Abstract
The North Sea bryozoan Flustra foliacea was investigated to determine its secondary metabolite content. Gas chromatography-mass spectrometry analysis of a dichloromethane extract of the bryozoan enabled 11 compounds to be identified. Preparative high-performance liquid chromatography of the extract resulted in the isolation of 10 brominated alkaloids (compounds 1 to 10) and one diterpene (compound 11). All of these compounds were tested to determine their activities in agar diffusion assays against bacteria derived from marine and terrestrial environments. Compounds 1, 3 to 7, 10, and 11 exhibited significant activities against one or more marine bacterial strains originally isolated from F. foliacea but only weak activities against all of the terrestrial bacteria. By using the biosensors Pseudomonas putida(pKR-C12), P. putida(pAS-C8), and Escherichia coli(pSB403) the antagonistic effect on N-acyl-homoserine lactone-dependent quorum-sensing systems was investigated. Compounds 8 and 10 caused reductions in the signal intensities in these bioassays ranging from 50 to 20% at a concentration of 20 μg/ml.
Many benthic marine organisms have developed chemical defense systems against potential predators. Microorganisms, such as bacteria, may settle and thrive on their prey, a process known as biofouling (21). In recent years, it has become apparent that bacteria coordinate their interactions and associations with higher organisms by using intercellular communication systems that rely on small diffusible molecules in a process known as quorum sensing (1, 9, 10). Among gram-negative bacteria the most intensively investigated and probably the most widespread signaling molecules are N-acyl-homoserine lactones (AHLs). AHL-based quorum-sensing systems ensure that certain phenotypic traits are expressed only when a particular population density has been attained. These regulatory systems control various functions, including bioluminescence, conjugative plasmid transfer, antibiotic synthesis, motility, production of virulence factors in animal, plant, and human pathogens, and the ability to form surface-associated consortia, commonly referred to as biofilms.
Marine organisms may inhibit the colonization and establishment of biofilm-forming microbiota by producing antimicrobial metabolites and/or metabolites that interfere with microbial communication systems (7). The marine bryozoan Flustra foliacea (L.), which is abundant in various parts of the North Sea, has been shown to contain biologically active brominated alkaloids (3) and monoterpenes (5). Electron microscopic investigation of F. foliacea revealed no microbial settling on the distal part of the zooid, but bacterial cells were detected near the bottom of the operculum of the zooid. A distinct biofilm was established at the proximal end of the zooid and on older parts of a bryozoan colony (22). Previous studies with extracts of F. foliacea showed that animals from Canadian waters produce compounds with strong activity against Bacillus subtilis (a gram-positive bacterium), and the alkaloid fraction, containing mainly the brominated alkaloids compounds 8 and 10 (Fig. 1), exhibited activity mainly against gram-negative bacteria like Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella enterica serovar Typhimurium, and Serratia marcescens, as well as against the low-G+C-content gram-positive organisms Staphylococcus aureus and Staphylococcus epidermidis (17). An extract of F. foliacea from the North Sea, however, was described as being devoid of any such activity; only the isolated compound flustramine E (compound 12) was determined to be active against Botrytis cinerea and Rhizotonia solani (15).
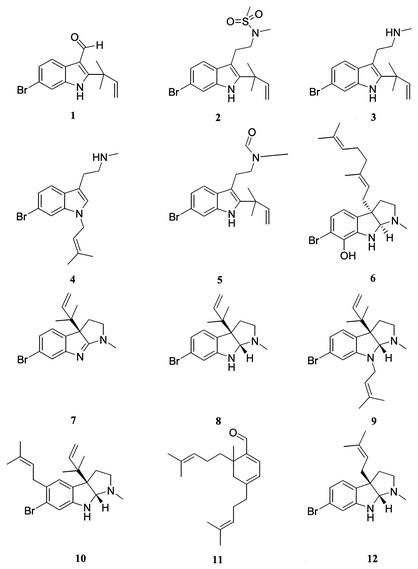
FIG. 1.
Structures of secondary metabolites isolated from F. foliacea.
The bioactive secondary metabolites produced by F. foliacea may be important for survival of the organism in its natural environment; i.e., they may have an impact on the microenvironment of this bryozoan (4). In the present study the major secondary metabolites of F. foliacea were analyzed by gas chromatograph (GC)-mass spectrometry (MS) and subsequently isolated in pure form by preparative high-performance liquid chromatography (HPLC). The activities of the compounds were assessed with bacteria, including bacterial isolates obtained from F. foliacea itself, and finally the influence of F. foliacea metabolites on quorum sensing was investigated by using several assay systems.
MATERIALS AND METHODS
Sampling of F. foliacea.
Samples of the bryozoan F. foliacea for chemical investigation were collected by divers who used SCUBA gear from Steingrund in the North Sea, which is 10 km northeast of Helgoland, in September 1998 from depths of 8 to 12 m. After collection samples were kept frozen until they were used. The sampling times and sites in the North Sea used for recovery of bacteria from bryozoan samples were as follows: April 1998 at a location west of the island Helgoland (54°10′50"N, 7°52′55"E) at a sampling depth of 10 m; April 1999 at Steingrund (sublitoral moraine, 9 sea miles northeast of Helgoland; 54°14.631′N, 008°03.365′E) at a sampling depth of 12 m; and February 2000 at Steingrund (54°13.577′N, 008°03.207′E) at a sampling depth of 15 m. Samples were collected by divers and placed directly in polyethylene bags containing seawater from the local sites. The temperature of the seawater was between 4.7 and 8°C.
Taxonomic identification of the bryozoan was performed by P. Haywood, School of Biological Sciences, University of Wales, Swansea, Wales. A museum sample has been retained at the Department for Pharmaceutical Biology, University of Bonn, Bonn, Germany.
Isolation and identification of bacteria from bryozoans.
Bacteria associated with F. foliacea and Bugula plumosa were isolated from the bryozoan samples as described by Pukall et al. (22). For isolation of cultivatable bacteria, bacterial suspensions were spread plated onto a variety of complex and minimal agar media supplemented with different compounds as energy sources (22). In this manner, different F. foliacea samples which were obtained from different sampling sites and at different times in the North Sea (west side of Helgoland, Steingrund) and, in addition, one sample of B. plumosa taken from the south pier at the harbor entrance of Helgoland were investigated. Analysis of the amplified ribosomal DNA restriction polymorphism was used to sort and generate different clusters of the bacterial strains isolated. In order to affiliate the amplified ribosomal DNA restriction polymorphism clusters with taxonomic units, representatives were characterized by analysis of the first 350 nucleotides of the 16S rRNA gene. A detailed analysis of strains was described by Pukall et al. (22).
Agar diffusion assays.
Agar diffusion assays were carried out at room temperature by using the methods of Schulz et al. (24). The following marine strains, representing members of different phylogenetic taxa, were used as test organisms for the agar diffusion assay: strain MBT3 (Paenibacillus pabuli; a low-G+C-content gram-positive strain), MBT21 (Roseobacter sp.; a member of the α subclass of the class Proteobacteria), SFLA51 (Sulfitobacter sp.; a member of the α subclass of the Proteobacteria), OI-14-4 (Psychroserpens-like bacterium; a member of the Cytophaga-Flexibacter-Bacteroides phylum), and A011 (Halomonas marina; a member of the γ subclass of the Proteobacteria). Strains whose designations begin with MBT-, SFLA-, and OI- were originally isolated from Flustra colonies sampled in February 2000 (Steingrund), April 1999 (Steingrund), and April 1998 (west side of Helgoland), respectively. Additionally, the test organisms Bacillus megaterium DSM 32T (a gram-positive strain) and Escherichia coli DSM 498 (a gram-negative strain) were used.
Sample solutions each contained 1 mg of a test sample per ml. One hundred microliters of each solution was pipetted onto a sterile antibiotic filter disk (diameter, 10 mm; Schleicher & Schuell), which after evaporation of the solvent was placed onto the appropriate agar medium and sprayed with a suspension of the test organism. For marine-derived bacteria Bacto Marine Agar 2216 (Difco) was used. The appropriate agar medium for E. coli and B. megaterium has been described previously (24). An inhibition zone that was ≥2 mm in diameter (measured from the edge of the filter disk) was considered a positive result.
Inhibition of AHL sensor strains.
Quorum-sensing inhibition of compounds 1 to 11 was investigated by using the following sensor strains: Pseudomonas putida harboring the sensor plasmid pKR-C12 (25); P. putida harboring the sensor plasmid pAS-C8 (25); and E. coli MT102 harboring the sensor plasmid pSB403 (26). The sensor strains were grown overnight in Luria-Bertani (LB) medium (2) at 30°C, diluted fourfold in fresh medium, and grown for another hour. After addition of the appropriate signal molecule (50 nM N-3-oxododecanoyl-l-homoserine lactone for pKR-C12; 50 nM N-octanoyl-l-homoserine lactone for pAS-C8; 100 nM N-oxohexanoyl-l-homoserine lactone for pSB403), 100-μl aliquots of cultures were pipetted into the wells of microtiter plates (FluoroNunc, Polysorp). F. foliacea compounds were dissolved in dimethyl sulfoxide (DMSO) (10 mg/ml) and added to the wells at final concentrations ranging from 300 ng/ml to 20 μg/ml. After this, the microtiter plates were incubated at 30°C for 4 h. Fluorescent or bioluminescent signals were measured with a Lambda Fluoro 320 Plus reader (Bio-Tek Instruments). Inhibitor-mediated reduction of the reporter strain signal was correlated to the value obtained when the compounds were not added.
Growth experiment.
To eliminate the possibility that the inhibition of the quorum-sensing sensor strains was due to inhibition of bacterial growth, 30-ml LB medium cultures of E. coli were supplemented with two different concentrations (20 and 100 μg/ml) of compounds 8 and 10. As controls, growth experiments were also performed in media supplemented with the corresponding amounts of the solvent DMSO. The cultures were grown at 37°C with vigorous shaking. One-milliliter samples were retrieved at various times, and cell density was measured at 600 nm.
Measurement of protease activity.
P. aeruginosa wild-type strain PAO1 (14) and the AHL-negative lasI rhlI mutant strain PAO1-JP2 (19) were grown in 5 ml of LB medium in the absence and presence (20 μg/ml) of compounds 8 and 10 at 37°C for 16 h. Overnight cultures were harvested by centrifugation at 10,000 × g for 10 min, and the supernatants were sterile filtered. The proteolytic activity assay was performed as described elsewhere (23). Briefly, 50 μl of supernatant and 250 μl of 2% (wt/vol) azocasein (in 50 mM Tris-HCl, pH 7.5) were mixed and incubated at 37°C for 1 h. The reaction was stopped by addition of 1.25 ml of 10% (wt/vol) trichloroacetic acid, and the mixture was incubated for 20 min at room temperature and centrifuged. After addition of 750 μl of 1 M NaOH to 600 μl of the supernatant, the absorbance (optical density at 440 nm) was determined.
Extraction and isolation.
For extraction for GC-MS analysis, 1 g of frozen F. foliacea was extracted three times with 20 ml of dichloromethane. The volume of the resultant combined extracts was reduced to 1 ml, and the preparation was analyzed by GC-MS. For extraction and isolation of compounds 1 to 11, frozen F. foliacea (wet weight, ca. 5 kg) was extracted three times with 5 liters of dichloromethane. The extract (12 g of brown oil) was fractionated by vacuum liquid chromatography over normal-phase silica gel, and this yielded 13 fractions (20). Solid-phase extraction (SPE; Bakerbond SiOH) of fraction 13 with a step gradient from dichloromethane to methanol yielded 13 mg of compound 4. All other pure compounds were obtained by normal and reversed-phase HPLC, as described previously (20).
Analysis of natural products.
GC-MS results were obtained at 70 eV (1 scan/s at 180°C) by using a Perkin-Elmer AutoSystem XL attached to a Perkin-Elmer AutoMass detector and autosampler. In method 1, the following temperature gradient program was used: increase from 90°C (at zero time) to 160°C at a rate of 6°C/min and increase from 160 to 300°C at a rate of 10°C/min. The sample solution contained 1 mg/ml, the injection volume was 1 μl, and the split ratio was 1/5. In method 2, the temperature gradient program included an increase from 50°C (at zero time) to 230°C at a rate of 4°C/min, followed by an increase from 230 to 300°C at a rate of 10°C/min. The sample solution contained 1 mg/ml, the injection volume was 1 μl, and the split ratio was 1/5. GC separation was carried out by using a Perkin-Elmer PE-1 column (30 m by 0.32 mm) and He (2 ml/min) as the carrier gas. HPLC was carried out by using a Waters system consisting of a 600 pump controller, a 996 photodiode array detector, a 717 Plus autosampler, and an in-line degasser. Isolated natural products 1 to 11 were analyzed by using a Eurospher 100-C18 column (5 μm; 250 by 8 mm) and a eluent system consisting of water, methanol, and dichloromethane in a gradient from 15 to 0% water in 10 min, 85 to 40% methanol in 30 min, and 0 to 60% dichloromethane in 30 min; the flow rate was 2 ml/min. Organic solvents were purchased from Biesterfeld and distilled prior to use.
RESULTS
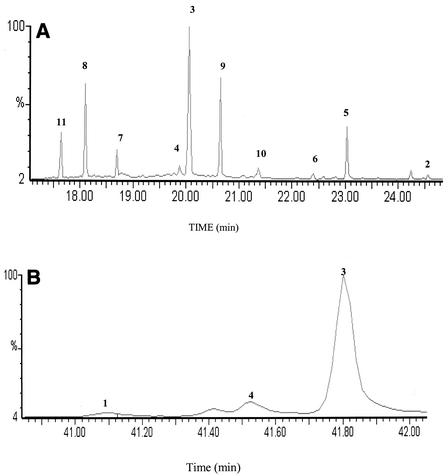
F. foliacea is a foliaceous, abundant bryozoan that is frequently found in the North Sea. Field and microscopic observations of samples of specimens of this animal collected from the North Sea revealed only a few epibionts on the surface, mostly on older parts of the colony (22), a fact that might be related to the production of brominated indole alkaloids in the active growing zone of the animals. In order to investigate the secondary metabolite content of F. foliacea, a sample of this animal was extracted with dichloromethane. GC-MS analysis of the extract enabled compounds 1 to 11 to be identified (Fig. 2) and the relative amounts of these compounds to be determined (Table 1). This analysis showed that the most abundant metabolite was the brominated alkaloid compound 3 (37% of the total indole alkaloid content), followed by compounds 5, 8, 9, and 11 (9 to 17%) and compounds 2, 4, 6, 7, and 10 (1 to 4%); compound 1 was the least abundant of the 11 compounds (<0.1%).
FIG. 2.
(A) GC-MS chromatogram showing separation of compounds 2 to 11 from the dichloromethane extract of F. foliacea. The GC parameters were as follows: an increase from 90°C (at time zero) to 160°C at a rate of 6°C/min and then an increase from 160 to 300°C at a rate of 10°C/min; sample concentration, 1 mg/ml; injection volume, 1 μl; split ratio, 1/5. (B) GC-MS chromatogram showing separation of compound 1 from the dichloromethane extract of F. foliacea. The GC parameters were as follows: an increase from 50°C (at zero time) to 230°C at a rate of 4°C/min and then an increase from 230 to 300°C at a rate of 10°C/min; sample concentration, 1 mg/ml; injection volume, 1 μl; split ratio, 1/5.
TABLE 1.
GC-MS and HPLC analyses of the secondary metabolites 1 to 11 found in F. foliacea
| Compound | GC-MS
|
Retention time in HPLC (min)c | |
|---|---|---|---|
| Retention time (min)a | Relative %b | ||
| 1 | 41.1d | <0.1 | 8.2 |
| 2 | 24.6e | 0.8 | 6.8 |
| 3 | 20.1e | 36.7 | 20.5 |
| 4 | 19.9e | 2.9 | 33.0 |
| 5 | 23.0e | 10.3 | 8.7 |
| 6 | 22.4e | 1.5 | 12.9 |
| 7 | 18.8e | 2.6 | 12.5 |
| 8 | 18.1e | 15.6 | 15.8 |
| 9 | 20.7e | 17.2 | 17.2 |
| 10 | 21.4e | 3.5 | 17.9 |
| 11 | 17.6e | 9.0 | 16.7 |
Sample solution 1 mg/ml; injection volume, 1 μl; split ratio, 1/5; Perkin-Elmer PE-1 column (30 m by 0.32 mm [inside diameter]); and He (2 ml/min) as the carrier gas.
The total amount of compounds 1 to 11 was defined as 100%.
Determined by using a Eurospher 100-C18 column (5 μm; 250 by 8 mm) and a solvent system consisting of H2O, methanol, and CH2Cl2 in a gradient from 15 to 0% H2O in 10 min, from 85 to 40% methanol in 30 min, and 0 to 60% CH2Cl2 in 30 min (flow rate, 2 ml/min).
Determined by using GC-MS method 2.
Determined by using GC-MS method 1.
HPLC separation of the extract yielded sufficient amounts of the pure natural products to evaluate their biological activities. Thus, HPLC separation of the dichloromethane extract with C18 reversed-phase material resulted in 10 bromo-tryptamine-based alkaloids (compounds 1 to 10) and a cyclohexadiene-carbaldehyde diterpene (compound 11). Compounds 1 to 4 and 6 were new natural products. Elucidation of the structures of compounds 1, 2, 3, and 6 was described previously by Peters et al. (20), and elucidation of the structure of the alkaloid compound 4 will be reported elsewhere. After isolation, the purity of all natural products was assessed by performing HPLC with gradient elution and diode array detection; Table 1 shows the HPLC retention times.
Bacteria associated with F. foliacea and B. plumosa were isolated from the bryozoan samples as described by Pukall et al. (22). The cultivatable bacteria isolated from F. foliacea could be assigned to the γ subclass of the Proteobacteria (Shewanella, Pseudoalteromonas), the α subclass of the Proteobacteria (Roseobacter, Sulfitobacter), the Cytophaga-Flexibacter-Bacteroides phylum (Flavobacterium, Psychroserpens-like bacterium), or the low-G+C-content gram-positive group (Bacillus). The cultivatable bacteria associated with B. plumosa consisted mainly of members of the γ subclass of the Proteobacteria (Vibrio splendidus group, Halomonas marina, Shewanella sp., Pseudoalteromonas sp.).
The antibacterial effects of compounds 1 to 11 were investigated by using four bacterial strains representing different phylogenetic taxa which were obtained from F. foliacea (P. pabuli, Roseobacter sp., Sulfitobacter sp., Psychroserpens sp.) and H. marina obtained from B. plumosa. For comparative purposes terrestrially derived bacterial strains of B. megaterium and E. coli were included in the assays. The results of agar diffusion assays are shown in Table 2. All compounds inhibited the growth of at least two of the bacteria obtained directly from F. foliacea. Strong activity against Sulfitobacter sp. and Psychroserpens sp. was observed for the brominated alkaloid compound 1, the least abundant metabolite isolated from the dichloromethane extract of the bryozoan. The major components of the dichloromethane extract, compounds 3 and 4, inhibited the growth of all test organisms, and the most prominent activity was the activity against Sulfitobacter sp. Compound 6 showed strong activity against P. pabuli and Sulfitobacter sp. Addition of compound 11 resulted in considerable growth inhibition of Roseobacter sp. and Sulfitobacter sp. Interestingly, the growth of H. marina, derived from a different bryozoan species, was not inhibited by the F. foliacea compounds tested. Activities of compounds 1 to 11 against the nonmarine bacteria E. coli and B. megaterium were moderate (compounds 3 and 4), weak (compounds 1, 6, 10, and 11), or nonexistent (compounds 2, 5, 7, 8, and 9).
TABLE 2.
Antibacterial activities of F. foliacea compounds 1 to 11 in agar diffusion assays
| Compounda | Inhibition zone (cm)
|
|||||
|---|---|---|---|---|---|---|
| E. coli | B. megaterium | Roseobacter sp.b | Sulfitobacter sp.b | P. pabulib | Psychroserpens sp.b | |
| 1 | 0.1 (W)c | —d | 0.1 (T) | 1.0 (T) | 0.3 (T) | 1.2 (T) |
| 2 | — | — | — | 0.2 (W) | — | 0.4 (W) |
| 3 | 0.1 (W) | 0.3 (T) | 0.1 (T) | 1.0 (T) | 0.2 (W) | 0.3 (T) |
| 4 | 0.2 (T) | 0.4 (T) | 0.2 (T) | 0.7 (T) | 0.7 (T) | 0.4 (T) |
| 5 | — | — | 0.2 (T) | 0.4 (W) | — | 0.5 (T) |
| 6 | — | 0.1 (T) | — | 0.8 (T) | 0.4 (T) | 0.2 (W) |
| 7 | — | — | 0.1 (W) | 0.3 (T) | — | — |
| 8 | — | — | — | 0.2 (W) | 0.4 (W) | 0.2 (W) |
| 9 | — | — | — | 0.5 (W) | 0.6 (W) | 0.3 (W) |
| 10 | — | 0.1 (W) | — | 0.3 (T) | 0.3 (T) | 0.2 (T) |
| 11 | — | 0.2 (T) | 0.5 (T) | 1.0 (T) | NTe | 0.3 (W) |
| Streptomycin sulfate | 0.5 (W) | 0.9 (T) | NT | 1.5 (T) | 1.4 (T) | 1.2 (T) |
| Benzylpenicillin | 0.3 (W) | 0.6 (T) | NT | 1.2 (T) | 0.6 (W) | — |
Concentration, 100 μg of compound per disk.
Bacterial strain obtained from F. foliacea.
W, inhibition of growth in the zone indicated; T, complete inhibition (no growth in the zone indicated).
—, no inhibition observed.
NT, not tested.
The potential of compounds 3, 6, and 8 to 10 to interfere with bacterial cell-cell communication systems was investigated by using three different AHL biosensors, which, depending on the components used for construction, respond to different types of AHL molecules. One of these sensors contains a translational fusion of the lasB elastase gene of P. aeruginosa to gfp(ASV) encoding an unstable version of the green fluorescent protein (25). Furthermore, the sensor contains the lasR gene, which encodes the cognate N-3-oxododecanoyl-l-homoserine lactone receptor protein, under control of a lac type of promoter. Since expression of lasB is controlled by the las quorum-sensing system, this sensor is most sensitive for N-3-oxododecanoyl-l-homoserine lactone and related long-chain AHLs. The second sensor is based on the cep genes of Burkholderia cepacia and contains a translational cepI:gfp(ASV) fusion together with the cepR regulator gene under control of Plac. Since expression of cepI is autoregulated, this sensor plasmid is particularly sensitive for N-octanoyl-l-homoserine lactone (25). The third sensor strain contains the Photobacterium fischeri luxR gene together with a transcriptional fusion of the luxI promoter region to the bioluminescence operon luxCDABE. Although this sensor exhibits the highest sensitivity for the P. fischeri signal N-oxohexanoyl-l-homoserine lactone, a relatively wide range of other AHL molecules can be detected by it (11, 26).
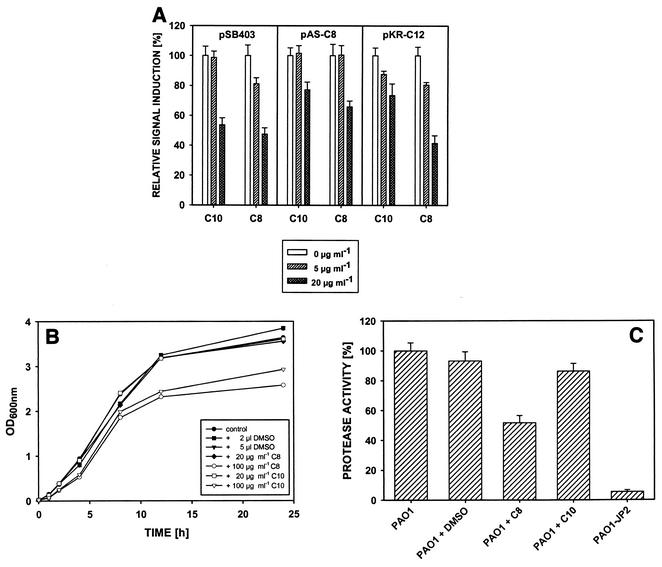
To analyze whether compounds 3, 6, and 8 to 10 were capable of antagonizing AHL-dependent quorum-sensing systems, decreasing concentrations of the substances were added to cultures of the different biosensors in the presence of the appropriate inducing AHL. In the case of AHL-inhibitory activity a reduction in expression of the reporter gene(s) (luxAB or gfp) was anticipated. In these assays two of the five compounds exhibited significant antagonistic effects. When the biosensors P. putida(pKR-C12) and E. coli(pSB403) were used, compound 8 caused more than 50% reduction in the signal intensities at a concentration of 20 μg/ml (Fig. 3A). In the case of the sensor P. putida(pAS-C8) the fluorescence signal was reduced by 30%. Similar but less pronounced effects were also observed with compound 10; the signal intensities were reduced by 50% for E. coli(pSB403) and by 20% for P. putida(pKR-C12), and for P. putida(pAS-C8) when the compound was added at a concentration of 20 μg/ml.
FIG. 3.
Interference of F. foliacea compounds 8 and 10 with AHL-dependent cell-cell communication. (A) Responses of different quorum-sensing reporter strains to the signal molecules and compounds 8 (C8) and 10 (C10). pKR-C12, P. putida harboring pKR-C12; pAS-C8, P. putida harboring pAS-C8; pSB403, E. coli harboring pSB403. The reporter strain signals (fluorescence and bioluminescence) in the absence of the compounds were defined as 100%. (B) Effects of compounds 8 and 10 on the growth of E. coli. The cultures were grown in the presence (20 or 100 μg/ml) or absence of compounds 8 and 10. Control cultures were supplemented with appropriate volumes of DMSO. OD600nm, optical density at 600 nm. (C) Effects of compounds 8 and 10 on expression of QS-regulated exoproteases of P. aeruginosa PAO1. Cultures of PAO1 were grown in the absence or presence of 20 μg of compound 8 or 10 per ml. P. aeruginosa lasI rhlI mutant PAO1-JP2, an AHL-deficient mutant, was used as a negative control. The protease activities of spent culture supernatants were determined with azocasein as the substrate. The data are means of three independent experiments. The proteolytic activity of PAO1 in the absence of the compounds was defined as 100%. The error bars indicate the standard errors of the means.
To exclude the possibility that the quorum-sensing antagonistic activity observed with compounds 8 and 10 was not caused by growth inhibition but reflected a specific interaction of the test samples with the quorum-sensing cascade, the growth curves of E. coli in the presence and absence of the substances were determined. At a concentration used in the quorum-sensing inhibition assays (20 μg/ml), the two compounds did not interfere with bacterial growth (Fig. 3B). At a concentration of 100 μg/ml some growth-inhibiting effects were observed with both compounds. These results indicate that compounds 8 and 10 are capable of specifically blocking AHL-regulated gene expression at concentrations below 20 μg/ml. At higher concentrations, however, the compounds had additional biocidal effects.
To further analyze the AHL-antagonistic activity of compounds 8 and 10, their effects on the production of extracellular proteases in P. aeruginosa, a phenotype that is under stringent control of the AHL-dependent quorum-sensing systems operating in this organism (18, 23), were investigated. To do this, P. aeruginosa PAO1 was grown in the absence and presence of compounds 8 and 10. As shown in Fig. 3C, addition of 20 μg of compound 8 per ml resulted in a 50% reduction in protease activity, and compound 10 had only marginal effects at the same concentration. Importantly, growth of P. aeruginosa PAO1 was not affected by the presence of the test compounds at a concentration of 20 μg/ml. These results strengthen the view that the two F. foliacea compounds specifically inhibit AHL-mediated cell-cell communication.
DISCUSSION
In 1991, Christophersen speculated that the alkaloids found in F. foliacea are a reflection of ecological and physiological stresses originating from the environment of the animal. F. foliacea invests a great deal of energy in the biosynthesis of its secondary metabolites (i.e., compounds 1 to 11) (Fig. 1), and these compounds may thus be envisaged as insurance for survival of the animal and hence propagation of the species (4).
Most sessile invertebrates, such as marine bryozoans, are extremely vulnerable to biofouling and predation by grazers. In attempts to combat these attacks species have developed an array of physical and/or chemical defenses that can act separately or in combination against such risk organisms. F. foliacea has essentially weak physical defenses against predator and/or fouling organisms. To grow indeterminantly with new increments added during each growing season and to survive for up to 12 years, F. foliacea may have developed chemical defense strategies. Dyrynda found that extracts of F. foliacea have toxic effects on larvae of various other modular invertebrates (e.g., other bryozoans), fish, and bacteria (8). Carté and Faulkner observed similar effects for tambjamines A to D isolated from the bryozoan Sessibugula translucens and also proposed that these secondary metabolites may be implicated in defense of the bryozoan, acting against grazers and bacteria (6).
In this context the strong activities (Table 2) of compounds 1, 3, 4, 6, and 11 against one or more bacteria assigned to the α subclass of the Proteobacteria, the Cytophaga-Flexibacter-Bacteroides phylum, and the low-G+C-content gram-positive bacteria obtained directly from F. foliacea suggests that they are ecologically important for the bryozoan; i.e., compounds 1, 3, 4, 6, and 11 may be implicated in the control of bacterial growth on the surface of this bryozoan. GC-MS analysis of seawater surrounding F. foliacea showed that alkaloids are released from the colonies (unpublished results). In contrast to the pronounced activity of Flustra metabolites against bacteria associated with the organism itself, the activities against ecologically unrelated bacteria (E. coli, B. megaterium) and H. marina obtained from a different bryozoan species are negligible. The growth-inhibiting activities of Flustra metabolites against the various bacteria obtained from F. foliacea are different (Table 2). This probably reflects the different ecological roles of individual compounds.
The biocidal activities of compounds 8 and 10 against bacteria, previously observed indirectly by Laycock et al. (17) during an investigation of alkaloid fractions from F. foliacea containing mainly compounds 8 and 10, are weak (Table 2 and Fig. 3B). These compounds, however, influence AHL-based quorum sensing and thus may affect bacteria associated with F. foliacea through an additional mechanism. The red alga Delisea pulchra is known to elaborate a range of metabolites known as halogenated furanones (6, 16). These compounds exhibit a broad range of biological activities, including feeding deterrence of herbivores, inhibition of settlement of fouling organisms, and interference with bacterial signal-mediated regulatory systems (7).
Previous work has shown that furanones from D. pulchra have the ability to specifically interfere with AHL-regulated processes (12, 13). Similar to the effect of the F. foliacea metabolites compounds 8 and 10, the AHL-antagonistic effect of the furanones is limited to a certain concentration range. At higher concentrations the compounds from both organisms often have biocidal effects.
Compounds 8 and 10 may serve as lead structures for the design of specific AHL blockers. In many pathogenic bacteria expression of virulence factors is not constitutive but is regulated in a cell density-dependent manner by quorum-sensing systems. This form of gene regulation ensures that the pathogen remains invisible to the immune system of the host until the opportunistic pathogen has reached a critical population density sufficient to overwhelm the host's defenses and to establish an infection. Thus, quorum-sensing systems represent highly attractive targets for the development of novel therapeutic strategies. The major advantage of this approach to drug development is that expression of pathogenic traits is specifically blocked while growth of the bacteria is unaffected. As attenuation of the pathogen does not impose any selective pressure on the cells, it can be anticipated that resistant mutants will not arise. The finding that the F. foliacea metabolite compound 8 is capable of suppressing the AHL-dependent production of proteolytic activity in P. aeruginosa, a major virulence factor of this opportunistic pathogen, is a promising starting point for the development of a novel anti-infective agent.
Acknowledgments
Financial support for this research came from the Land Niedersachsen und Nordrhein-Westfalen, Forschungsschwerpunkt Meeresbiotechnologie, and the Fond der Chemischen Industrie (grant 164 357) and is gratefully acknowledged.
REFERENCES
- 1.Andersen, J. B., A. Heydorn, M. Hentzer, L. Eberl, O. Geisenberger, B. B. Christensen, S. Molin, and M. Givskov. 2001. gfp-based N-acyl homoserine-lactone sensor systems for detection of bacterial communication. Appl. Environ. Microbiol. 67:575-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlé, J. S., and C. J. Christophersen. 1979. Bromo-substituted physostigmine alkaloids from a marine bryozoa, Flustra foliacea. J. Am. Chem. Soc. 101:4012-4013. [Google Scholar]
- 4.Christophersen, C. 1991. Evolution in molecular structure and adaptive variance in metabolism. Comp. Biochem. Physiol. B Comp. Biochem. 98:427-432. [DOI] [PubMed] [Google Scholar]
- 5.Christophersen, C., and J. S. Carlé. 1978. Chemical signals from a marine bryozoa. Naturwissenschaften 65:440-441. [Google Scholar]
- 6.De Nys, R., A. D. Wright, G. M. König, and O. Sticher. 1993. New halogenated furanones from the marine alga Delisea pulchra (cf. fimbriata). Tetrahedron 49:11213-11220. [Google Scholar]
- 7.Dworjanyn, S. A., R. De Nys, and P. D. Steinberg. 1999. Localisation and surface quantification of secondary metabolites in the red alga Delisea pulchra. Mar. Biol. 133:727-736. [Google Scholar]
- 8.Dyrynda, P. E. J. 1985. Functional allelochemistry in temperate waters: chemical defences of bryozoans, p. 95-100. In C. Nielsen and G. P. Larwood, (ed.), Bryozoa: Ordovician to recent. Olsen and Olsen, Fredensborg, Germany.
- 9.Eberl, L. 1999. N-acyl-homoserine lactone-mediated gene regulation in Gram-negative bacteria. Syst. Appl. Microbiol. 22:493-506. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the luxR-luxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 11.Geisenberger, O., M. Givskov, K. Riedel, N. Hoiby, B. Tümmler, and L. Eberl. 2000. Production of N-acyl-l-homoserine lactones by P. aeruginosa isolates from chronic lung infections associated with cystic fibrosis. FEMS Microbiol. Lett. 184:273-278. [DOI] [PubMed] [Google Scholar]
- 12.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hentzer, M., K. Riedel, T. B. Rasmussen, A. Heydorn, J. B. Andersen, M. R. Parsek, S. A. Rice, L. Eberl, S. Molin, N. Høiby, S. Kjelleberg, and M. Givskov. 2002. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148:87-102. [DOI] [PubMed] [Google Scholar]
- 14.Holloway, B. W. 1955. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13:572-581. [DOI] [PubMed] [Google Scholar]
- 15.Holst, P. B., U. Anthoni, C. Christophersen, and P. H. Nielsen. 1994. Two alkaloids, flustramine E and debromoflustramine B, from the marine bryozoan Flustra foliacea. J. Nat. Prod. 57:997-1000. [DOI] [PubMed] [Google Scholar]
- 16.Kazlauskas, R., P. Murthy, R. Quinn, and R. Wells. 1977. A new class of halogenated lactones from the red alga Delisea fumbriata. Tetrahedron Lett. 1:37-40. [Google Scholar]
- 17.Laycock, M. V., J. L. C. Wright, J. A. Findlay, and A. D. Patil. 1986. New physostigmine related bromoalkaloids from the marine bryozoan Flustra foliacea. Can. J. Chem. 64:1312-1316. [Google Scholar]
- 18.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 19.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters, L., G. M. König, H. Terlau, and A. D. Wright. 2002. Four new bromo-tryptamine derivatives from the marine bryozoan Flustra foliacea. J. Nat. Prod. 65:1633-1637. [DOI] [PubMed] [Google Scholar]
- 21.Ponasik, J. A., S. Conova, D. Kinghorn, W. A. Kinney, D. Rittschof, and B. Ganem. 1998. Pseudoceratidine, a marine natural product with antifouling activity: synthetic and biological studies. Tetrahedron 54:6977-6986. [Google Scholar]
- 22.Pukall, R., I. Kramer, M. Rohde, and E. Stackebrandt. 2001. Microbial diversity of cultivatable bacteria associated with the North Sea bryozoan Flustra foliacea. Syst. Appl. Microbiol. 24:623-633. [DOI] [PubMed] [Google Scholar]
- 23.Riedel, K., T. Ohnesorg, K. A. Krogfelt, T. S. Hansen, K. Omori, M. Givskov, and L. Eberl. 2001. N-Acyl-l-homoserine lactone-mediated regulation of the lip secretion system in Serratia liquefaciens MG1. J. Bacteriol. 183:1805-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz, B., J. Sucker, H. J. Aust, K. Krohn, K. Ludewig, P. G. Jones, and D. Döhring. 1995. Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res. 99:1007-1015. [Google Scholar]
- 25.Steidle, A., K. Sigl, R. Schuhegger, A. Ihring, M. Schmid, S. Gantner, M. Stoffels, K. Riedel, M. Givskov, A. Hartmann, C. Langebartels, and L. Eberl. 2001. Visualization of N-acylhomoserine lactone-mediated cell-cell communication between bacteria colonizing the tomato rhizosphere. Appl. Environ. Microbiol. 67:5761-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winson, M. K., S. Swift, P. J. Hill, C. M. Sims, G. Griesmayr, B. W. Bycroft, P. Williams, and G. S. Stewart. 1998. Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol. Lett. 163:193-202. [DOI] [PubMed] [Google Scholar]