Abstract
Objective To review the clinical effectiveness of oseltamivir and zanamivir for the treatment and prevention of influenza A and B.
Design Systematic review and meta-analyses of randomised controlled trials.
Data sources Published studies were retrieved from electronic bibliographic databases; supplementary data were obtained from the manufacturers.
Selection of studies Randomised controlled, double blind trials that were published in English, had data available before 31 December 2001, evaluated treatment or prevention of naturally occurring influenza with zanamivir or oseltamivir (if given using the formulation and dosage licensed for clinical use), and reported at least one end point of relevance.
Review methods The main outcome measures were the median time to the alleviation of symptoms (for treatment trials) and number of flu episodes avoided (for prevention trials). Three population groups were defined: children aged 12 years and under; otherwise healthy individuals aged 12 to 65 years; and “high risk” individuals (those with certain chronic medical conditions or aged 65 years and older).
Results Seventeen treatment trials and seven prevention trials identified met the inclusion criteria. All trials included compared one of the drugs against placebo or standard care. Treatment of children, otherwise healthy individuals, and high risk populations with zanamivir reduced the median duration of symptoms in days respectively by 1.0 (95% confidence interval 0.5 to 1.5), 0.8 (0.3 to 1.3), and 0.9 (-0.1 to 1.9) for the intention to treat population. The corresponding results, in days, for oseltamivir were 0.9 (0.3 to 1.5), 0.9 (0.3 to 1.4), and 0.4 (-0.7 to 1.4). The effect of giving zanamivir and oseltamivir prophylactically resulted in a relative reduction of 70-90% in the odds of developing flu, depending on the strategy adopted and the population studied.
Conclusions Evidence from randomised controlled trials consistently supports the view that both oseltamivir and zanamivir are clinically effective for treating and preventing flu. However, evidence is limited for the treatment of certain populations and for all prevention strategies.
Introduction
Influenza epidemics occur almost every winter and are associated with considerable morbidity and mortality.1 All age groups are susceptible, but increasing age, certain chronic medical conditions, and residential care increase the risk of complications and death. Two interventions can lessen the impact of flu: immunisation with inactivated vaccines and treatment and prophylaxis with antivirals. Current policy in the United Kingdom recommends that individuals at high risk of serious illness or death from flu should be vaccinated against the virus annually (see www.doh.gov.uk/flu.htm). Antivirals represent a rational approach to flu management to complement vaccination, particularly in “high risk” individuals, but until recently only the M2 inhibitors, amantadine and rimantadine, were available. Limitations of these drugs include rapid emergence of resistance,2,3 lack of antiviral activity against influenza B, and frequent adverse central nervous system events, particularly in elderly people.4,5
The goal of providing activity against influenza A and B with few adverse events came with the development of the neuraminidase inhibitors for flu, zanamivir (Relenza, GlaxoSmithKline) and oseltamivir (Tamiflu, Roche). In this systematic review, commissioned by the National Institute for Clinical Excellence (NICE), we examined randomised controlled trials of zanamivir and oseltamivir, both for treatment and prophylaxis, in three populations—children, high risk adults, and otherwise healthy adults—to assess the evidence for the clinical effectiveness of these two drugs. The results of this systematic review were incorporated into an economic decision model to produce the NICE guidance on zanamivir and oseltamivir, which was issued in February 2003.6
Methods
Searching
We searched Medline (1966 to December 2001), Embase (1980 to December 2001), Integrated Science Citation Index (1981 to December 2001), and the National Library of Medicine (PubMed). In addition, we searched cited literature in retrieved articles, previous systematic reviews and meta-analyses of neuraminidase inhibitors,7–10 and manufacturers' trial databases. We contacted drug companies for information on unpublished trials.
Selection
We selected randomised controlled, double blind trials that met all the following criteria: were published in English, had data available before 31 December 2001, evaluated treatment or prevention of naturally occurring influenza with zanamivir or oseltamivir (if these were given using the formulation and dosage licensed for clinical use), and reported at least one end point of relevance (see below).
Validity assessment
We used a validated instrument developed previously11 to assess the methodological quality of the treatment and prevention trials according to the method of randomisation, concealment of allocation, blinding of trial investigators and patients, and completeness of follow up.
Data abstraction
Summary outcome data were initially extracted from trial publications and final trial reports. Additional data were also requested from the drug companies—for example, separate data on individual population groups when publications reported combined results for otherwise healthy and high risk individuals, standard errors of the median “time to an event,” and, where appropriate, re-analysis of the data to allow for censored observations (whereby the event has not occurred by the end of the trial).
Study characteristics
We considered three populations: children aged 12 years and under; otherwise healthy individuals aged 12 to 65 years; and high risk individuals. We defined high risk individuals as those aged 65 years and older or those having certain chronic medical conditions, such as respiratory disease, heart disease, and pulmonary disorders.
The primary treatment end points were “time to the alleviation of symptoms” and “complications requiring antibiotics.” We also considered “time to return to normal activities” and “admissions to hospital”; these are reported elsewhere.12 In this paper, we present results for both the intention to treat (ITT) populations and the populations with a laboratory confirmed “flu positive” diagnosis.
The primary prevention end point was the number of individuals with laboratory confirmed symptomatic flu at the end of the trial. We reported our assessment of adverse events elsewhere.12
Quantitative data synthesis
The meta-analyses reported here are presented to the standards set out in the QUOROM statement.13 We performed meta-analyses separately for each neuraminidase inhibitor and used random effect models to take into account heterogeneity between trials.14
Results
We present results of trials comparing each neuraminidase inhibitor with placebo or standard care for treatment and prevention. We identified no “head to head” (zanamivir v oseltamivir) trials. Varying proportions of randomised individuals were vaccinated before entry into the trials (table 1 and 2). The full list of trials included in this review is available on bmj.com (as “web extra”).
Table 1.
Description of all 17 treatment trials of neuraminidase inhibitors in systematic review
| Trial | Age group (years) | High risk subjects (%) | Flu positive subjects (%) | Vaccinated subjects (%) | Interventions | Treatment duration (days) | Follow up (days) | Jadad score11* |
|---|---|---|---|---|---|---|---|---|
| Zanamivir | ||||||||
| NAIA2005, NAIB2005 | ≥13 | 0 | 63 | 0 | Placebo, inhaled and intranasal (n=144); 10 mg inhaled plus placebo intranasal twice daily (n=132); 10 mg inhaled, plus 6.4 mg intranasal twice daily (n=141) † | 5 | 28 | 4 |
| NAIB2007 | ≥13 | 13 | 62 | Not reported | Placebo (n=183); 10 mg inhaled twice daily (n=188); 10 mg inhaled plus 6.4 mg intranasal twice daily (n=183) † | 5 | 5 | Lack of information |
| NAIB3001 | ≥12 | 17 | 71 | 6 | Placebo (n=228); 10 mg inhaled twice daily (n=227) | 5 | 28 | 5 |
| NAIA3002 | ≥12 | 14 | 73 | Not reported | Placebo (n=365); 10 mg inhaled twice daily (n=412) | 5 | 28 | Lack of information |
| NAIB3002 | ≥12 | 9 | 78 | 4 | Placebo (n=182); 10 mg inhaled twice daily (n=174) | 5 | 28 | 5 |
| NAI30008 | ≥12 | 100 | 60 | 23 | Placebo (n=263); 10 mg inhaled twice daily (n=262) | 5 | 28 | 5 |
| NAI30009 | 5 to 12 | 8 | 73 | 2 | Placebo (n=247); 10 mg inhaled twice daily (n=224) | 5 | 28 | 3 |
| NAI30010 | ≥5 | >7 | 49 | 10 | Placebo (n=158); 10 mg inhaled twice daily (n=163) | 5 | 28 | 4 |
| Oseltamivir | ||||||||
| WV15670 | 18-65 | 0 | 65 | 0 | Placebo (n=238); 75 mg twice daily (n=243); 150 mg twice daily (n=245) † | 5 | 21 | 5 |
| WV15671 | 18-65 | 0 | 59 | 0 | Placebo (n=209); 75 mg twice daily (n=210); 150 mg twice daily (n=208) † | 5 | 21 | 5 |
| WV15730 | 18-65 | 0 | 66 | 0 | Placebo (n=27); 75 mg twice daily (n=31) | 5 | 21 | 5 |
| WV15812 | NA | NA | NA | NA | NA | NA | NA | NA |
| WV15872 | NA | NA | NA | NA | NA | NA | NA | NA |
| WV15819 | NA | NA | NA | NA | NA | NA | NA | NA |
| WV15876 | NA | NA | NA | NA | NA | NA | NA | NA |
| WV15978 | NA | NA | NA | NA | NA | NA | NA | NA |
| WV15758 | 1-12 | 0 | 67 | 2 | Placebo (n=351); 2 mg/kg twice daily (to maximum of 100 mg/dose) (n=344) | 5 | 28 | 4 |
NA=not available (data from the five trials of high risk subjects (WV15812, WV15819, WV15872, WV15876, and WV15978) were supplied by the manufacturer as “commercial in confidence” and were excluded from table).
Data from trials NAIA2005 and NAIB2005 were amalgamated by the drug company before analysis.
The higher the score (maximum 5), the higher the methodological quality. Jadad scores were not included for trials NAIB2007 and NAIA3002 as the drug company supplied the data and full reports were not available.
Included for completeness (only licensed dosage considered by the review).
Table 2.
Description and results of all seven prevention trials of neuraminidase inhibitors in systematic review, by strategy
| Trial | Age group (years) | High risk subjects (%) | Vaccinated subjects (%) | Trial design arms (No of subjects in each arm) | Treatment duration | No of flu positive cases | Odds ratio (95% CI) | Jadad score11* |
|---|---|---|---|---|---|---|---|---|
| Zanamivir | ||||||||
| Seasonal prophylaxis in a healthy population:
|
|
|
|
|
|
|
|
|
| NAIA3005 | 18-64 | 0 | 15 | Placebo (n=554); 10 mg inhaled once daily (n=553) | 4 weeks | Placebo 34; intervention 11 | 0.31 (0.14 to 0.64) | 4 |
| Post-exposure prophylaxis in household setting †
|
|
|
|
|
|
|
|
|
| NAIA2009, NAIB2009 | 13-65 | Not reported | 0 | Placebo spray and inhalation (n=144); placebo inhaled plus active spray (n=141) §, 10 mg inhaled plus placebo spray (n=144); 5 mg inhaled twice daily plus intranasal sprays (16 mg/ml) per nostril (0.1 ml per spray) (n=146) § | 5 days | Placebo 9; 10 mg inhaled 3 | 0.27 (0.07 to 1.05) | 3 |
| NAI30010 | Families | >6 | 16 | Contact cases ¶: Placebo (n=423); 10 mg inhaled once a day (n=414) | 10 days | Placebo 40; intervention 9 | 0.16 (0.07 to 0.37) | 4 |
| Oseltamivir | ||||||||
| Seasonal prophylaxis in elderly residential home:
|
|
|
|
|
|
|
|
|
| WV15825 | 64-96 | 100 | 80 | Placebo (n=272); 75 mg once daily (n=276) | 6 weeks | Placebo 12; intervention 1 | 0.08 (0.01 to 0.61) | 4 |
| Seasonal prophylaxis in a healthy population ‡
|
|
|
|
|
|
|
|
|
| WV15673 | 18-65 | 0 | 0 | Placebo (n=268); 75 mg once daily (n=268); 75 mg twice daily (n=267) § | 6 weeks | Placebo 19; intervention 3 | 0.15 (0.04 to 0.51) | 5 |
| WV15697 | 18-65 | 0 | 0 | Placebo (n=251); 75 mg once daily (n=252); 75 mg twice daily (n=253) | 6 weeks | Placebo 6; intervention 3 | 0.49 (0.12 to 1.99) | 5 |
| Post-exposure prophylaxis in the household setting
|
|
|
|
|
|
|
|
|
| WV15799 | 12-85 | 40 | 13 | Placebo (n=462); 75 mg once daily (n=493) | 7 days | Placebo 34; intervention 4 | 0.10 (0.04 to 0.29) | 4 |
Data from trials NAIA2009 and NAIB2009 were amalgamated by the drug company before analysis.
The higher the score (maximum 5), the higher the methodological quality.
Pooled odds ratio 0.19 (0.09 to 0.38)).
Pooled odds ratio 0.26 (0.08 to 0.84)).
Included for completeness (only licensed dosage considered by the review).
Members of household in which one individual had contracted influenza-like illness (index case) and who were given a neuraminidase inhibitor prophylactically while the index case was treated with a neuraminidase inhibitor.
Treatment
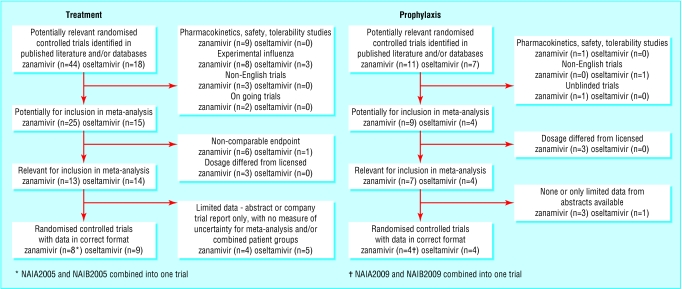
We identified 44 studies evaluating zanamivir for the treatment of flu and 18 for oseltamivir (fig 1). Of these, eight randomised controlled trials of zanamivir and nine of oseltamivir met our eligibility criteria (table 1).
Fig 1.
Flow diagram of systematic review
Zanamivir
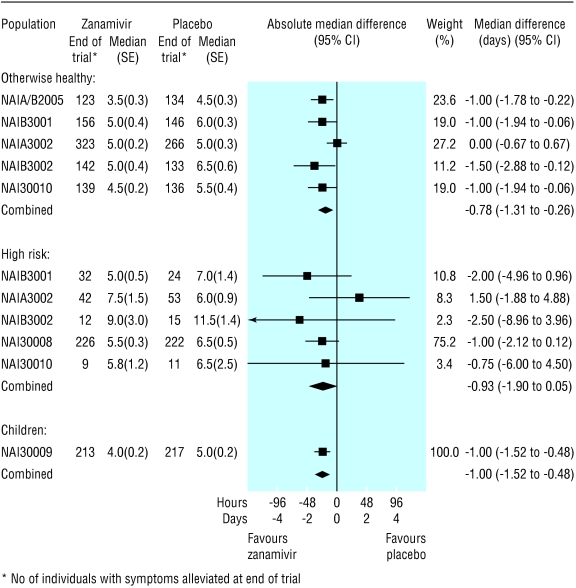
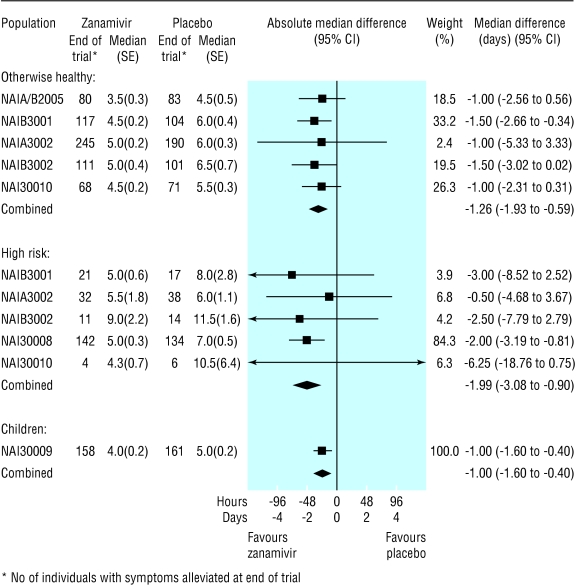
Time to alleviation of symptoms—In the ITT population, the reduction in the median time to alleviation of symptoms in the treatment groups, when compared with placebo, ranged from 0.8 (95% confidence interval 0.3 to 1.3) days for otherwise healthy adults to 1.0 (0.5 to 1.5) days for children (fig 2). In the flu positive population, this reduction ranged from 1.0 (0.4 to 1.6) days for children to 2.0 (0.9 to 3.1) days for high risk adults (fig 3).
Fig 2.
Forest plot of difference in time to alleviation of symptoms between zanamivir and placebo arms, by the three intention to treat populations
Fig 3.
Forest plot of difference in time to alleviation of symptoms between zanamivir and placebo arms, by the three flu positive populations
Complications requiring antibiotics—Few trial level data on complications requiring antibiotics were obtained from the literature; however, two published, pooled marginal analyses were identified.15,16 When considering all three ITT populations combined, Monto and colleagues observed a 29% (10% to 44%) relative reduction (zanamivir v placebo) in the odds of complications requiring antibiotics.15 However, among the high risk flu positive population, they found a non-significant relative reduction (45%) in the odds of antibiotic use. Lalezari and colleagues,16 who focused on high risk adults and on children, obtained similar results.
Oseltamivir
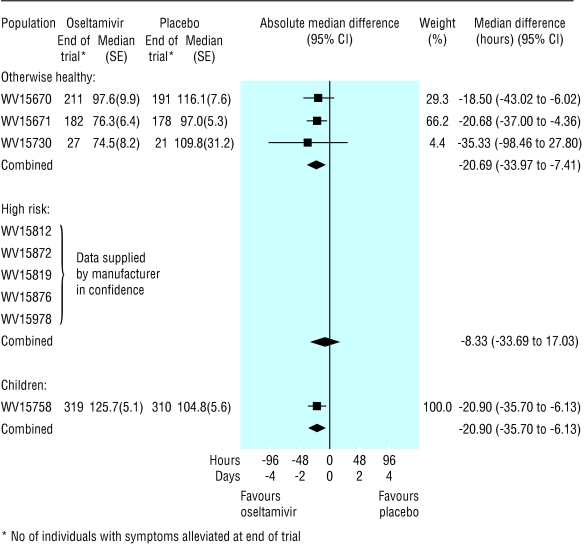
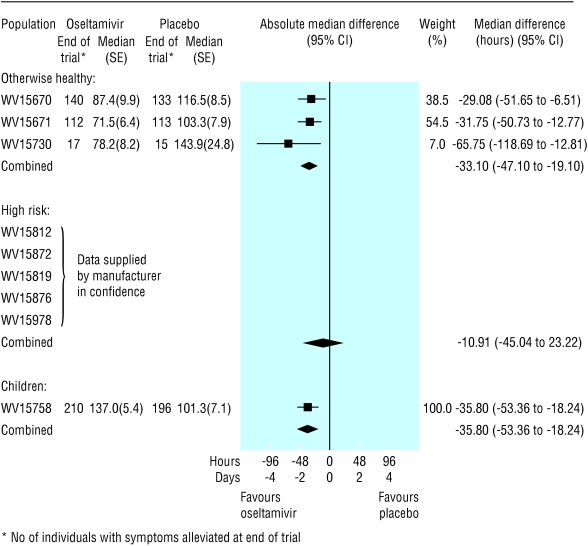
Time to symptom alleviation—In the ITT population, the reduction in median time to alleviation of symptoms, when oseltamivir was compared with placebo, ranged from 0.4 (-0.7 to 1.4) days for high risk adults to 0.9 (0.3 to 1.5) days for children (fig 4). In the flu positive population, the reduction in time to alleviation of symptoms ranged from 0.4 (1.0 to 1.9) days for high risk adults to 1.5 (0.8 to 2.2) days for children (fig 5).
Fig 4.
Forest plot of difference in time to alleviation of symptoms between oseltamivir and placebo arms, by the three intention to treat populations
Fig 5.
Forest plot of difference in time to alleviation of symptoms between oseltamivir and placebo arms, by the three flu positive populations
Complications requiring antibiotics—Only one study (WV15670) in otherwise healthy adults reported a non-significant relative reduction (oseltamivir v placebo, 43%) in the odds of complications requiring antibiotics in the ITT population and a significant relative reduction (87%) in the flu positive population.17 Among children, a 35% relative reduction in the odds of complications requiring antibiotics was observed in one study (WV15758).18
Prevention
We identified 11 randomised controlled trials that evaluated zanamivir for the prevention of flu and seven for oseltamivir (fig 1). Of these, three trials of zanamivir and four of oseltamivir met our eligibility criteria (table 2).
Zanamivir
Seasonal prophylaxis of a healthy population (NAIA3005)—A 69% (36% to 86%) relative reduction (zanamivir v placebo) in the odds of laboratory confirmed symptomatic flu was observed (table 2).
Post-exposure prophylaxis in households (NAIA/B2009, NAI30010)—A meta-analysis of these two trials showed an 81% (62% to 91%) relative reduction (zanamivir v placebo) in the odds of laboratory confirmed symptomatic flu.
Oseltamivir
Seasonal prophylaxis of a healthy population (WV15673, WV15697)—A meta-analysis of these two trials showed a 74% (16% to 92%) relative reduction (oseltamivir v placebo) in the odds of laboratory confirmed symptomatic flu.
Post-exposure prophylaxis in households (WV15799)— A 90% (71% to 96%) relative reduction (oseltamivir v placebo) in the odds of laboratory confirmed symptomatic flu was observed (table 2).
Seasonal prophylaxis in residential care (WV15825)— In a mostly vaccinated elderly population receiving residential care there was a 92% (39% to 99%) relative reduction (oseltamivir v placebo) in the odds of laboratory confirmed symptomatic flu (table 2). Similar benefits were observed in those previously vaccinated.
Discussion
The results of our systematic review show that treating otherwise healthy adults and children with zanamivir and oseltamivir reduces the duration of symptoms in the intention to treat population by between 0.4 and 1.0 days and provides 29% to 43% relative reduction in the odds of complications requiring antibiotics when these are given within 48 hours of onset of symptoms. The results were less conclusive in the high risk population (as defined in the methods) though these were based on fewer patients. Caution is required when comparing the results because the definition of symptoms assessed for alleviation in the treatment trials varied among trials of the two compounds, and between adults and children for each compound. Moreover, the time to event outcomes were measured on different scales (days and hours). Also, the rates of flu positive (≥49%) individuals who were enrolled in the trials may be higher than the rates identified routinely in clinical practice. Thus, the treatment effects estimated for the ITT trial populations may not be achievable in routine practice.
The data on complications reported above were not ideal because they relied primarily on pooled marginal analyses and thus did not take into account any heterogeneity between trials.19,20 It is not clear how well complications requiring antibiotics correlate with the incidence of more serious complications of flu. Little evidence exists either on serious complications requiring admission to hospital or causing death or on adverse events. Both of these are evidently rare (at least in otherwise healthy individuals) but are potentially important in the evaluation of treatments; the trials were underpowered in terms of such outcomes. Insufficient data are available from clinical trials to assess adequately the risk of emergence of resistance to neuraminidase inhibitors.
A lack of evidence exists for use of neuraminidase inhibitors for preventing flu in children and in frail elderly people in residential care. We found that neuraminidase inhibitors given for flu prevention led to a relative reduction of 70% to 90% in the odds of developing flu, depending on the strategy adopted and the population studied.
In conclusion, although evidence from randomised controlled trials consistently supports the clinical effectiveness of both oseltamivir and zanamivir for the treatment and prevention of flu, evidence is limited for the treatment of high risk populations and for all prevention strategies. Research is needed into the comparative effectiveness of neuraminidase inhibitors with one another and the potential “added value” of these drugs compared with or in combination with flu vaccine.
What is already known on this topic
Neuraminidase inhibitors (zanamivir and oseltamivir) may be useful in treating and preventing flu
Data are limited, however, for certain population groups and for prophylactic use
What this study adds
Unlike previous systematic reviews, this review considered three distinct populations (children, high risk adults, and otherwise healthy adults)
For the use of neuraminidase inhibitors for flu prevention, different strategies evaluated by randomised controlled trials were reviewed
Oseltamivir and zanamivir are clinically effective for treating and preventing flu, but evidence is limited for certain population groups and for all prevention strategies
Supplementary Material
 The full list of trials included in this review is available on bmj.com
The full list of trials included in this review is available on bmj.com
Editorial by Stöhr
We thank GlaxoSmithKline (in particular, Stephen Sharp) and Roche (in particular, Paul Mahoney) for providing additional trial data.
Contributors: All authors participated in designing the review, checking the data, and revising the manuscript, which was drafted by NJC. NJC, AJS, AW, and DAT decided on trial inclusion or exclusion, extracted data, and assessed study quality. NJC, AJS, and KRA performed the statistical analyses. KRA and KGN were the principal advisers, guiding and interpreting the review. NJC is the guarantor for the paper.
Funding: This work was commissioned by the NHS Health Technology Assessment programme and the National Institute for Clinical Excellence. NJC is funded by University Hospitals of Leicester NHS Trust. DAT and AW are funded by the Trent Institute for Health Services Research. The guarantor accepts full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish.
Competing interests: KGN has received travel sponsorship and honorariums from GlaxoSmithKline, the manufacturer of zanamivir, and Roche, which makes oseltamivir, for consultancy and speaking at international respiratory and infectious diseases symposiums. His research group has received research funding from GlaxoSmithKline and Roche to participate in multicentre trials of neuraminidase inhibitors; Berna Biotech and Chiron for trials of flu vaccines; and Wyeth for work on the epidemiology of flu in young children. KGN was a founder member and vice chairman of the European Scientific Working Group on Influenza (ESWI), a group of European scientists promoting the study of flu. ESWI is supported by the vaccine manufacturers, Roche and GlaxoSmithKline, but is scientifically independent.
References
- 1.Nicholson KG, Webster RG, Hay AJ. Textbook of influenza. Oxford: Blackwell Science, 1998.
- 2.Cochran K, Maasasab H, Tsunoda A, Berlin BS. Studies on the antiviral activity of amantadine hydrochloride. Ann N Y Acad Sci 1965;130: 432-9. [DOI] [PubMed] [Google Scholar]
- 3.Tsunoda A, Maasab HF, Cochran KW, Eveland WC. Antiviral activity of alpha-methyl-1-admantanemethylamine hydrochloride. Antimicrobial Agents Chemother 1965;5: 553-60. [DOI] [PubMed] [Google Scholar]
- 4.Hayden F, Hoffman H, Spyker D. Differences in side effects of amantadine hydrochloride and rimantadine hydrochloride relate to differences in pharmacokinetics. Antimicrobial Agents Chemother 1983;23: 458-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millet VM, Dreisbach M, Bryson YJ. Double-blind controlled study of central nervous system side effects of amantadine, rimantadine, and chlorpheniramine. Antimicrobial Agents Chemother 1982;21: 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Clinical Excellence. Technology appraisal guidance No 58. London: NICE, 2003. www.nice.org.uk/pdf/58_Flu_fullguidance.pdf (accessed 24 Apr 2003)
- 7.Burls A, Clark W, Stewart A, Preston C, Bryan S, Jefferson T, et al. Zanamivir for the treatment of influenza in adults. Health Technology Assessment 2000:6. (No 9.) [DOI] [PubMed]
- 8.Husereau DR, Brady B, McGreer A. Oseltamivir for the treatment of suspected influenza: a clinical and economic assessment. Ottawa: Canadian Coordinating Office for Health Technology Assessment, 2001.
- 9.Brady B, McAuley L, Shukla VK. Economic evaluation of zanamivir for the treatment of influenza. Ottowa: Canadian Coordinating Office for Health Technology Assessment, 2001.
- 10.Jefferson T, Demicheli V, Deeks J, Rivetti D. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. 2000. Cochrane Database Syst Rev 2000;(2): CD001265. [DOI] [PubMed]
- 11.Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials 1996;17: 1-12. [DOI] [PubMed] [Google Scholar]
- 12.Turner D, Wailoo A, Nicholson K, Cooper NJ, Sutton AJ, Abrams KR. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. NICE Assessment Report 2002. Available at www.nice.org.uk/pdf/influenzaassrep.pdf [DOI] [PubMed]
- 13.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet 1999;354: 1896-900. [DOI] [PubMed] [Google Scholar]
- 14.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: Wiley, 2000.
- 15.Monto AS, Webster A, Keene O. Randomized, placebo-controlled studies of inhaled zanamivir in the treatment of influenza a and b: pooled efficacy analysis. J Antimicrobial Chemother 1999;44: 23-9. [DOI] [PubMed] [Google Scholar]
- 16.Lalezari J, Camion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 2001;161: 212-7. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson KG, Aoki FY, Osterhaus ADME, Trottier S, Carewicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 2000;355: 1845-50. [DOI] [PubMed] [Google Scholar]
- 18.Whitley RJ, Hayden FG, Reisinger KS, Young N, Dutkowski R, Ipe D, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001;20: 127-33. [DOI] [PubMed] [Google Scholar]
- 19.Simpson EH. The interpretation of interaction in contingency tables. J R Stat Soc Series B 1951;13: 238-41. [Google Scholar]
- 20.Lievre M, Cucherat M, Leizorovicz A. Pooling, meta-analysis, and the evaluation of drug safety. Curr Control Trials Cardiovasc Med 2002;3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.