Abstract
Reductions in annual rainfall in some regions and increased human consumption have caused a shortage of water resources at the global level. The recycling of treated wastewaters has been suggested for certain domestic, industrial, and agricultural activities. The importance of microbiological and parasitological criteria for recycled water has been repeatedly emphasized. Among water-borne pathogens, protozoa of the genera Giardia and Cryptosporidium are known to be highly resistant to water treatment procedures and to cause outbreaks through contaminated raw or treated water. We conducted an investigation in four wastewater treatment plants in Italy by sampling wastewater at each stage of the treatment process over the course of 1 year. The presence of the parasites was assessed by immunofluorescence with monoclonal antibodies. While Cryptosporidium oocysts were rarely observed, Giardia cysts were detected in all samples throughout the year, with peaks observed in autumn and winter. The overall removal efficiency of cysts in the treatment plants ranged from 87.0 to 98.4%. The removal efficiency in the number of cysts was significantly higher when the secondary treatment consisted of active oxidation with O2 and sedimentation instead of activated sludge and sedimentation (94.5% versus 72.1 to 88.0%; P = 0.05, analysis of variance). To characterize the cysts at the molecular level, the β-giardin gene was PCR amplified, and the products were sequenced or analyzed by restriction. Cysts were typed as assemblage A or B, both of which are human pathogens, stressing the potential risk associated with the reuse of wastewater.
At the global level, there has been a growing shortage of freshwater reserves, mainly those of good quality, as a result of increasing human consumption and, in some regions, decreases in the annual rainfall or annual rainfall consisting mostly of heavy rain, which is poorly absorbed by the soil (19, 20, 21). To address this problem, dual water networks in which treated wastewater can be used for domestic, industrial, and agricultural purposes, for which the use of water with a low level of chemical or microbiological contaminants would not represent a threat to human health have been proposed (18).
The importance of microbiological and parasitological criteria for controlling the contamination of recycled water has been repeatedly emphasized. In industrialized countries, the most common human parasitic protozoa transmitted by water belong to the genera Giardia and Cryptosporidium (34). Giardiasis and cryptosporidiosis are also common infections of domestic and wild animals, which shed a large number of cysts and oocysts in the environment. These cysts are insensitive to disinfectants at the concentration commonly used in water treatment plants to reduce bacterial contamination, although it has been shown that at higher concentrations of chlorine and ozone, Giardia cysts are less resistant than Cryptosporidium oocysts (35). Moreover, Giardia cysts have been shown to survive in water for up to 2 months at temperatures as low as 8°C (26), and Cryptosporidium oocysts can survive for up to 1 year at 4°C in artificial seawater (36). Furthermore, the infectious dose has been estimated to be as low as 10 cysts for Giardia (1) and 30 oocysts for Cryptosporidium (12).
Numerous water-borne outbreaks of giardiasis and cryptosporidiosis have been documented in the past several decades, mainly in the United States, Europe, and Australia (33, 34). This has led the U.S. Environmental Protection Agency (EPA) to regulate the level of Giardia cysts and Cryptosporidium oocysts allowed in drinking water (40). To detect oocysts, many methods have been developed based on the filtration of large volumes of water, followed by centrifugation, clarification (either by density gradients or immunomagnetic separation), and microscopic screening of the sample after staining with monoclonal antibodies (28). Although these methods have proven to be very useful for determining whether or not waters are contaminated with parasites, they cannot distinguish among the different species or genotypes. To this end, PCR assays have been developed (33), yet the efficiency of amplification techniques is often reduced by the presence of inhibitory substances in water samples, such as humic and fulvic acids (37, 42).
In Italy, there are few published data on the prevalence of Giardia and Cryptosporidium in wastewaters. The objectives of the present study were to evaluate the prevalence of these parasites in four wastewater treatment plants, to estimate the efficiency of treatment plants in removing these parasites, to develop a reliable method for DNA extraction from concentrated water samples, and to determine the species and genotype of these parasites by means of a molecular assay.
MATERIALS AND METHODS
Sample collection and processing.
Samples were collected at four wastewater treatment plants. One plant was located in northern Italy (plant 1, located in the city of Bergamo, Lombardy region), and three plants were located in southern Italy (plant 2, city of Naples, Campania region; plant 3, city of Cagliari, Sardinia region; and plant 4, city of Palermo, Sicily region). Samples (15 to 20 liters) of untreated wastewater (influent) and of primary, secondary, and final effluent were collected during the spring, summer, autumn, and winter of the year 2000. To be able to examine the same wastewater at various points in the treatment process, when collecting the samples, the holding times of each step in the process were respected.
The specific steps in the treatment process used in each of the four plants are described in Table 1. The treatment carried out at plant 1 differed from that at the other three plants. Specifically, primary treatment did not include sedimentation, and secondary treatment consisted of oxidation with O2 and sedimentation, whereas in the other three plants it consisted of activated sludge and sedimentation. Furthermore, no disinfection was used in plant 1. In plant 1, since it was not physically possible to collect samples at the end of the primary treatment, the first sample of treated wastewater was collected shortly after the oxidation process had begun. In plant 4, samples were not collected after the primary treatment.
TABLE 1.
Main features of treatment plants and removal of Giardia cysts
| Plant | Population served | Primary treatment | Secondary treatment | Disinfection | % Removal of Giardia cysts (mean geometric value) |
|---|---|---|---|---|---|
| 1 | 153,000 | Screening and grit separation | Oxidation with O2 and Sedimeutation | None | 94.5 |
| 2 | 300,000 | Screening, grit separation, and sedimentation | Activated sludge and sedimentation | Chlorination (0.05-1 ppm) | 87.0 |
| 3 | 330,000 | Screening, grit separation, and sedimentation | Activated sludge and sedimentation | Chlorination (0.05-1 ppm) | 96.0 |
| 4 | 100,000 | Screening, grit separation and sedimentation | Activated sludge and sedimentation | Filtration (60-μm pore) and peracetic acid (4 ppm) | 98.4 |
The water samples were filtered through a 50-mesh sieve (300 μm) to remove large particles and then concentrated by filtration on cellulose-acetate filters (0.8-μm pore size, 142-mm diameter; Nucleopore-Whatman, Clifton, N.J.) (2). The filter was placed in a 50-ml conical polypropylene centrifuge tube and dissolved with acetone. After centrifugation at 4,620 × g for 10 min at 4°C, the supernatant was discarded, and about 5 ml of pellet was left at the bottom of the tube. The pellet was resuspended in 50 ml of 95% alcohol, centrifuged, resuspended in 50 ml of 70% alcohol, centrifuged, resuspended in 50 ml of phosphate-buffered saline containing 0.1% Tween 80, 0.1% sodium dodecyl sulfate, and 0.001% antifoam agent B (Sigma, St. Louis, Mo.), and centrifuged, leaving a 5-ml pellet. An aliquot of 50 μl of the pellet was serially diluted (1:10, 1:50, and 1:100) and examined by immunofluorescence with anti-Giardia and anti-Cryptosporidium monoclonal antibodies conjugated to fluorescein isothiocyanate according to the manufacturer's protocol (Meridian Diagnostics, Inc., Cincinnati, Ohio).
Giardia cysts were identified based on their size, shape, and the pattern and intensity of immunofluorescent assay staining (i.e., bright green fluorescence of the cyst wall). Cryptosporidium oocysts were identified based on their size, shape, and the presence of a suture on the oocyst wall at a magnification of 1,000×. The number of oocysts was counted for each sample in triplicate.
Statistical analysis.
To evaluate the removal efficiency of Giardia cysts at the four plants, the logarithm of the number of cysts in the influent and in the effluent before disinfection was evaluated to overcome the biases due to the variable number of cysts in the influent of the four plants and the fact that, independently of the efficiency of a plant, the higher the number of cysts in the influent, the larger the difference between the number of cysts in the influent and that in the effluent. The removal efficiency of plant 1 (i.e., that with an active oxidation with O2 and sedimentation) was compared with those of plants 2, 3, and 4, which used activated sludge and sedimentation, by analysis of variance, having specified the active oxidation with O2 and sedimentation as nested within the plant.
DNA extraction and PCR amplification.
DNA extraction was performed according to the method of Da Silva et al. (11). Briefly, 0.4 ml of concentrated wastewater was homogenized with the FP120 FastPrep cell disruptor (Q-Biogene, Carlsbad, Calif.). The DNA released from disrupted cysts was purified with the FastDNA kit (Q-Biogene, Carlsbad, Calif.), and stored at 4°C.
A 753-bp product from the β-giardin gene of Giardia was amplified with the forward primer G7 (5′-AAGCCCGACGACCTCACCCGCAGTGC-3′) and the reverse primer G759 (5′-GAGGCCGCCCTGGATCTTCGAGACGAC-3′) (7). The variable region of the small-subunit rRNA gene of Cryptosporidium was amplified with the forward primer 5′-AAGCTCGTAGTTGGATTTCTG-3′ and the reverse primer 5′-TAAGGTGCTGAAGGAGTAAGG-3′ (17). The PCR mix consisted of 1× buffer containing 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphate mix, 25 pmol of each primer, 1.25 U of AmpliTaq DNA polymerase (Applied Biosystems, Branchburg, N.J.), and 1 to 5 μl of purified DNA in a final volume of 50 μl. Negative and positive controls were included in each batch of experiments.
PCR was performed as follows: after an initial denaturation of 5 min at 94°C, a set of 35 to 40 cycles was run, each consisting of 30 s at 94°C, 30 s at 65°C, and 60 s at 72°C, followed by a final extension of 5 min at 72°C. PCR products were detected by agarose gel electrophoresis and visualized by ethidium bromide staining. The products were purified with the QiaQuick kit (Qiagen, Hilden, Germany).
Molecular identification of cysts by sequence analysis or PCR-restriction fragment length polymorphism analysis.
PCR products were sequenced with the ABI Prism BigDye terminator cycle sequencing kit (Applied Biosystems, Branchburg, N.J.) and a set of internal primers. Sequencing reactions were analyzed on an ABI 310 automatic DNA sequencer (Applied Biosystems, Branchburg, N.J.). Sequences were assembled with the program SeqMan II (DNAStar).
Nucleic acids were extracted from aliquots (0.4 ml) of concentrated influent samples collected at the four plants; each aliquot contained approximately 600 to 2,000 cysts. To identify Giardia cysts, aliquots of β-giardin PCR products were digested for 4 h at 37°C with 10 U of HaeIII (New England BioLabs, Beverly, Mass.) in a final volume of 20 μl. The predicted restriction patterns were fragments of 202, 201, 150, 126, and 74 bp for assemblage A, and fragments of 202, 176, 150, 117, 84, and 24 bp for assemblage B. Restriction fragments were separated by electrophoresis on a 3% Metaphor gel (FMC, Rockland, Maine) and visualized after ethidium bromide staining.
RESULTS
Prevalence of protozoa in wastewater samples.
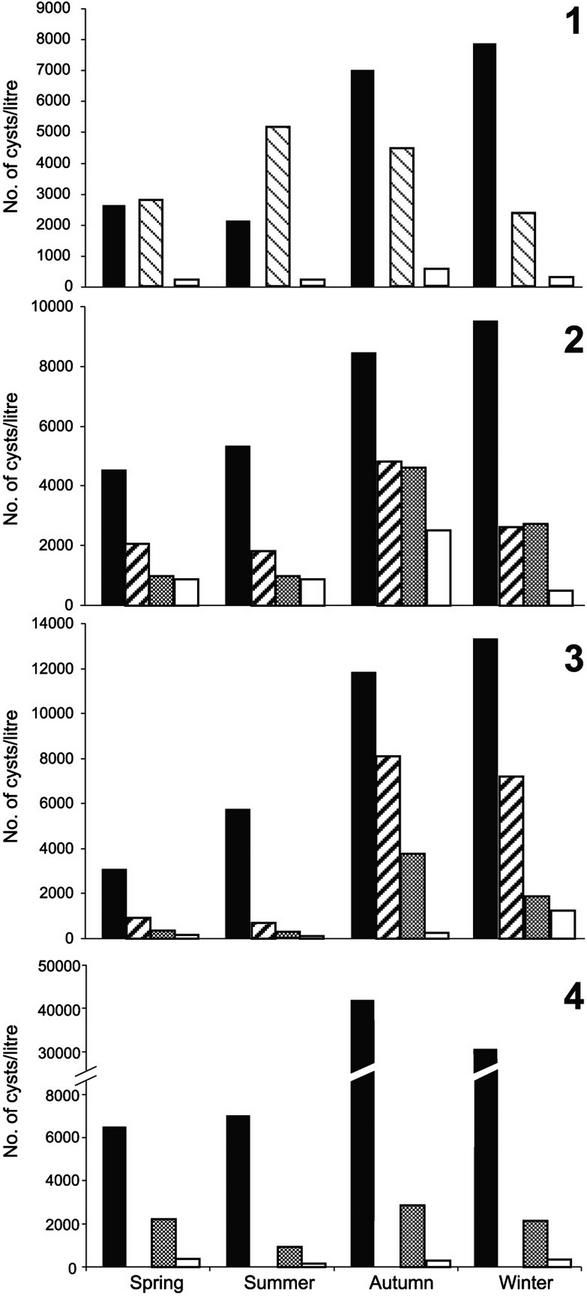
Giardia cysts were found in the influents of all plants throughout the year. The estimated mean number of cysts per liter ranged from 2.1 × 103 to 4.2 × 104. In all plants, the highest number of cysts was found in autumn and winter (Fig. 1). Cryptosporidium oocysts were only detected in two plants: twice in the influents of plant 1 (40 and 2.5 oocysts/liter) and once in the influent of plant 4 (277 oocysts/liter), always during the spring and only before primary treatment.
FIG. 1.
Number of Giardia cysts per liter in wastewater samples collected at different steps in the treatment process at treatment plants in Bergamo (plant 1), Naples (plant 2), Cagliari (plant 3), and Palermo (plant 4) in the spring, summer, autumn, and winter of the year 2000. Solid bars, influent samples; thick striped bars, samples after primary treatment; gray bars, samples after secondary treatment; white bars, effluent samples. In plant 1, the thin striped bars show the number of Giardia cysts shortly after oxidation with O2 had begun, and the white bars show the number of Giardia cysts after oxidation with O2 and sedimentation was completed. In plant 4, no samples were collected after primary treatment.
The removal efficiency of Giardia cysts after primary treatment, which was evaluated at plants 2 and 3, was 50.2 and 65.2%, respectively (geometric means for the four seasons). At plant 1, where the first treated sample was collected shortly after the oxidation process had begun, the number of cysts was observed to have increased by 17.5% in the spring and by 132.9% in the summer.
In plants 2 and 3, the removal efficiency of the secondary treatment (i.e., activated sludge and sedimentation) was 43.9 and 61.0%, respectively. In a single sample collected during the winter at plant 2, an increase of 4% in the number of cysts was observed (Fig. 1). The removal efficiency of the disinfection process, which was carried out at plants 2, 3, and 4, was 53.2, 70.8, and 87.0%, respectively (Fig. 1).
The overall removal efficiency of Giardia cysts was 94.5, 87.0, 96.0, and 98.4% in plants 1, 2, 3, and 4, respectively (Fig. 1). The removal efficiency when comparing untreated wastewater samples to those after secondary treatment was 94.5, 72.1, 86.4, and 88.0% for plants 1, 2, 3, and 4, respectively. At plant 1, the secondary treatment consisted of active oxidation with O2 and sedimentation (i.e., the final treatment), because a disinfection process was not applied at this plant. This treatment resulted in a higher removal efficiency in comparison to that observed in the other three plants, and the difference was significant (P = 0.05, analysis of variance).
Molecular identification of parasites.
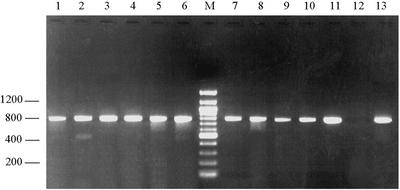
PCR amplification of the 753-bp fragment of the β-giardin gene was performed on these templates, and products of the expected size were obtained with 1 to 2 μl of template, which corresponds to 10 to 50 cysts (Fig. 2). At least one PCR product from each plant was sequenced (Table 2), whereas all 16 influent samples were analyzed with a PCR-restriction fragment length polymorphism assay (Fig. 3).
FIG. 2.
Electrophoretic separation of β-giardin amplification products from wastewater samples. Lanes 1 to 3, influent samples from the plant 1; lanes 4 to 6, influent samples from plant 2; lane M, 100-bp molecular ladder; lanes 7 to 9, influent samples from plant 3; lanes 10 and 11, influent samples from plant 4; lane 12, negative control; lane 13, positive control.
TABLE 2.
Genetic typing of Giardia cysts detected in four wastewater treatment plants in Italy
| Treatment plant no. | Assemblage(s) present
|
|||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| 1 | A + B | A + B | Aa | A + Ba |
| 2 | A | Aa | A | A + Ba |
| 3 | A + Ba | A | A + B | A |
| 4 | A | A + Ba | A | A + Ba |
Samples were sequenced.
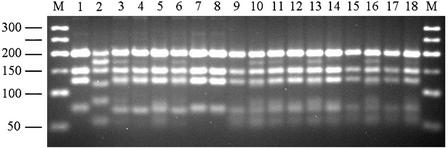
FIG. 3.
Electrophoretic separation of β-giardin PCR products after restriction with the endonuclease HaeIII. Lanes M, 50-bp molecular ladder; lane 1, positive control for assemblage A; lane 2, positive control for assemblage B; lanes 3 to 6, samples of influent from plant 1; lanes 7 to 10, samples of influent from plant 2; lanes 11 to 14, samples of influent from plant 3; lane 15 to 18, samples of influent from plant 4. For each plant, influent samples from spring, summer, autumn, and winter were typed and are shown in that order. Note the concomitant presence of restriction fragments specific for assemblages A and B in samples 3, 4, 5, 10, 11, 13, 16, and 18.
As shown in Table 2, cysts of assemblage A were detected in eight samples, whereas in the other eight samples cysts of both assemblages A and B were detected by sequencing and/or by PCR-restriction fragment length polymorphism (Fig. 3). Amplification of Cryptosporidium DNA from the three positive samples was not obtained.
DISCUSSION
Giardia and Cryptosporidium spp. can be transmitted to humans through contaminated water and food, in addition to the classical oral-fecal route. Transmission is sustained by both a zoonotic and an anthroponotic cycle (14, 38). The infected hosts, whether animals or humans, shed very large numbers of oocysts with their feces, thereby increasing the environmental contamination. Moreover, oocysts can withstand normal water disinfection processes, and they have been found in significant quantities in the final effluents of sewage treatment works (e.g., see reference 31).
Our investigation of the four plants revealed that Giardia cysts were ubiquitous, whereas Cryptosporidium oocysts were quite rare. Similar prevalence rates were reported in wastewater collected at a treatment plant in Bari, a city in southern Italy, where the number of Giardia cysts was 100-fold than the number of Cryptosporidium oocysts (5). Since the parasites detected in our study were probably of human origin, given that the wastewater was from cities and not from agricultural areas, these results suggest that the prevalence of cryptosporidiosis is lower than that of giardiasis; this is also supported by the results of surveys of intestinal parasites in Italy's general population (3, 10, 23). That the prevalence of cryptosporidiosis is relatively low in Italy compared to other countries is supported by the results of previous studies, in which the prevalence among persons with AIDS before the introduction of highly active antiretroviral therapy, which is considered to reflect the prevalence among the general population, was 1.9% (27), compared to 5 to 6% in the United States (9). A prevalence of 1.9% was also reported among immunocompetent children in Italy (4).
Although Giardia cysts were found in all of the wastewater samples in the four treatment plants throughout the year, the greatest number of cysts was found in the autumn and winter. Although a similar seasonal pattern has been reported by some authors (16, 41), it has not been confirmed by others (15, 30); thus, it is not clear whether or not seasonality is a general feature of Giardia contamination.
As shown in Fig. 1, an increase in Giardia cysts was observed three times during the purification process in the plants. As Giardia does not reproduce outside the host, this was probably due to the fact that the aggregated protozoa desegregated before sedimentation, thus increasing the concentration of free parasites in the sample, as also observed by other authors (6).
The overall removal efficiency ranged from 87.0 to 98.4% at the different plants, which is consistent with estimates from other treatment plants that use similar processes (8, 31). The highest removal efficiency was at plant 4 (98.4%), perhaps as a consequence of filtration, which was applied after the secondary treatment and before disinfection; although the filter had 60-μm pores and Giardia cysts measure 15 to 18 μm, aggregated cysts could have been trapped. However, the process of oxidation with O2 and sedimentation used at plant 1 resulted in greater cyst reduction than that obtained by the activated sludge and sedimentation methods used at the other three plants (P = 0.05, analysis of variance). To determine whether active oxidation with O2 is truly more effective than activated sludge in reducing the number of Giardia cysts, additional research will be needed. In fact, the present results may be biased by several factors, including the limited number of samples examined, the different volume of water treated in each plant (from 500 to 6,000 m3/hour), and the seasonality in the number of Giardia cysts.
Most studies on Giardia contamination of water have been limited to estimating the prevalence (15, 16, 22), and little information has been published on the specific contaminating species. However, this is of particular importance, since only Giardia duodenalis is associated with human infection (38), and only two of the seven G. duodenalis assemblages (i.e., assemblages A and B) have been found in humans (39). Therefore, the simple presence of Giardia cysts in the absence of data on the species or assemblage does not imply a risk of transmission to humans. Most studies have been conducted by spiking water samples with a known number of cysts, followed by evaluation of procedures for recovery and typing of the organism (32). In the few instances when nonspiked water samples were studied, the sensitivity and specificity of the PCR assays were low. In a study of drinking water samples performed after an outbreak of giardiasis in Canada, the direct typing of cysts by PCR amplification of the triose phosphate isomerase gene was unsuccessful, possibly because of the small number of cysts (25). In a study on sewage samples from Finland, nonspecific PCR amplifications were observed with primers targeting the glutamate dehydrogenase gene, and a further characterization of the Giardia cysts was not possible (29).
In our study, nucleic acids were efficiently extracted from concentrated wastewater samples with a method that had been developed for detecting protozoa present in fecal samples (7, 11). This method is rapid, in that it allows up to 12 samples to be simultaneously processed in about 1 h, and the DNA extracted is essentially free of inhibitors and can thus be efficiently amplified by PCR (Fig. 2). Moreover, we have shown that the β-giardin PCR assay yields robust and specific amplification products and that it allows the rapid identification of genotypes by sequence analysis or restriction analysis (Fig. 3). The better performance of this assay is probably due to the amplification target chosen, since giardin proteins are considered unique to Giardia (13), and primers that do not cross-react with other organisms can be designed (24).
The results indicate that water processed at the four treatment plants could be a potential source of human infection with G. duodenalis, although the viability of the cysts was not investigated. In Italy, about 80% of drinking water is from ground water, and only 20% originates from surface water, which is more easily contaminated with parasitic protozoa. However, the release of contaminated effluents into the environment could increase the risk of human infection with these pathogens through the consumption of vegetables. Moreover, the results stress the importance of the microbiological control of effluents from wastewater treatment plants and the need for regulations that establish the acceptable concentrations of oocysts based on the use of wastewaters, i.e., if they should be recycled in the cities for public and/or in-house dual systems, for agricultural purposes, which could be limited to certain crops only, or for industry.
Acknowledgments
We thank D. Tonanzi for technical assistance. We are also grateful to P. Pezzotti for statistical analysis and to G. Aurisicchio, S. Fiorillo, M. Gallo, A. Goffredi, L. Marossi, G. Marras, R. Oliveri, and P. Spanu, who supplied the wastewater samples.
This study received financial support from research project 1156/RI, entitled “Infezioni Da Cryptosporidium e Giardia attraverso alimenti e acque: metodi di identificazione ed epidemiologia molecolare,” of the Istituto Superiore di Sanità, Rome, Italy, and from research grant PR 28/IS of the Ministero dell'Ambiente of Italy.
REFERENCES
- 1.Adam, R. D. 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev. 14:447-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldom, J. E., and A. H. Chagla. 1995. Recovery of Cryptosporidium oocysts from water by a membrane filter dissolution method. Lett. Appl. Microbiol. 20:186-187. [DOI] [PubMed] [Google Scholar]
- 3.Ballone, E., P. Fazii, G. Riario Sforza, E. Scassa, M. Di Nicola, N. Ippolito, C. Di Mascio, and F. Schioppa. 2001. Survey on giardiasis propagation in Pescara. Ann. Igiene 13:111-120. [PubMed] [Google Scholar]
- 4.Brandonisio, O., A. Marangi, M. A. Panaro, R. Marzio, M. I. Natalicchio, P. Zizzadoro, and U. De Santis. 1996. Prevalence of Cryptosporidium in children with enteritis in southern Italy. Eur. J. Epidemiol. 12:187-190. [DOI] [PubMed] [Google Scholar]
- 5.Brandonisio, O., F. Portincasa, G. Torchetti, N. Lacarpia, A. Rizzi, L. Fumarola, F. Donadio, and D. Carnimeo. 2000. Giardia and Cryptosporidium in water: evaluation of two concentration methods and occurrence in wastewater. Parassitologia 42:205-209. [PubMed] [Google Scholar]
- 6.Bukhari, Z., H. V. Smith, N. Sykes, S. W. Humphreys, C. A. Paton, R. W. A. Girdwood, and C. R. Fricker. 1997. Occurrence of Cryptosporidium spp. oocysts and Giardia spp cysts in sewage influents and effluents from treatment plants in England. Water Sci. Technol. 35:385-390. [Google Scholar]
- 7.Cacciò, S. M., M. De Giacomo, and E. Pozio. 2002. Sequence analysis of the β-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int. J. Parasitol. 32:1023-1030. [DOI] [PubMed] [Google Scholar]
- 8.Casson, L. W., C. A. Sorber, J. L. Sykora, P. D. Gavaghan, M. A. Shapiro, and W. Jakubowski. 1990. Giardia in wastewater-effect of treatment. Water Pollut. Control Fed. Res. J. 62:670-675. [Google Scholar]
- 9.Colford, J. M., Jr., I. B. Tager, A. M. Hirozawa, G. F. Lemp, T. Aragon, and C. Petersen. 1996. Cryptosporidiosis among patients infected with human immunodeficiency virus. Factors related to symptomatic infection and survival. Am. J. Epidemiol. 144:807-816. [DOI] [PubMed] [Google Scholar]
- 10.Crotti, D., and M. L. D'Annibale. 2001. Dientamoeba fragilis e dientamoebosi: aspetti di parassitologia clinica e diagnostica di laboratorio. Parassitologia 43:135-138. [PubMed] [Google Scholar]
- 11.da Silva, A. J., F. J. Bornay-Llinares, I. N. S. Moura, S. B. Slemenda, J. L. Tuttle, and N. J. Pieniazek. 1999. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol. Diagn. 4:57-64. [DOI] [PubMed] [Google Scholar]
- 12.DuPont, H. L., C. L. Chappell, C. R. Sterling, P. C. Okhuysen, J. B. Rose, and W. Jakubowski. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332:855-859. [DOI] [PubMed] [Google Scholar]
- 13.Faubert, G. 2000. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 13:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1305-1322. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto, A., T. Hirata, and S. Kunikane. 2001. Occurrence of Cryptosporidium oocysts and Giardia cysts in a conventional water purification plant. Water Sci. Technol. 43:89-92. [PubMed] [Google Scholar]
- 16.Isaac-Renton, J., W. Moorehead, and A. Ross. 1996. Longitudinal studies of Giardia contamination in two community drinking water supplies: cyst levels, parasite viability, and health impact. Appl. Environ. Microbiol. 62:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, D. W., N. J. Pieniazek, D. W. Griffin, L. Misener, and J. B. Rose. 1995. Development of a PCR protocol for sensitive detection of Cryptosporidium oocysts in water samples. Appl. Environ. Microbiol. 61:3849-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahana, Y., and A. Tal. 2000. Technological solutions in support of sustainable use of water in metropolitan areas. Interdisciplinary Center for Technological Analysis and Forecasting, Tel Aviv University, Tel Aviv, Israel.
- 19.Karl, T. R., and R. W. Knight. 1998. Secular trends of precipitation amount, frequency, and intensity in the United States. Bull. Am. Meteorol. Soc. 79:231-241. [Google Scholar]
- 20.Karl, T. R., R. W. Knight, D. R. Easterling, and R. G. Quayle. 1996. Indices of climate changes for the United States. Bull. Am. Meteorol. Soc. 77:279-303. [Google Scholar]
- 21.Karl, T. R., R. W. Knight, and N. Plummer. 1995. Trends in high-frequency climate variability in the twentieth century. Nature 377:217-220. [Google Scholar]
- 22.Le Chevallier, M. W., W. D. Norton, and R. G. Lee. 1991. Occurrence of Giardia and Cryptosporidium species in surface water supplies. Appl. Environ. Microbiol. 57:2610-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libanore M., R. Bicocchi, M. R. Rossi, P. Montanari, L. Sighinolfi, F. Macario, and F. Ghinelli. 1991. Incidenza della giardiasi in pazienti adulti con enterite acuta. Minerva Med. 82:375-380. [PubMed] [Google Scholar]
- 24.Mahbubani, M. H., A. K. Bej, M. H. Perlin, F. W. Schaefer, I. I. I., W. Jakubowski, and R. M. Atlas. 1992. Differentiation of Giardia duodenalis from other Giardia spp. using polymerase chain reaction and gene probes. J. Clin. Microbiol. 30:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntyre, L., L. Hoang, C. S. L. Ong, P. Lee, and J. L. Isaac-Renton. 2000. Evaluation of molecular techniques to biotype Giardia duodenalis collected during an outbreak. J. Parasitol. 86:172-177. [DOI] [PubMed] [Google Scholar]
- 26.Meyer, E. A., and E. J. Jarroll. 1980. Giardiasis. Am. J. Epidemiol. 111:1-12. [DOI] [PubMed] [Google Scholar]
- 27.Pezzotti, P., D. Serraino, G. Rezza, L. Dal Maso, E. Vaccher, A. C. Lepri, and S. Franceschi. 1999. The spectrum of AIDS-defining diseases: temporal trends in Italy prior to the use of highly active antiretroviral therapies, 1982-1996. Int. J. Epidemiol. 28:975-981. [DOI] [PubMed] [Google Scholar]
- 28.Quintero-Betancourt, W., E. R. Peele, and J. B. Rose. 2002. Cryptosporidium parvum and Cyclospora cayetanensis: a review of laboratory methods for detection of these water-borne parasites. J. Microbiol. Methods 49:209-224. [DOI] [PubMed] [Google Scholar]
- 29.Rimhanen-Finne, R., P. Ronkainen, and M. L. Haenninen. 2001. Simultaneous detection of Cryptosporidium parvum and Giardia in sewage sludge by IC-PCR. J. Appl. Microbiol. 91:1030-1035. [DOI] [PubMed] [Google Scholar]
- 30.Robertson, L. J., and B. Gjerde. 2001. Occurrence of Cryptosporidium oocysts and Giardia cysts in raw waters in Norway. Scand. J. Public Health 29:200-207. [PubMed] [Google Scholar]
- 31.Robertson, L. J., C. A. Paton, A. T. Campbell, P. G. Smith, M. H. Jackson, R. A. Gilmour, S. E. Black, D. A. Stevenson, and H. V. Smith. 2000. Giardia cysts and Cryptosporidium oocysts at sewage treatment works in Scotland, UK. Water Res. 34:2310-2322. [Google Scholar]
- 32.Rochelle, P. A., D. M. Ferguson, T. J. Handojo, R. De Leon, M. H. Stewart, and R. L. Wolfe. 1997. An assay combining cell culture with reverse transcriptase PCR to detect and determine the infectivity of water-borne Cryptosporidium parvum. Appl. Environ. Microbiol. 63:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose, J. B., D. E. Huffman, and A. Gennaccaro. 2002. Risk and control of water-borne cryptosporidiosis. FEMS Microbiol. Rev. 26:113-123. [DOI] [PubMed] [Google Scholar]
- 34.Slifko, T. R., H. V. Smith, and J. B. Rose. 2000. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 30:1379-1393. [DOI] [PubMed] [Google Scholar]
- 35.Sterling, C. R. 1990. Waterborne cryptosporidiosis, p. 51-58. In J. P. Dubey, C. A. Speer, and R. Fayer (ed.), Cryptosporidiosis of man and animals. CRC Press, Boca Raton, Fla.
- 36.Tamburrini, A., and E. Pozio. 1999. Long-term survival of Cryptosporidium parvum oocysts in seawater and in experimentally infected mussels (Mytilus galloprovincialis). Int. J. Parasitol. 29:711-715. [DOI] [PubMed] [Google Scholar]
- 37.Tebbe, C. C., and W. Vahjen. 1993. Interference of humic acids and DNA extracted directly from soil in detection and transformation of recombinant DNA from bacteria and a yeast. Appl. Environ. Microbiol. 59:2657-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson, R. C. A. 2000. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 30:1259-1267. [DOI] [PubMed] [Google Scholar]
- 39.Thompson, R. C., R. M. Hopkins, and W. L. Homan. 2000. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol. Today 16:210-213. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Environmental Protection Agency. 1999. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. EPA-821-R-99-006. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 41.Wallis, P. M., S. L. Erlandsen, J. L. Isaac-Renton, M. E. Olson, W. J. Robertson, and H. van Keulen. 1996. Prevalence of Giardia cysts and Cryptosporidium oocysts and characterization of Giardia spp. isolated from drinking water in Canada. Appl. Environ. Microbiol. 62:2789-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeates, C., M. R. Gillings, A. D. Davinson, N. Altavilla, and D. A. Veal. 1997. PCR amplification of crude microbial DNA extracted from soil. Lett. Appl. Microbiol. 25:303-307. [DOI] [PubMed] [Google Scholar]