geiger B. Cell biology: encounters in space. Science. 2001;294:1661–1663. doi: 10.1126/science.1066919. [DOI] [PubMed] [Google Scholar]
- Rüdiger M. Vinculin and alpha-catenin: shared and unique functions in adherens junctions. BioEssays. 1998;20:733–740. doi: 10.1002/(SICI)1521-1878(199809)20:9<733::AID-BIES6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, del Pozo A, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropec JA, Hollinger MK, Woolkalis MJ. Regulation of VE-cadherin linkage to the cytoskeleton in endothelial cells exposed to fluid shear stress. Exp Cell Res. 2002;273:240–247. doi: 10.1006/excr.2001.5453. [DOI] [PubMed] [Google Scholar]
- Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985;107:341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- Valiron O, Chevrier V, Usson Y, Breviario F, Job D, Dejana E. Desmoplakin expression and organization at human umbilical vein endothelial cell-to-cell junctions. J Cell Sci. 1996;109:2141–2149. doi: 10.1242/jcs.109.8.2141. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MKK, Gotlieb AI. In vitro reendothelialization of a single-cell wound. Role of microfilament bundles in rapid lamellipodia-mediated wound closure. Lab Invest. 1984;51:75–81. [PubMed] [Google Scholar]
- Kim DW, Gotlieb AI, Langille BL. In vivo modulation of endothelial F-actin microfilaments by experimental alterations in shear stress. Arteriosclerosis. 1989;9:439–445. doi: 10.1161/01.atv.9.4.439. [DOI] [PubMed] [Google Scholar]
- Langille BL, Graham JJK, Kim D, Gotlieb AI. Dynamics of shear-induced redistribution of F-actin in endothelial cells in vivo. Arterioscler Thromb. 1991;11:1814–1820. doi: 10.1161/01.atv.11.6.1814. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Azuma N, Akasaka N, Ikeda M, Gahtan V, Sasajima T, Sumpio BE. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol. 2001;280:H189–H197. doi: 10.1152/ajpheart.2001.280.1.H189. [DOI] [PubMed] [Google Scholar]
- Kim DW, Langille BL, Wong MKK, Gotlieb AI. Patterns of endothelial microfilament distribution in the rabbit aorta in situ. Circ Res. 1989;64:21–31. doi: 10.1161/01.res.64.1.21. [DOI] [PubMed] [Google Scholar]
- Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Andersen SSL. Balanced regulation of microtubule dynamics during the cell cycle: a contemporary view. BioEssays. 1999;21:53–60. doi: 10.1002/(SICI)1521-1878(199901)21:1<53::AID-BIES7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Davies PF, Robotewskyj A, Griem ML. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J Clin Invest. 1994;93:2031–2038. doi: 10.1172/JCI117197. [DOI] [PMC free article] [PubMed] [Google Scholar]