Abstract
The yeast Torula corallina is a strong erythritol producer that is used in the industrial production of erythritol. However, melanin accumulation during culture represents a serious problem for the purification of erythritol from the fermentation broth. Melanin biosynthesis inhibitors such as 3,4-dihydroxyphenylalanine and 1,8-dihydroxynaphthalene (DHN)-melanin inhibitors were added to the T. corallina cultures. Only the DHN-melanin inhibitors showed an effect on melanin production, which suggests that the melanin formed during the culturing of T. corallina is derived from DHN. This finding was confirmed by the detection of a shunt product of the pentaketide pathway, flaviolin, and elemental analysis. Among the DHN-melanin inhibitors, tricyclazole was the most effective. Supplementation with tricyclazole enhanced the production of erythritol while significantly inhibiting the production of DHN-melanin and DHN-melanin biosynthetic enzymes, such as trihydroxynaphthalene reductase. The erythrose reductase from T. corallina was purified to homogeneity by ion-exchange and affinity chromatography. Purified erythrose reductase was significantly inhibited in vitro in a noncompetitive manner by elevated levels of DHN-melanin. In contrast, the level of erythrose reductase activity was unaffected by increasing concentrations of tricyclazole. These results suggest that supplemental tricyclazole reduces the production of DHN-melanin, which may lead to a reduction in the inhibition of erythrose reductase and a higher yield of erythritol. This is the first report to demonstrate that melanin biosynthesis inhibitors increase the production of a sugar alcohol in T. corallina.
Erythritol, a four-carbon polyol, is a naturally occurring substance and is widely distributed in nature (11). Like most other polyols, it acts as a metabolite or storage compound for seaweed, mushrooms, and fruits. Erythritol occurs frequently in fermented foods, including wines and beers, and in processed vegetables, such as soy sauce and oriental miso bean paste (35, 36, 49). A 10% (wt/vol) solution of erythritol has 60 to 70% of the sweetness of sucrose. Erythritol has very high negative enthalpy, thus providing a strong cooling effect when dissolved (11). Since erythritol tastes sweet but lacks aftertaste, it is used in combination with intense sweeteners that have a bitter aftertaste, such as aspartame. Erythritol has been used safely as a noncariogenic food sweetener in many countries. The property of noncariogenicity is due to the inability of bacteria that cause dental caries to use erythritol as a fermentation substrate (10).
Erythritol can be synthesized from dialdehyde starch by chemical reaction at high temperatures in the presence of a nickel catalyst (30). However, this process has not been industrialized because of its low efficiency. Erythritol can also be produced by microbial methods with osmophilic yeasts and some bacteria (1, 9, 12, 13, 14, 18, 20, 22, 25, 27, 48) and has been produced commercially with a mutant of Aureobasidium that produces erythritol with a high yield (44%, wt/wt) (14). Erythritol is synthesized from erythrose-4-phosphate, which is an intermediate in the pentose phosphate cycle, by dephosphorylation and the subsequent reduction of erythrose. Erythrose reductase, which catalyzes the final step in this pathway, is a key enzyme in the biosynthesis of erythritol (15, 39).
Recently, we isolated a novel erythritol-producing microorganism, which we identified as the yeast strain Torula corallina KCCM-10171 (17). A mutant of T. corallina overproduced erythritol with a yield of 48.9% (wt/wt) and did not produce glycerol and ribitol by-products, which made this strain highly suitable for industrial-scale production of erythritol (20, 21, 25). However, a serious problem was encountered during purification, in that the fermentation broth contained contaminants, particularly melanin. Melanin represents a serious hindrance to the industrial production of erythritol by T. corallina. Melanin lowers the yield of erythritol, necessitates several additional purification steps (including chromatography), lowers the purification yield, and thus increases production costs.
Melanins, which are dark brown to black pigments of high molecular weight, are formed by oxidative polymerization and are widely dispersed among animals, plants, and microorganisms (2, 8, 29). The pigment is not essential for growth and development but enhances the survival and competitiveness of the species under conditions of UV and electromagnetic irradiation, desiccation, and extreme temperature (2). Most fungal melanins are derived from the precursor molecule 1,8-dihydroxynaphthalene (DHN) and are known as DHN-melanins; the biosynthetic pathway which furnishes DHN has been termed the pentaketide pathway (2). The biosynthesis of DHN-melanin via the pentaketide pathway has been studied intensively. The late stages of the pathway, in which 1,3,6,8-tetrahydroxynaphthalene (4HN) is converted in succession to scytalone, vermelone, and DHN, are well understood (24, 26, 45, 46, 47). The tyrosine ring represents a second source of precursor molecules, either by itself or as dihydroxyphenylalanine (DOPA), and since the oxidized product of DOPA, dopaquinone, is able to cyclize to form a 5,6-dihydroxyindole ring, tyrosine or DOPA melanin typically contains indole rings.
Many microorganisms accumulate melanin (2, 5, 8, 29, 31), which can account for a major portion of the dry weight of the cell. Although melanins impede the recovery of some metabolites, such as sugar alcohols and antibiotics, relatively little is done to minimize melanin production during culture, and little is known about the effects of melanin on sugar alcohol production. We reported previously that erythritol production was improved by controlling the glucose concentration in a fed-batch culture of T. corallina (25) and by supplementing the culture with inositol, phytic acid (21), and Mn2+ and Cu2+ (20). The inhibition and effects of by-products, such as melanin, however, have not yet been studied.
In this study, we decreased melanin production by using melanin biosynthesis inhibitors. Thus, we attempted to facilitate erythritol purification while increasing overall erythritol production by blocking the carbon flow into melanin synthesis. We discovered that DHN-melanin was a major by-product of erythritol production by T. corallina. We quantified DHN-melanin production during the fermentation process and examined its effect on the activity of erythrose reductase, a key enzyme in erythritol biosynthesis.
MATERIALS AND METHODS
Microorganism, media, and chemicals.
T. corallina was isolated from a 40% sucrose solution at the Bolak Co. R&D Center (Osan, Korea) (17). Growth medium (200 g of glucose and 10 g of yeast extract per liter) was used for initial shake flask cultivation at 30°C. The production medium contained 200 g of glucose, 10 g of yeast extract, 10 mg of MnSO4 · 4H2O, and 2 mg of CuSO4 · 5H2O per liter (20) and melanin biosynthesis inhibitors (Table 1). Medium components were purchased from Difco (Detroit, Mich.) and Wako Pure Chemical Industries (Osaka, Japan). The DOPA-melanin biosynthesis inhibitors such as niacin, hydroquinone, and kojic acid were purchased from Sigma Chemical Co. (St. Louis, Mo.). Tricyclazole, pyroquilon, and phthalide were obtained from Eli Lilly Research Laboratories (Greenfield, Ind.), Ciba-Geigy Ltd. (Basel, Switzerland), and Kureha Chemical Ind. Co. Ltd. (Tokyo, Japan), respectively. Tricyclazole and pyroquilon were dissolved in ethanol. The concentration of ethanol in the culture medium was not more than 0.1% (vol/vol).
TABLE 1.
Comparison of erythritol production and melanin formation by T. corallina with various melanin biosynthesis inhibitors in flask culturesa
| Inhibitor | Erythritol (g/liter) at inhibitor concn (mg/liter):
|
Melanin (mg/liter)c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 10 | 20 | 30 | 40 | 50 | ||
| None (control)b | 54.8 | 60.5 | |||||||
| Arbutin | 43.6 | 19.4 | 16.2 | 7.1 | —d | — | — | — | |
| Kojic acid | 51.2 | 23.0 | 16.4 | 7.3 | — | — | — | — | |
| EDTA | 54.0 | 55.2 | 55.5 | 53.3 | 52.3 | 48.5 | 18.8 | 60.0 | |
| Niacin | 53.4 | 55.0 | 53.2 | 54.6 | 54.1 | 48.8 | 43.9 | 59.8 | |
| Hydroquinone | 53.3 | 52.9 | 54.6 | 54.7 | 53.8 | 53.4 | 52.6 | 58.5 | |
| Tricyclazole | 54.5 | 62.4 | 66.8 | 69.2 | 82.7 | 75.2 | 64.7 | 12.3 | |
| Phthalide | 53.4 | 55.6 | 58.4 | 66.1 | 65.6 | 58.6 | 51.5 | 16.4 | |
| Pyroquilon | 55.2 | 60.3 | 67.2 | 70.5 | 63.5 | 54.4 | 45.1 | 15.9 | |
Each value represents the mean of triplicate measurements and varied from the mean by not more than 10%.
Ethanol (0.1%, vol/vol) without inhibitors was added to the medium.
The concentration of melanin biosynthesis inhibitors was set at 30 mg/liter.
—, not detectable.
Culture conditions.
A single colony of T. corallina was inoculated into a 20-mm-diameter test tube containing 5 ml of growth medium and incubated for 48 h at 30°C with agitation at 250 rpm. Five milliliters of the broth was transferred to a 500-ml baffled flask containing 100 ml of growth medium, and the culture was incubated at 30°C and 250 rpm for 24 h. This seed culture was then transferred into a baffled flask. Flask experiments were performed with 500-ml baffled flasks that contained 100 ml of production medium, and the cultures were grown at 34°C and 250 rpm in the dark for 144 h. The initial pH of the production medium was adjusted to 5.5 (17).
Isolation and determination of melanins.
Cells were harvested from the culture broth by centrifugation, and the supernatant was assayed for extracellular melanin and melanogenic enzyme activity. Melanin pigment in the culture supernatant was determined by measuring the A585 in a Beckman DU30 spectrophotometer (26).
Cell wall-associated melanin was extracted as described previously (33, 43). Melanized and nonmelanized T. corallina cells were collected from a 5-day-old culture, washed once with 1.0 M sorbitol in 0.1 M sodium citrate (pH 5.0), and resuspended in 5 ml of the same solution. Novo-zyme 234 was added to the cell suspension at a concentration of 10 g per liter, and the suspension was incubated for 1 h at 30°C to generate protoplasts. The protoplasts from melanized and nonmelanized cells were collected by centrifugation and suspended in 4 M guanidinium isothiocyanate for 30 min at room temperature. The cell debris was collected by centrifugation and suspended in 6 N HCl for 30 min at 100°C. After guanidinium isothiocyanate and HCl treatments, the nonmelanized cells dissolved completely. However, a black pellet remained in the melanized cells. The black pellet contained particulate material that was dialyzed against distilled water for 10 days and lyophilized. Although the purity of the melanin could not be established unequivocally because of the lack of knowledge about the structure of any melanin, the melanins used in this study consistently gave the same C/N/O ratios for the same melanin in quantitative analyses that were carried out according to the methods of Rosas et al. (34), which strongly suggests that the preparations were free of other cellular components. Carbon and nitrogen quantitative analysis was performed with a Perkin-Elmer 2400 CHN elemental analyzer (PerkinElmer, Shelton, Conn.).
Cell extract preparation and membrane isolation.
Cells from the culture broth were harvested by centrifugation. After washing with 0.1 M Tris-HCl buffer (pH 7.8), cells were resuspended in disruption buffer containing 20 mM Tris-HCl (pH 7.8), 10 mM MgCl2, and 1 mM phenylmethylsulfonyl fluoride. The cell suspension was disrupted by grinding with glass beads (0.5 mm in diameter; Sigma Chemical Co.). The cell extract was obtained by removing the ruptured cells by centrifugation at 10,000 × g for 30 min and used for the determination of erythrose reductase activity and melanogenic enzyme activity. The membranes were isolated by ultracentrifugation of lysates at 100,000 × g for 2.0 h, suspended in 20 mM Tris-HCl buffer (pH 7.8), and then subjected to successive washes with buffer containing 1.0, 2.0, and 4.0 M NaCl. After each wash, the suspension was centrifuged for 2 h at 100,000 × g, and the membrane fraction was resuspended to a protein concentration of 12 mg/ml in buffer without NaCl. n-Dodecyl-β-d-maltoside (1.5%, wt/vol) was then added, and the membranes were extracted with a tissue homogenizer and centrifuged at 120,000 × g for 2.0 h, and the supernatant was used for further analysis (41).
Purification of erythrose reductase.
Cell extracts were fractionated by ammonium sulfate precipitation. The fraction precipitated by between 40 and 70% saturation with ammonium sulfate was collected by centrifugation and dissolved in 50 mM Tris-HCl buffer (pH 7.8). Following removal of insoluble material by centrifugation at 10,000 × g for 1 h, the enzyme solution was dialyzed against the same buffer at 4°C for 24 h. Dialyzed enzyme solution was placed on a DEAE-Toyopearl 650S column (1.4 by 20 cm) equilibrated with 50 mM Tris-HCl buffer at pH 7.8. Protein was eluted by a linear 0 to 0.5 M NaCl gradient in the same buffer. The active fractions were pooled. The enzyme solution was put on a Cibacron Blue 3GA affinity column (1.4 by 20 cm) which had been equilibrated with the same buffer. The column was eluted with a linear gradient of 0 to 1 M NaCl in the same buffer. The active fractions were pooled, concentrated, and dialyzed against the same buffer, concentrated with Centricon (Amicon, Bedford, Mass.), and then used as the purified enzyme in the following experiments.
Enzyme assay.
Trihydroxynaphthalene reductase (3HNR) activity was assayed by the method of Jordan et al. (16). The assay mixture (1 ml total volume) contained 100 mM morpholinepropanesulfonic acid (MOPS)-NaOH, 0.1 mM NADPH, the 3HNR enzyme extract (0.1 ml), and 20 μM phenanthrene quinone (PQ), at pH 7.0 and 25°C. Dimethyl sulfoxide (5 μl) was added, in the absence or presence of inhibitor, 10 s before the reactions were initiated with PQ in 5 μl of dimethyl sulfoxide. The initial rates were determined by monitoring NADPH consumption at 340 nm with a spectrophotometer. One unit of enzyme represents the amount of enzyme that caused an initial rate of decrease of 1 μmol of NADPH per minute.
Laccase activity was assayed with syringaldazine as the chromogenic substrate (28). Syringaldazine was added to the crude cellular extracts, and the solution was incubated at 37°C for 10 min. Laccase activity was measured as the oxidized product of syringaldazine at an absorbance of 525 nm. One unit was defined as the amount of enzyme that oxidized 1 μmol of syringaldazine per min at 37°C.
Peroxidase activity was measured by the assay method with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as the substrate (7). Peroxidase samples (20 μl) were added to a spectral cuvette with a 1-cm optical path length containing 0.91 mM ABTS in 0.1 M phosphate buffer (pH 7.0) and 2.5 mM H2O2 in a final volume of 2 ml. The rate of change in absorbance at 403 nm was measured spectrophotometrically at 25°C. One unit of enzyme represented the amount of enzyme that caused an initial rate of decrease of 1 μmol of ABTS per min.
Erythrose reductase activity was determined by either monitoring the oxidation of NADPH in a spectrophotometric cuvette at 340 nm and 37°C (6) or measuring erythrose and erythritol directly in the reaction mixture by high-pressure liquid chromatography (HPLC) coupled to a Bio-LC ED50A electrochemical detector (Dionex, Calif.). The cuvette for spectrophotometric assay contained 0.5 ml of 50 mM Tris-HCl buffer (pH 7.8), 0.1 ml of 0.1 M 2-mercaptoethanol, 0.1 ml of enzyme solution, and 0.2 ml of 3.4 mM NADPH. This reaction mixture was allowed to stand for 1 min to eliminate the endogenous oxidation of NADPH. The reaction was started by the addition of 0.1 ml of 0.2 M d-erythrose. One unit of enzyme represents the amount of enzyme that caused an initial rate of decrease of 1 μmol of NADPH per minute. Activities are expressed as units per milligram of protein. All of the spectrophotometric assays were performed with a Beckman model DU3 spectrophotometer. Tricyclazole and melanin were dissolved in ethanol and NaOH, respectively. Ethanol (0.1%, vol/vol) and NaOH (5 mM) in the reaction mixture were confirmed not to interfere with the assay procedure.
PAGE.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (19). Native PAGE was performed on a 10% polyacrylamide gel without SDS. Protein bands were stained with Coomassie brilliant blue R250. Erythrose reductase activity staining on the polyacrylamide gel was done by use of a modification of the procedure of Birken (4). The staining mixture used for the detection of NADP-erythritol activity consisted of 40 ml of 0.1 M Tris-HCl buffer (pH 7.8), 25 mg of tetranitroblue tetrazolium, 3 mg of phenazine methosulfate, 30 mg of NADP, and 2 ml of 0.5 M erythritol. Gels were incubated in appropriate activity staining solution for 15 min, washed in water, and stored in 7% acetic acid.
Analytical methods.
Protein concentrations were determined by the method of Lowry et al. (22) with bovine serum albumin as the standard. The concentrations of erythritol and glucose were determined by HPLC coupled to a refractive index detector (model 2410; Waters, Milford, Mass.) and a KR100-10NH2 column (4.6 by 250 mm; Kromasil, Sweden). The mobile phase was acetonitrile-water (80:20, vol/vol), and the flow rate was 1.5 ml/min. The intracellular levels of tricyclazole were measured by HPLC coupled to a UV detector (model 486; Waters) on a Wakosil-II 5C18 AR column (4.6 by 150 mm; Wako, Japan). Water and acetonitrile were used as the mobile phase in gradient mode (0 to 15 min, CH3CN 30 to 60%; 15 to 20 min, CH3CN 60%). For chromatographic standards, the melanin metabolites flaviolin and 2-hydroxyjuglone were synthesized as described previously (45). Then the culture extracts were analyzed for the presence of flaviolin and 2-hydroxyjuglone by ethyl acetate extraction and thin-layer chromatography as described previously (44).
RESULTS
Effect of melanin biosynthesis inhibitors on erythritol production.
Reduced formation of melanin during T. corallina fermentation is desirable because melanin is a contaminating by-product that both lowers the yield of erythritol and interferes with its purification. In order to inhibit melanin biosynthesis and to increase erythritol production, various melanin biosynthesis inhibitors (DOPA-melanin and DHN-melanin inhibitors) were added to separate flasks containing production medium, which consisted of 200 g of glucose, 10 g of yeast extract, 10 mg of MnSO4 · 4H2O, and 2 mg of CuSO4 · 5H2O per liter (20). Supplementation with DOPA-melanin inhibitors such as arbutin (23), kojic acid (23), EDTA (2), niacin (42), and hydroquinone (23) decreased erythritol production but had no effect on melanin production in T. corallina cultures.
The reduced production of erythritol caused by DOPA melanin inhibitors seemed to be the result of decreased growth of T. corallina. On the other hand, supplementation with DHN-melanin inhibitors, such as tricyclazole, phthalide, and pyroquilon (44), increased erythritol production significantly (Table 1). The optimal concentrations of tricyclazole, phthalide, and pyroquilon that gave reductions in melanin formation and increases in erythritol production were found to be 30, 30, and 20 mg per liter, respectively. Among the melanin biosynthesis inhibitors tested, tricyclazole was the most effective compound in terms of decreased formation of melanin and increased erythritol production. Cultures that were supplemented with concentrations of up to 10 mg of tricyclazole per liter were black at stationary phase, while the black color did not appear (or the medium turned pale brown) at concentrations above 10 mg of tricyclazole per liter. This suggests that tricyclazole inhibited the formation of melanin in T. corallina cultures.
Melanization of T. corallina cultures in the presence and absence of tricyclazole.
Treatment with 4 M guanidinium isothiocyanate dissolved the majority of the nonmelanized cells but did not dissolve the melanized cells. Since melanin is highly insoluble and resistant to detergents, the melanized cells were resistant to solubilization due to the melanin in their cell walls. Since melanins are also resistant to acid hydrolysis, we digested the guanidinium isothiocyanate-treated melanized and nonmelanized cells with hot 6 N HCl. This treatment resulted in complete solubilization of the cell debris from nonmelanized yeast cells but not of the black particulate material that remained from melanized cells. Five-day-old cultures of T. corallina were grown at 34°C in the presence and absence of 30 mg of tricyclazole per liter, then washed, and divided in half. One portion was lyophilized to obtain the dry cell weight, and the other portion was treated with guanidinium isothiocyanate and HCl for the preparation of particulate melanin. From calculations of the weight of the lyophilized particulate melanin material divided by the weight of lyophilized cells, we estimated that melanin constituted 11.2% (112 mg) and 0.9% (9 mg) of the dry weight of the cells (1 g) in the absence and presence of 30 mg of tricyclazole per liter, respectively.
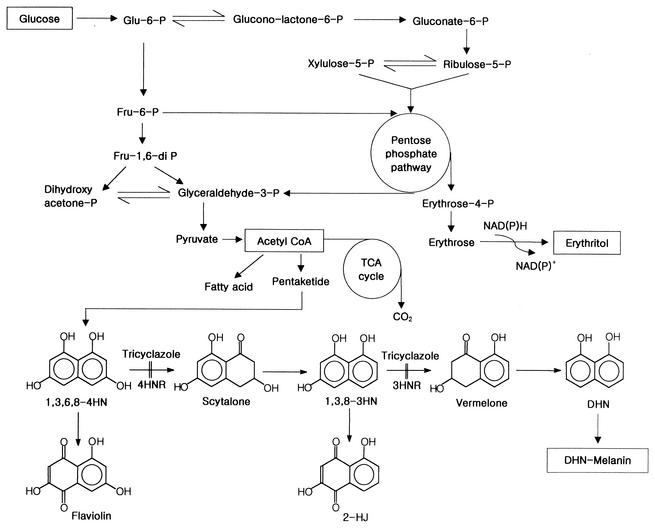
Elemental quantitative analyses were performed to determine the relative elemental composition of the melanins. The absence of nitrogen (<0.1% N) in melanin was confirmed by elemental analysis. Tricyclazole inhibits two reductase reactions in the melanin pathways of fungi (Fig. 1). One of the reactions reduces 1,3,6,8-4HN to scytalone, and the other reduces 1,3,8-3HN to vermelone. Inhibition of these steps causes the accumulation of melanin intermediates and the shunt product (38). In the present study, T. corallina cultures were treated with tricyclazole to block melanin biosynthesis. The culture medium turned pale brown, and small amounts of flaviolin, which is a shunt product of the DHN-melanin pathway, were detected by the procedure described previously (40). Though this level of flaviolin cannot account for the carbon flux that does not make it to melanin, shunt metabolites, such as flaviolin and 2-hydroxyjuglone, are considered to be indicators of pentaketide melanin biosynthesis, since they have been observed in virtually all species of tricyclazole-treated fungi in which melanin biosynthesis occurs via the DHN pathway (2, 5). Our findings that (i) only DHN inhibitors inhibited melanin formation, (ii) flaviolin accumulated in tricyclazole-treated cultures, and (iii) melanin lacked nitrogen suggest that the melanin that accumulated during T. corallina culture was derived from the pentaketide pathway.
FIG. 1.
Proposed erythritol biosynthetic pathway (15, 33, 37) and the pentaketide pathway for melanin biosynthesis in fungi (25, 42, 43, 44). P, phosphate; CoA, coenzyme A; TCA, tricarboxylic acid; 4HNR, tetrahydroxynaphthalene reductase; 2-HJ, 2-hydroxyjuglone.
Increased erythritol production in the presence of supplemental tricyclazole.
Flask cultures were performed in production medium containing various concentrations of tricyclazole (0 to 50 mg per liter) to investigate the effects of tricyclazole on cell growth, erythritol production, and melanin production. Tricyclazole supplementation decreased the cell mass but increased erythritol production, which peaked at 30 mg of tricyclazole per liter (Table 2). At 50 mg of tricyclazole per liter, cell growth was significantly decreased and about 40 g of glucose per liter remained after 120 h, while the glucose was completely consumed in cultures that contained up to 30 mg of tricyclazole per liter. The observed decrease in erythritol production at 50 mg of tricyclazole per liter compared to production at 30 mg per liter may have been due to a sharp decrease in cell growth.
TABLE 2.
Effect of tricyclazole on cell mass, erythritol production, and melanin formation in T. corallina culturesa
| Tricylazole (mg/liter) | Mean ± SD
|
|||
|---|---|---|---|---|
| Cell mass (g/liter) | Erythritol concn (g/liter) | Melanin concn
|
||
| Intracellular (mg/g of protein) | Extracellular (mg/liter) | |||
| 0.0b | 16.7 ± 1.1 | 54.4 ± 4.2 | 20.7 ± 1.8 | 60.4 ± 5.6 |
| 1.0 | 16.4 ± 1.3 | 54.0 ± 4.8 | 19.3 ± 1.7 | 56.1 ± 5.2 |
| 5.0 | 16.5 ± 0.9 | 62.1 ± 5.0 | 16.7 ± 1.2 | 45.4 ± 4.0 |
| 10.0 | 16.3 ± 1.1 | 66.2 ± 5.2 | 13.4 ± 0.9 | 36.8 ± 2.7 |
| 20.0 | 15.8 ± 0.7 | 73.0 ± 6.5 | 7.1 ± 0.6 | 20.8 ± 1.6 |
| 30.0 | 14.9 ± 0.9 | 82.6 ± 7.0 | 4.3 ± 0.4 | 12.6 ± 1.1 |
| 40.0 | 13.0 ± 1.1 | 75.1 ± 6.0 | 3.4 ± 0.3 | 8.7 ± 0.9 |
| 50.0 | 11.2 ± 1.0 | 63.8 ± 5.1 | 3.1 ± 0.3 | 7.6 ± 0.7 |
Values are means for triplicate samples.
Ethanol (0.1%, vol/vol) without tricyclazole was added to the medium.
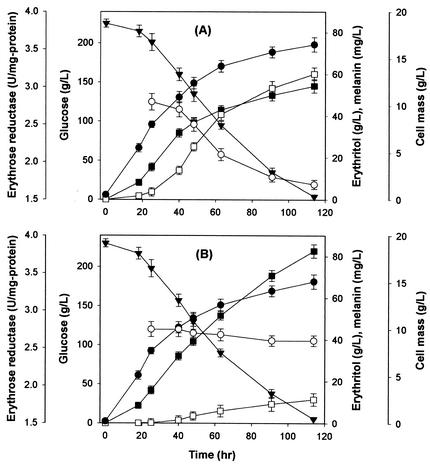
Time course experiments were performed to investigate in more detail the effects of tricyclazole on the production of erythritol and melanin. A control culture that lacked the melanin biosynthesis inhibitor and a culture that contained 30 mg of tricyclazole per liter were established (Fig. 2). There were no significant differences in glucose consumption between the two cultures. The cultures without tricyclazole exhibited a slightly higher cell growth, which suggests that tricyclazole inhibits the growth of T. corallina. The erythritol level in the culture with tricyclazole was approximately the same as that in the culture without tricyclazole for the first 40 h. After 40 h of culture, erythritol production in the culture with tricyclazole (Fig. 2B) was significantly enhanced, whereas melanin formation was noticeably suppressed from early growth phase compared with the culture that lacked tricyclazole (Fig. 2A). The decrease in erythritol production and erythrose reductase activity seemed to be correlated with the increase in fungal melanin formation (Fig. 2A). The final concentration of erythritol that was produced in the presence of supplemental tricyclazole increased by 52%, while the final melanin concentration was reduced to 19%.
FIG. 2.
Cell growth, glucose consumption, erythritol production, and DHN-melanin formation in a culture without tricyclazole (A) and with 30 mg of tricyclazole per liter (B) by T. corallina. Symbols: •, cell growth; ○, erythrose reductase; ▾, glucose consumption; ▪, erythritol production; □, melanin formation. Each value represents the mean of triplicate measurements and varied from the mean by not more than 10%. Ethanol (0.1%, vol/vol) without tricyclazole was added to the control culture (A).
Effect of tricyclazole on melanin and erythritol biosynthetic enzymes during cell culture.
The effects of tricyclazole on melanin and erythritol biosynthetic enzymes such as 3HNR, laccase, peroxidase, and erythrose reductase were determined for cultures of T. corallina. The activity of 3HNR, an enzyme involved in DHN-melanin biosynthesis, was much lower in cultures containing 30 mg of tricyclazole per liter than in control cultures. The concentration of tricyclazole in the cell extracts of the cultures treated with 10, 20, 30, and 50 mg of tricyclazole per liter was 0.37, 0.87, 1.28, and 1.92 mg per liter, respectively. 3HNR activity decreased as the intracellular level of tricyclazole increased. Although other reductases besides 3HNR may be partially involved in the reduction of PQ, this result is consistent with the mechanism that tricyclazole inhibits 3HNR dose dependently (37).
Laccase and peroxidase polymerizing DHN into DHN-melanin also showed lower activities (Table 3). Laccase and peroxidase in the assays treated with 5.0 mg of tricyclazole per liter showed 45 and 40%, respectively, of the activity of the enzymes in the assay not treated with tricyclazole. Although the assays were carried out with the crude enzymes, the results suggest that tricyclazole may inhibit the enzymes in T. corallina. Further investigation with purified laccase and peroxidase from T. corallina is required to understand the mechanism by which tricyclazole inhibits the enzymes. On the other hand, the activity of erythrose reductase, which converts erythrose to erythritol, increased with increasing concentrations of tricyclazole. The specific activity of erythrose reductase peaked at 50 mg of tricyclazole per liter, whereas the total reductase activity was maximal at 30 mg of tricyclazole per liter. Since the specific activity multiplied by the cell concentration gives the total activity, the lower concentration of cells at 50 mg of tricyclazole per liter resulted in a lower value for the total erythrose reductase activity. Therefore, erythritol production was maximal at 30 mg of tricyclazole per liter, as shown in Table 2.
TABLE 3.
Effect of tricyclazole on the activity of enzymes involved in melanin and erythritol biosyntheses in T. corallina culturesa
| Tricyclazole (mg/liter) | Mean sp act (U/mg of protein) ± SD
|
|||
|---|---|---|---|---|
| 3HNR | Laccase | Peroxidase | Erythrose reductase | |
| 0.0b | 45.4 ± 2.7 | 37.5 ± 2.6 | 397.8 ± 27.8 | 1.84 ± 0.10 |
| 10.0 | 13.3 ± 0.6 | 33.1 ± 2.3 | 288.9 ± 17.3 | 1.75 ± 0.13 |
| 20.0 | 7.3 ± 0.5 | 27.3 ± 1.9 | 197.3 ± 13.8 | 1.90 ± 0.16 |
| 30.0 | 2.1 ± 0.2 | 18.7 ± 1.3 | 139.3 ± 11.1 | 2.37 ± 0.20 |
| 50.0 | 1.2 ± 0.1 | 12.6 ± 1.0 | 127.8 ± 10.2 | 2.58 ± 0.19 |
Values are means for triplicate samples.
Ethanol (0.1 %, vol/vol) without tricyclazole was added to the medium.
Purification of erythrose reductase.
Crude extracts of T. corallina were prepared from 4-day-old cultures, which had high levels of erythrose reductase. Fractionation with ammonium sulfate increased by twofold the specific activity of erythrose reductase, with 76% recovery of activity. The active fractions were applied to a DEAE-Toyopearl 650S column, and erythrose reductase was eluted with a 0 to 0.5 M NaCl linear gradient. The erythrose reductase activity was recovered at approximately 0.2 M NaCl. Three peaks with protein content and one peak with erythrose reductase activity were resolved by Cibacron Blue 3GA affinity column chromatography (data not shown). The first large peak and the third peak did not have erythrose reductase activity, whereas the second small peak, which eluted as a single peak at approximately 0.7 M NaCl, showed erythrose reductase activity.
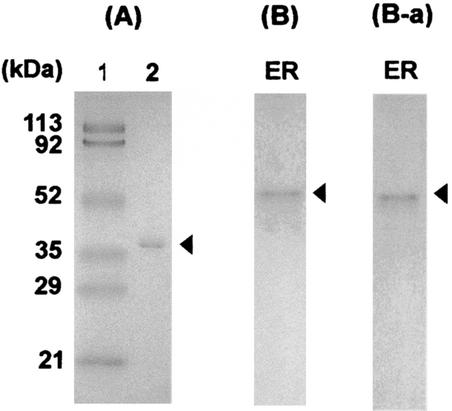
The active fractions were collected, concentrated, and dialyzed in a stirred cell (Amicon, Bedford, Mass.). The chromatographic procedure resulted in a 53.5-fold purification of erythrose reductase, a recovery rate of 10.8%, and specific activity of 13.8 units/mg of protein. As shown in Fig. 3, the purified enzyme produced a single protein band by PAGE in both the presence (A) and absence (B) of SDS. The molecular weight of the erythrose reductase as determined by SDS-PAGE and gel filtration chromatography on a Shodex Protein KW-802.5 (Shiseido, Tokyo, Japan) column attached to an HPLC system was 35.4 kDa and 71.0 kDa, respectively, indicating that the enzyme is homodimeric. Activity staining (4) of the nondenaturing gel showed a single band with the same mobility as that of the protein band (Fig. 3B-a). These results indicate that the purified enzyme is homogeneous. This enzyme solution was used in the erythrose reductase activity assays.
FIG. 3.
(A) SDS-PAGE (10% acrylamide) shows a homogeneous enzyme preparation after the final purification step. Lane 1, molecular size markers; lane 2, purified erythrose reductase. (B) Native PAGE of the fraction obtained by affinity chromatography. (B-a) In vitro activity staining after native PAGE. Arrowheads indicate the erythrose reductase.
Effects of tricyclazole and DHN-melanin on erythrose reductase.
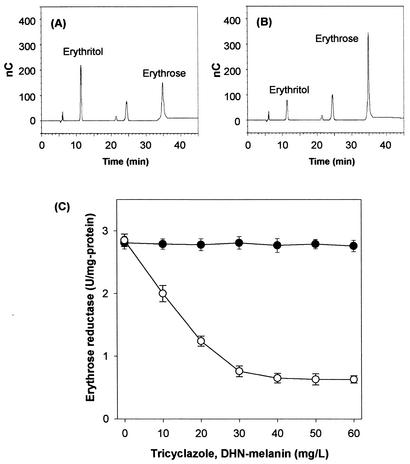
Erythritol production was significantly inhibited when the concentration of melanin exceeded 20 mg per liter (Fig. 2). In order to investigate in detail the role of tricyclazole and melanin in erythritol production, the in vitro activity of purified erythrose reductase was assayed in reaction mixtures that contained increasing concentrations of tricyclazole or DHN-melanin (Fig. 4). Erythrose reductase activity remained unchanged with increasing concentrations of tricyclazole but was inhibited significantly as the concentration of DHN-melanin increased (Fig. 4). DHN-melanin inhibitors such as tricyclazole, phthalide, and pyroquilon did not affect enzyme activity when added at concentrations of 10 to 50 mg per liter. These results suggest that the supplementation of T. corallina cultures with tricyclazole, phthalide, and pyroquilon did not directly affect the activity of erythrose reductase.
FIG. 4.
HPLC analysis of the reaction products of T. corallina erythrose reductase to determine enzyme inhibition by DHN-melanin. The enzyme (1 U) was incubated in a mixture of buffer and erythrose (1 mM) at 37°C for 1 min without (A) and with 30 μg of melanin per ml (B). The reaction mixture was diluted, filtered through a 0.2-μm-pore-size membrane, and subjected to HPLC analysis (Bio-LC ED50A electrochemical detector, Bio-LC Quaternary Gradient Pump, CarboPacTM MA1 column; Dionex). The sample was eluted isocratically with 500 mM aqueous NaOH at a flow rate of 0.4 ml/min. The peaks were assigned based on the retention times of the standard samples. nC, nanocoulomb. (C) Effect of tricyclazole (•) and DHN-melanin (○) on the activity of purified erythrose reductase from T. corallina. Tricyclazole and DNH-melanin were dissolved in ethanol and NaOH, respectively. Control experiments (0.1% ethanol or 5 mM NaOH) without inhibitor were performed.
DHN-melanin was found to be an inhibitor of erythrose reductase. Since DHN-melanin inhibits erythrose reductase, erythrose can accumulate in cultures not treated with tricyclazole. Erythrose, however, did not accumulate in the cultures not treated with tricyclazole, as determined by HPLC coupled to a Bio-LC ED50A electrochemical detector (Dionex), probably because intracellular erythrose formed via the pentose phosphate pathway is recycled through the pentose phosphate pathway and consumed to synthesize several biomaterials such as aromatic amino acids and vitamin B6.
The cellular location of the enzyme was investigated as previously described (41). Approximately 43.6% (4.8 U of total activity, 0.045 U/mg of protein) and 56.4% (6.2 U of total activity, 0.032 U/mg of protein) of the erythrose reductase were found in the cytoplasm and membrane fraction, respectively. The distribution of the erythrose reductase activity between the cytoplasm and membrane fractions probably indicates that the enzyme is weakly bound to the membrane. Although further study is required to fully elucidate how melanin contacts the erythrose reductase, these results imply that melanin can contact the enzyme near the membrane.
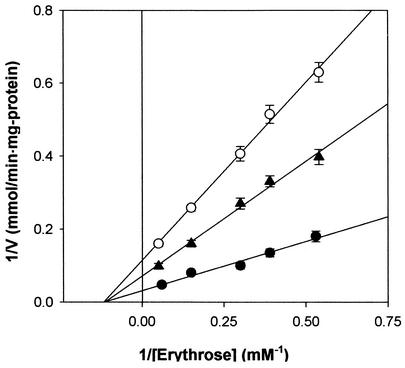
The kinetic parameters of erythrose reductase were determined by initial rate determination followed by Lineweaver-Burk plotting. The Km and Vmax values for the reduction of erythrose in the plot were found to be 7.12 mM and 26 μmol/min per mg of protein, respectively. The effect of DHN-melanin concentration on erythrose reductase inhibition was studied with a Lineweaver-Burk plot. In vitro assays demonstrated that DHN-melanin specifically inhibited erythrose reductase in a noncompetitive manner and that the Ki value for DHN-melanin was 12.5 mg per liter by the secondary plot for noncompetitive inhibition with erythrose (Fig. 5).
FIG. 5.
Lineweaver-Burk plots of inhibition of erythrose reductase by DHN-melanin in T. corallina. Symbols: •, without DHN-melanin; ▴, with 10 mg of DHN-melanin per liter; ○, with 20 mg of DHN-melanin per liter. Each value represents the mean of triplicate measurements and varied from the mean by not more than 10%.
DISCUSSION
Many organisms produce melanin as a metabolic by-product of oxidative metabolism. Certain fungi produce significant quantities of melanin, which can account for a major part of the dry weight of the cell, e.g., as much as 30% for spores of Agaricus bisporus (31) and 15% for cells of Cryptococcus neoformans (43), which represents a considerable pressure on material and energy resources. Melanin is suggested to play an important role in the pathogenesis of infections caused by certain pathogenic fungi. Although melanin accumulates to high levels extracellularly or within cell walls and the melanin biosynthetic pathway has been uncovered in various species, most studies have been conducted into its role in pathogenesis. Few studies have been conducted into the effect of melanin on the production of useful metabolites, such as erythritol.
We report here that the DHN pathway in T. corallina is responsible for cell wall melanization and the inhibition of a key enzyme in erythritol production. DHN-melanin is present in many fungi and originates from acetate via the pentaketide pathway (Fig. 1) (2, 29, 45, 46). The starting molecule of the pentaketide (DHN-melanin) pathway, 4HN, is formed by the head-to-tail joining and cyclization of acetate molecules. Thus, the inhibition of DHN-melanin formation should allow the conservation of acetyl-coenzyme A for use elsewhere, for example, in the supply of maintenance energy and reduction equivalent [NAD(P)H] and in the metabolic scheme for erythritol production (Fig. 1), thereby facilitating the efficient production and purification of erythritol.
Catabolite inhibition is a general phenomenon, even though the scale of the inhibitory effects varies widely. The inhibition of by-product formation during biosynthetic processes has attracted interest as a way to minimize the effects of catabolite repression. Large amounts of melanin accumulate in T. corallina cultures during the production of erythritol. Furthermore, the levels of melanin are reduced significantly in cultures that are supplemented with DHN-melanin biosynthesis inhibitors compared to those of inhibitor-deficient cultures. We have demonstrated that tricyclazole stimulates the synthesis of erythritol in T. corallina, and we propose that this stimulatory effect may be mediated by the inhibition of DHN-melanin, which is a noncompetitive inhibitor of erythrose reductase. Regulation of erythritol biosynthesis by DHN-melanin biosynthesis inhibitors (or DHN-melanin levels) has not been reported previously.
We tried to generate a melanin-deficient mutant and compare its erythritol production with that of wild-type T. corallina as an alternative approach to adding a chemical inhibitor that will increase production costs. T. corallina was mutagenized with N-methyl-N′-nitro-N-nitrosoguanidine (100 mg per liter). Many of the mutated cells were morphologically different from the parent but tended to produce melanin. A few colonies that showed albino characteristics were isolated, but these mutants were highly unstable. These results imply that site-directed mutagenesis with genetic tools rather than chemical mutagenesis might be investigated in the future to introduce deficiency in melanin production as a less toxic means. To this end, cloning and characterization of the genes involved in melanin biosynthesis in T. corallina should begin.
In conclusion, supplementation of T. corallina cultures with tricyclazole reduces the production of DHN-melanin, an inhibitor of erythrose reductase. As a result, erythrose reductase activity may be less repressed, which results, at least in part, in a higher yield of erythritol. To the best of our knowledge, this is the first report to propose that melanin biosynthesis inhibitors increase the production of erythritol, a sugar alcohol, in T. corallina. Although DHN-melanin acts as an inhibitor of the erythrose reductase from T. corallina, the mechanism by which DHN-melanin inhibits erythrose reductase in vivo remains to be investigated. Studies along these lines would provide a better understanding of erythritol biosynthesis in microorganisms.
Acknowledgments
This research was supported by a grant (IMT 2000 AIT-143) from the Ministry of Information and Communication, Korea. This work was also supported by a grant (M1011100007-01A160000510) from the Ministry of Science and Technology, Korea.
REFERENCES
- 1.Aoki, M. A. Y., G. M. Pastore, and K. Park. 1993. Microbial transformation of sucrose and glucose to erythritol. Biotechnol. Lett. 15:383-388. [Google Scholar]
- 2.Bell, A. A., and M. H. Wheeler. 1986. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 24:411-451. [Google Scholar]
- 3.Bell, A. A., R. D. Stipanovic, and J. E. Puhalla. 1976. Pentaketide metabolities of Verticillium dahliae: identification of (+)-scytalone as a natural precursor to melanin. Tetrahedron 32:1353-1356. [Google Scholar]
- 4.Birken, S., and M. A. Pisano. 1976. Purification and properties of a polyol dehydrogenase from Cephalosporium chrysogenus. J. Bacteriol. 125:225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, M. J., and A. W. Day. 1998. Fungal melanins: a review. Can. J. Microbiol. 44:1115-1136. [Google Scholar]
- 6.Chang, C., and S. G. Knight. 1966. d-Xylose reductase and xylitol dehydrogenase from Penicillium chrysogenum. Methods Enzymol. 9:188-193. [Google Scholar]
- 7.Childs, R. E., and W. G. Bardsley. 1975. The steady state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulfonic acid) as chromogen. Biochem. J. 145:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Hoog, G. S. 1993. Evolution of black yeasts: Possible adaptation to the human host. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 63:48-56. [DOI] [PubMed] [Google Scholar]
- 9.Fernanda, N. M., and M. S. Da Costa. 1985. The accumulation of polyols by the yeast Debaryomyces hansenii in response to water stress. Can. J. Microbiol. 31:1061-1064. [Google Scholar]
- 10.Goldberg, I. 1994. Functional foods. Chapman and Hall, New York, N.Y.
- 11.Goossen, J., and H. Röper. 1994. Erythritol, a new sweetener. Confectionery Production 24:182-188. [Google Scholar]
- 12.Hanjny, G. J., J. H. Smith, and J. C. Garver. 1964. Erythritol production by a yeast-like fungus. Appl. Microbiol. 12:240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattori, K., and T. Suzuki. 1974. Production of erythritol by n-alkane grown yeasts. Agric. Biol. Chem. 38:581-586. [Google Scholar]
- 14.Ishizuka, H., H. Wako, T. Kasumi, and T. Sasaki. 1989. Breeding of a mutant of Aureobasidium sp. with high erythritol production. J. Ferment. Bioeng. 68:310-314. [Google Scholar]
- 15.Ishizuka, H., K. Tokuoka, T. Sasaki, and H. Taniguchi. 1992. Purification and some properties of an erythrose reductase from an Aureobasidium sp. Biosci. Biotechnol. Biochem. 56:941-945. [DOI] [PubMed] [Google Scholar]
- 16.Jordan, D. B., G. S. Basarab, D. I. Liao, W. M. Jornson, K. N. Winzenberg, and D. A. Winkler. 2001. Structure-based design of inhibitors of the rice blast fungal enzyme trihydroxynaphthalene reductase. J. Mol. Graph. Model. 19:434-447., 470-471. [DOI] [PubMed] [Google Scholar]
- 17.Kim, K. A., B. S. Noh, J. K. Lee, S. Y. Kim, and D. K. Oh. 2000. Optimization of culture conditions for erythritol production by Torula sp. J. Microbiol. Biotechnol. 10:69-74. [Google Scholar]
- 18.Kim, S. Y., K. H. Lee, J. H. Kim, and D. K. Oh. 1997. Erythritol production by controlling osmotic pressure in Trigonopsis variabilis. Biotechnol. Lett. 19:729-733. [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. K., S. J. Ha, S. Y. Kim, and D. K. Oh. 2000. Increased erythritol production in Torula sp. by Mn2+ and Cu2+. Biotechnol. Lett. 22:983-986. [Google Scholar]
- 21.Lee, J. K., S. J. Ha, S. Y. Kim, and D. K. Oh. 2001. Increased erythritol production in Torula sp. by phytic acid. Biotechnol. Lett. 23:497-500. [Google Scholar]
- 22.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 23.Maeda, K., and M. Fukuda. 1996. Arbutin: mechanism of its depigmenting action in human melanocyte culture. J. Pharm. Exp. Ther. 276:765-769. [PubMed] [Google Scholar]
- 24.Martinez, R. R., M. H. Wheeler, A. G. Plata, G. Rico, and H. T. Guerrero. 2000. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 68:3696-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh, D. K., C. H. Cho, J. K. Lee, and S. Y. Kim. 2001. Increased erythritol production in Torula sp. by fed batch culture. J. Ind. Microbiol. Biotechnol. 26:248-252. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto, S., M. Sakurada, Y. Kubo, G. Tsuji, I. Fujii, Y. Ebizuka, M. Ono, H. Nagasawa, and S. Sakuda. 2001. Inhibitory effect of aflastatin A on melanin biosynthesis by Colletotrichum lagenarium. Microbiology 147:2623-2628. [DOI] [PubMed] [Google Scholar]
- 27.Onishi, H. 1967. Production of polyalcohols by yeasts. Jpn. Ferment. Technol. 25:495-506. [Google Scholar]
- 28.Palmieri, G., P. Giardina, C. Bianco, A. Scaloni, A. Capassol, and G. Sannia. 1997. A novel white laccase from Pleurotus astreatus. J. Biol. Chem. 272:31301-31307. [DOI] [PubMed] [Google Scholar]
- 29.Pavlenko, G. V., M. S. Loitsyanskaya, and N. I. Nemirovskaya. 1982. Melanin pigment of Gluconobacter oxydans. Microbiol. USSR 50:539-542. [Google Scholar]
- 30.Pfeifer, V. F., V. E. Sohns, H. E. Conway, E. B. Lancaster, S. Dabic, and E. L. Griffin, Jr. 1960. Two-stage process for dialdehyde starch with electrolytic regeneration of periodic acid. Ind. Eng. Chem. 52:201-205. [Google Scholar]
- 31.Rast, D. M., and G. O. Hollenstein. 1977. Architecture of the Agaricus bisporus spore wall. Can. J. Bot. 55:2251-2262. [Google Scholar]
- 32.Richter, H., D. Vlad, and G. Unden. 2001. Significance of pantothenate for glucose fermentation by Oenococcus oeni and for suppression of the erythritol and acetate production. Arch. Microbiol. 175:26-31. [DOI] [PubMed] [Google Scholar]
- 33.Riley, P. A. 1991. Melanogenesis: a realistic target for antimelanoma therapy. Eur. J. Cancer 27:1172-1177. [DOI] [PubMed] [Google Scholar]
- 34.Rosas, A. L., J. D. Nosanchuk, B. L. comez, W. A. Edens, J. M. Henson, and A. Casadevall. 2000. Isolation and serological analyses of fungal melanins. J. Immunol. Methods 244:69-80. [DOI] [PubMed] [Google Scholar]
- 35.Shindoh, T., Y. Sasaki, A. Miki, T. Eguchi, K. Hagiwara, and T. Ichikawa. 1988. Determination of erythritol in fermented foods by high performance liquid chromatography. J. Food Hyg. Soc. Jpn. 29:419-422. [Google Scholar]
- 36.Shindoh, T., Y. Sasaki, A. Miki, K. Hagiwara, and T. Ichikawa. 1988. Determination of erythritol in fruits and fermented foods by high performance liquid chromatography. Jpn. Agr. Biol. Chem. 62:623-626. [Google Scholar]
- 37.Thompson, J. E., G. S. Basarb, A. Andersson, Y. Lindqvist, and D. B. Jordan. 1997. Trihydroxynaphthalene reductase from Magnaporthe grisea: realization of an active center inhibitor and elucidation of the kinetic mechanism. Biochemistry 36:1852-1860. [DOI] [PubMed] [Google Scholar]
- 38.Tokousbalides, M. C., and H. D. Sisler. 1979. Sites of inhibition by tricyclazole in the melanin biosynthetic pathway of Verticillium dahliae. Pestic. Biochem. Physiol. 11:64-73. [Google Scholar]
- 39.Tokuoka, K., H. Ishizuka, K. Wako, and H. Taniguchi. 1992. Comparison of three forms of erythrose reductase from an Aureobasidium sp. mutant. J. Gen. Appl. Microbiol. 38:145-155. [DOI] [PubMed] [Google Scholar]
- 40.Tsai, H. F., M. H. Wheeler, Y. C. Chang, and K. J. Kwon-Chung. 1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vickers, M. F., S. Y. M. Yao, S. A. Baldwin, J. D. Young, and C. E. Cass. 2000. Nucleoside transporter proteins of Saccharomyces cerevisiae. Demonstration of a transporter (FUI1) with high uridine selectivity in plasma membranes and a transporter (FUN26) with broad nucleoside selectivity in intracellular membranes. J. Biol. Chem. 275:25931-25938. [DOI] [PubMed] [Google Scholar]
- 42.Viradar, V. M., N. Kobayashi, J. Matsunaga, and V. J. Hearing. 1999. A standardized protocol for assessing regulators of pigmentation. Anal. Biochem. 270:207-219. [DOI] [PubMed] [Google Scholar]
- 43.Wang, Y., P. Aisen, and A. Casadevall. 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect. Immun. 64:2420-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler, M. H., and M. A. Klich. 1995. The effects of tricyclazole, pyroquilon, phthalide, and related fungicides on the production of conidial wall pigments by Penicillium and Aspergillus species. Pestic. Biochem. Physiol. 52:125-136. [Google Scholar]
- 45.Wheeler, M. H., and R. D. Stipanovic. 1985. Melanin biosynthesis and metabolism of flaviolin and 2-hydroxyjuglone in Wangiella dermatitidis. Arch. Microbiol. 142:232-241. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler, M. H. 1982. Melanin biosynthesis in Verticillium dahliae: dehydration and reduction in cell-free homogenates. Exp. Mycol. 6:171-179. [Google Scholar]
- 47.Wooloshuk, C. P., H. D. Sisler, M. C. Tokousbalides, and S. R. Dutky. 1980. Melanin biosynthesis in Pyricularia oryzae: site of tricyclazole inhibition and pathogenicity of melanin deficient mutants. Pestic. Biochem. Physiol. 14:256-264. [Google Scholar]
- 48.Yang, S. W., J. B. Park, N. S. Han, and J. H. Seo. 1999. Production of erythritol from glucose by an osmophilic mutant of Candida magnoliae. Biotechnol. Lett. 49:1569-1572. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida, H., T. Sugawara, and J. Hayashi. 1984. Studies in free sugars and free sugar alcohols of mushrooms. Jpn. Food Ind. 31:765-771. [Google Scholar]