Abstract
In previous work, some members of our group isolated mutant strains of Synechocystis sp. strain PCC 6803 in which point mutations had been inserted into the psaC gene to alter the cysteine residues to the FA and FB iron-sulfur clusters in the PsaC subunit of photosystem I (J. P. Yu, I. R. Vassiliev, Y. S. Jung, J. H. Golbeck, and L. McIntosh, J. Biol. Chem. 272:8032-8039, 1997). These mutant strains did not grow photoautotrophically due to suppressed levels of chlorophyll a and photosystem I. In the results described here, we show that suppressor mutations produced strains that are capable of photoautotrophic growth at moderate light intensity (20 μmol m−2 s−1). Two separate suppressor strains of C14SPsaC, termed C14SPsaC-R62 and C14SPsaC-R18, were studied and found to have mutations in a previously uncharacterized open reading frame of the Synechocystis sp. strain PCC 6803 genome named sll0088. C14SPsaC-R62 was found to substitute Pro for Arg at residue 161 as the result of a G482→C change in sll0088, and C14SPsaC-R18 was found to have a three-amino-acid insertion of Gly-Tyr-Phe following Cys231 as the result of a TGGTTATTT duplication at T690 in sll0088. These suppressor strains showed near-wild-type levels of chlorophyll a and photosystem I, yet the serine oxygen ligand to FB was retained as shown by the retention of the S ≥ 3/2 spin state of the [4Fe-4S] cluster. The inactivation of sll0088 by insertion of a kanamycin resistance cartridge in the primary C14SPsaC mutant produced an engineered suppressor strain capable of photoautotrophic growth. There was no difference in psaC gene expression or in the amount of PsaC protein assembled in thylakoids between the wild type and an sll0088 deletion mutant. The sll0088 gene encodes a protein predicted to be a transcriptional regulator with sequence similarities to transcription factors in other prokaryotic and eukaryotic organisms, including Arabidopsis thaliana. The protein contains a typical helix-turn-helix DNA-binding motif and can be classified as a negative regulator by phylogenetic analysis. This suggests that the product of sll0088 has a role in regulating the biogenesis of photosystem I.
Photosynthetic organisms contain membrane-bound photochemical reaction centers, which are complex metalloenzymes composed of protein subunits encoded, in the case of higher plants, in two different genomes, the nucleus and the plastid. Among other functions, the protein subunits harbor inorganic and organic cofactors that allow light-driven redox chemistry to drive the biosynthetic and energy-requiring processes of the cell. The studies described in this paper are concerned with the biogenesis of photosystem I (PS I), an iron-sulfur-quinone complex responsible for the reduction of ferredoxin, a metalloprotein that reduces NADP+ to NADPH (16).
The PS I reaction center contains the primary electron donor P700 (a chlorophyll a-a′ special pair); the primary electron acceptor A0 (a chlorophyll a monomer); an intermediate electron acceptor, A1 (phylloquinone); and three bound [4Fe-4S] clusters, FX, FA, and FB (for reviews see references 7 and 43). The core of the PS I reaction center consists of 82-, 84-, and 9.3-kDa polypeptides, encoded by the psaA, psaB, and psaC genes, respectively. FX is an interpolypeptide [4Fe-4S] cluster residing between the PsaA and PsaB heterodimers, and FA and FB are [4Fe-4S] clusters within the PsaC polypeptide (2, 7, 19).
In previous reports, we investigated the effects of site-specific mutations on the assembly and function of the PS I reaction center. We mutated the psaC gene of Synechocystis sp. strain PCC 6803 (46), targeting specifically the ligands of the [4Fe-4S] clusters in order to assign coordination of the FA and FB clusters to specific cysteine residues (21). Mutants of Synechocystis sp. strain PCC 6803 were recovered in which the cysteine ligands C14PsaC of FB and C51PsaC of FA were modified to alanine (C14APsaC and C51APsaC), aspartic acid (C14DPsaC and C51DPsaC), and serine (C14SPsaC and C51SPsaC) residues. The results from these studies were critical in arguing that PsaC is oriented with the FA cluster proximal to FX and the FB cluster proximal to the soluble ferredoxin binding site (15).
The aspartate and serine PsaC mutants have dramatically lower steady-state levels of chlorophyll a and PS I complexes per cell (46) and grow only mixotrophically under low light intensities (2.2 μmol m−2 s−1). Isolated PS I complexes from the mutants show competent charge separation between P700 and FA-FB and high rates of electron transfer from cytochrome c6 to flavodoxin. The lower PS I content rather than any inherent problem with primary photochemistry is apparently the underlying reason for the lack of photoautotrophic growth. It is reasonable to assume that the biogenesis of PS I was affected by these subtle PsaC mutations, possibly at a level beyond simple subunit assembly.
The biogenesis of many membrane complexes has been studied through the use of suppressor mutations, for example, the cytochrome bc1 complex of Saccharomyces cerevisiae (5), the rhodopsin I-transducer complex (20), and folding studies of yeast cytochrome b (11). A similar approach toward analysis of PS I complexes in cyanobacteria is complicated by the presence of multiple copies of the genome in each cell, but it has been shown elsewhere to be feasible for Synechocystis sp. strain PCC 6803 (45). In this work, we employ site-directed mutants of PsaC (46) to select for suppressors, strains that are capable of photoautotrophic growth. This genetic approach has been successful for the recovery of suppressor mutants in Synechocystis sp. strain PCC 6803 for the photosystem II (PS II) chlorophyll binding protein CP47 (41) and for defining the QA “niche” on the PS II reaction center protein D2 (13).
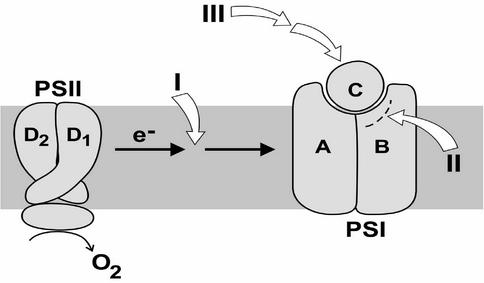
In earlier work, members of our group isolated PsaC mutants incapable of photoautotrophic growth (46) and then showed that they could easily isolate pseudorevertants to these primary mutations based on photoautotrophic growth selection on solid medium plates (45). In this study, we have attempted to analyze the molecular mechanisms by which suppressor mutations to PsaC mutations could allow for normal assembly and function of PS I. At least three classes of possible suppressor mutations are proposed to exist (Fig. 1). One type of mutant could limit electron flow to PS I. This would have the effect of not allowing the quinone pool to become completely reduced, thereby lowering oxidative stress in the presence of reduced PS I capacity. This class could be predicted from our data (Table 1) (21, 46), which show that most of the PsaC mutants could grow under reduced light intensities. The light sensitivity of these primary mutants suggests that overreduction of the quinone pool may be responsible, in part, for the inability to achieve photoautotrophic wild-type growth. This was further substantiated when it was shown that addition of N-(3,4-dichlorophenyl)-N′-dimethylurea (DCMU) would allow mixotrophic wild-type growth of some mutants under normal light conditions (22 μmol m−2 s−1 [46]). A second class of mutations could be structural mutations in an adjoining subunit(s), allowing for stable assembly of the mutated PsaC subunit. A number of such mutants were localized to the carboxy-terminal region of the PsaB subunit by genetic complementation of sections of the psaB gene into suppressor lines (J. Yu and L. McIntosh, unpublished results). A third class of mutations was found that shows no changes within the PS I complex itself, indicating a possible regulatory function at some unrecognized level. Two examples of this class of mutations are the subject of this paper.
FIG. 1.
A model is presented for the classes of proposed pseudorevertant mutations that could allow stable assembly and photosynthetic activity of PS I. Class I, suppressor mutations lessening the flow of electrons to PS I, limiting reduction of the quinone pool and photooxidative damage to PS I; class II, intergenic mutations in a reaction center subunit, PsaB, allowing for assembly of a genetically altered PsaC subunit; class III, an extragenic suppressor that is not within PS I but influences its expression, stability, or activity.
TABLE 1.
Physiological characterization of photoautotrophically grown cells of Synechocystis sp. strain PCC 6803a
| Cell typeb | Doubling time (h) | Chlorophyll/cellc | Whole-chain O2 evolutiond |
|---|---|---|---|
| WT-Gmr | 26.6 ± 3.2 | 1.65 ± 0.13 | 300.3 ± 42.4 |
| WT-sll0088K | 27.2 ± 5.3 | 2.96 ± 0.01 | 177.0 ± 21.1 |
| C14SPsaC-R18 | 34.1 ± 3.3 | 1.32 ± 0.06 | 298.8 ± 26.3 |
| C14SPsaC-R62 | 36.0 ± 3.4 | 1.40 ± 0.18 | 263.5 ± 44.7 |
| C14SPsaC-sll0088K | 37.7 ± 4.0 | 1.38 ± 0.14 | 142.4 ± 39.8 |
Results shown are averages and standard deviations from at least three independent cultures.
WT-Gmr, wild-type cells with an inserted gentamicin resistance gene; WT-sll0088K, wild-type merodiploid with a kanamycin resistance gene inserted in sll0088; C14SPsaC-R18 and C14SPsaC-R62, suppression mutant lines of C14SPsaC; C14SPsaC-sll0088K, C14S with a kanamycin resistance gene inserted into sll0088.
Shown as micrograms of chlorophyll per milliliter per unit of optical density at 730 nm at mid-log phase.
Shown as micromoles of O2 per milligram of chlorophyll per hour.
MATERIALS AND METHODS
Strains and growth conditions.
Experiments were performed with a glucose-tolerant strain of Synechocystis sp. strain PCC 6803 that was acclimated for wild-type growth on solid medium in the dark. Photoautotrophic, mixotrophic, and light-activated heterotrophic wild-type growth (LAHG) conditions, all at 30°C, were created as previously described (2). Antibiotics were added in the indicated concentrations: kanamycin, 5 mg/liter, and gentamicin, 1 mg/liter. Transformations were performed as described previously (30) except in the case of strain ΔC-RCPT (46). ΔC-RCPT was employed in the lines in which site-specific mutations (such as C14SPsaC) to psaC were constructed, and gentamicin was used in these constructs. Since they are light sensitive, the mutant strains were carefully maintained in dim light (5 μmol m−2 s−1 for 2 h of incubation) throughout the procedure (mixotrophic wild-type growth in the presence of glucose is inhibited at a white light intensity greater than 10 μmol m−2 s−1). Dot transformation was performed as described previously (45). Tests for photoautotrophic and mixotrophic growth were performed with solid media with or without supplemental glucose. Cool-white fluorescent bulbs made by General Electric Co. were used to provide continuous light. The light intensity was varied by covering plates with layers of cheesecloth, and the intensity was monitored with an L1-185A photometer (LICOR, Lincoln, Nebr.). Large cultures were grown in carboys (15 liters) under LAHG conditions and were bubbled with air. Doubling times were measured for established cultures in BG-11 medium supplemented with the appropriate antibiotic, when applicable. The cultures were incubated under 20 μmol of light m−2 s−1 and at 30°C with air bubbling.
DNA manipulations.
Nucleic acids were manipulated by standard methods (37) unless otherwise stated. For amplification of DNA fragments from cyanobacterial strains, cells picked from a medium-size colony or an equivalent number of cells collected from liquid culture were washed once with water and used as a template. Amplification products were purified with a PCR purification kit (Promega Corporation, Madison, Wis.). The procedure for preparation of cyanobacterial DNA was adapted from reference 29 with two loopfuls of cells scraped from plates or cells from 10 ml of liquid culture being used to extract DNA.
Transformation of Synechocystis sp. strain PCC 6803 and selection-growth conditions.
Synechocystis sp. strain PCC 6803 that was maintained under LAHG conditions for at least two subcultures (cells were subcultured once a week) was transformed with plasmids containing genes for kanamycin or gentamicin resistance. Selection for antibiotic-resistant colonies was performed under LAHG conditions. Resistant colonies were restreaked to obtain single colonies with at least five serial transfers to obtain full segregation of the mutation. Complete segregation was verified by restriction enzyme analysis of PCR products, direct sequencing of PCR products, Southern hybridizations, and growth tests.
Immunoblot analysis of thylakoid membrane proteins.
Thylakoid membranes were isolated from Synechocystis sp. strain PCC 6803 cells, and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and immunoblotting were performed on these membranes as described previously (39). To resolve the PsaA-PsaB proteins, SDS-10% polyacrylamide gels were used; to resolve the PsaC, PsaD, PsaE, and PsaF proteins, SDS-17% polyacrylamide gels were used. The D2 protein of PS II was resolved with an SDS-17% polyacrylamide gel to serve as a control. Protein assays were performed by the method of reference 27. Equal amounts of protein (150 μg) were loaded in each lane for thylakoid preparations. Rabbit antiserum to PsaC was raised against recombinant PsaC protein from Synechococcus sp. strain PCC 7002 expressed in Escherichia coli (25). Chlorophyll was extracted from whole cells at late log phase with methanol and quantified by visible spectrophotometry with published extinction coefficients (26).
Identification of second-site mutations.
The mutant C14SPsaC, in which a cysteine ligand to FB is changed to serine, was introduced into Synechocystis sp. strain PCC 6803 by site-directed mutagenesis (21, 46). To isolate pseudorevertants, 108 cells from a mid-exponential-phase culture grown under LAHG conditions (2) were spread onto 90-cm-diameter plates with solid BG-11 medium supplemented with gentamicin. The plates were placed at 30°C under 10 μmol of white light m−2 s−1 for 2 days and then transferred to 22 μmol of white light m−2 s−1 for up to 40 days. Colonies appeared spontaneously under these selective conditions at frequencies between 10−6 and 10−8 in several tests (45). Approximately 100 colonies were serially streaked three to four times. Two isolates, C14SPsaC-R18 and C14SPsaC-R64, were selected for further characterization. No special criteria were used, other than photoautotrophic growth, to choose suppressor lines for study. Due to the large number of other revertant strains found, it cannot be determined if they are related to sll0088 until they are analyzed at the molecular level. This work is under way.
The presence of the primary mutation C14SPsaC was checked by restriction analysis and direct sequencing of the psaC fragment amplified with the following primers: (i) ATATTATTTTTTTCGACTTTA and (ii) GATCAAAAATTGGAATAATG. Although true revertants have been found (codon TCT for serine reverted to TGT for cysteine), both C14SPsaC-R18 and C14SPsaC-R64 contained the primary mutation, and no second-site mutations were found in the psaC gene. DNA applied directly to the surface of a lawn of Synechocystis sp. strain PCC 6803 embedded in agar can result in the transformation (dot transformation) of these cells (12). Total DNA isolated from the two pseudorevertants was used to transform C14SPsaC. Clusters of transformed colonies appeared in 40 days under selective conditions, demonstrating specific DNA modifications (45).
The dot transformation technique was modified as follows to identify second-site mutations anywhere in the genome. (i) Genomic DNA isolated from a pseudorevertant was completely digested with HindIII, and gel slices with different sizes of restriction fragments were cut from a low-melting-point agarose gel. (ii) Each gel slice was melted, C14SPsaC was dot transformed, and a slice that confers photoautotrophic growth was identified. (iii) A pUC18 plasmid minilibrary was made from DNA purified from that slice, and each plasmid was tested in dot transformation. (iv) The identified plasmid was sequenced for the second-site mutation, or smaller restriction fragments from the insert were tested in dot transformation to narrow down the candidates.
Oxygen evolution.
LAHG-condition-grown cells were washed once in 40 mM HEPES buffer, pH 7.0, and cells containing 10 μg of chlorophyll a were resuspended in 1 ml of the same buffer and illuminated by saturating light at 25°C. Rates of oxygen evolution were determined with a Rank-type oxygen electrode unit. Whole-chain electron transport was measured in the presence of 10 mM NaHCO3. PS II electron transport was measured in the presence of 1 mM 2,6-dichlorobenzoquinone.
Low-temperature X-band EPR spectroscopy.
X-band electron paramagnetic resonance (EPR) experiments were performed with a Bruker ECS 106 X-band EPR spectrometer and either an ER4012 ST resonator or an ER4116 DM resonator. Cryogenic temperatures were maintained with an Oxford liquid helium cryostat and an ITC4 temperature controller. The magnetic field was calibrated with α,α′-diphenyl-β-picryl hydrazyl (DPPH) as standard. The microwave frequency was measured with a Hewlett-Packard 5340A frequency counter. The samples were resuspended to 500 μg of chlorophyll a ml−1 in 50 mM Tris buffer, pH 8.3, containing 1 mM ascorbic acid and 50 μM dichlorophenolindophenol (DCPIP) as electron donors.
RESULTS
Growth characteristics of pseudorevertants to the primary mutation C14SPsaC.
As described previously (46), the mutant strain C14SPsaC, in which the cysteine at position 14 of PsaC is changed to a serine, does not grow photoautotrophically and grows mixotrophically only under low (2.2 μmol m−2 s−1) light intensity. Physiological characterization has shown that these cells have a decreased PS I capacity, making PS I limiting for whole-chain photosynthesis. The doubling time of the mutant cells is increased, denoting diminished growth potential, while whole-chain oxygen evolution capacity is reduced. Mixotrophic growth (in the presence of glucose) is likewise inhibited by white light (22 to 60 μmol m−2 s−1) (46). As demonstrated previously, these changes mirror the lowered amounts of chlorophyll a and PS I complexes present in thylakoid membranes (28). Isolated PS I complexes show competent charge separation between P700 and FA-FB and high rates of electron transfer from cytochrome c6 to flavodoxin. Thus, it appears that the cells have difficulty growing photoautotrophically due to the inability of the reduced amount of PS I to accommodate electron flow from the unaltered PS II in the mutant cell lines. Reduced light and mixotrophic growth apparently lessen PS II electron flow so that the remaining PS I complexes remain intact and function as “leaky” mutants.
Pseudorevertants to this primary mutation were selected by their ability to grow photoautotrophically at moderate light intensity (20 μmol m−2 s−1). We selected two separate pseudorevertants to C14SPsaC for characterization: C14SPsaC-R18 and C14SPsaC-R62. The DNA sequence of the psaC gene was checked in both pseudorevertants to confirm that the primary mutation (45) was still present. Growth of the two pseudorevertants was similar to that of wild-type cells albeit with a slightly increased doubling time (Table 1). The chlorophyll a content was slightly reduced below that of wild-type cells. Whole-chain oxygen evolution was like that of wild type within the measured parameters (Table 1). These results indicate that, although the primary mutation has been retained, the cells are very close to the wild type in terms of growth and photosynthetic capacity.
EPR analysis of suppressor lines.
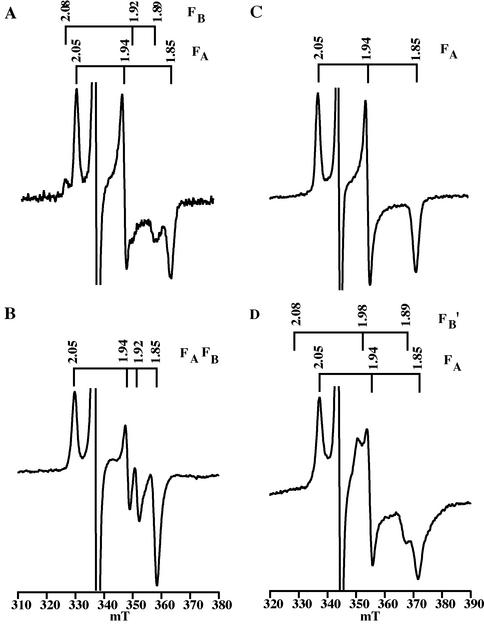
The EPR spectrum of the FA and FB iron-sulfur clusters was measured to confirm that the C14SPsaC-R18 and C14SPsaC-R62 suppressor strains retain the serine ligand to the FB cluster. In PS I complexes isolated from the wild type, FA and FB have a net ground spin state of S = 1/2. When PS I complexes are frozen in darkness and illuminated with white light at 15 K (Fig. 2A), the one electron in P700 is partitioned in a 1:3 ratio between FB (g = 2.07, 1.93, and 1.88) and FA (g = 2.05, 1.94, and 1.85). In PS I complexes isolated from the C14SPsaC mutant, the FB cluster has a proposed net ground spin state of S ≥ 3/2 due to the substitution of cysteine for serine at position 14 (15). The large spin anisotropy of the high-spin state leads to an extremely broad spectrum, and a virtual disappearance of the resonances from the mixed-ligand cluster in the g = 2 region. Hence, when PS I complexes isolated from the C14SPsaC mutant are frozen at 15 K and illuminated, the only visible resonances are at g = 2.05, 1.94, and 1.86 from FA−. As shown in Fig. 2B, these resonances are present in PS I complexes isolated from the C14SPsaC-R18 pseudorevertant. Furthermore, there are no resonances visible at g = 2.07, 1.93, and 1.88 from FB−, implying that the serine at position 14 of PsaC has been retained as the ligand to the FB cluster. When PS I complexes are illuminated during freezing, more than one electron can be promoted from DCPIP and P700, and both clusters become reduced. In the wild type, this results in a complex “interaction” spectrum of FA− and FB− with resonances at g = 2.05, 1.94, 1.92, and 1.88 (Fig. 2C). In the C14SPsaC mutant, the presence of reduced FA causes the FB cluster to undergo a spin-state crossover from S ≥ 3/2 to S = 1/2, and the sample shows resonances at g = 2.05, 1.94, and 1.85 from FA− and at g = 2.08, 1.98, and 1.89 from FB−′, where the prime denotes an altered set of resonances from wild-type FB. As shown in Fig. 2D, these resonances are retained in PS I complexes isolated from the C14SPsaC-R18 pseudorevertant, confirming that serine oxygen is retained as the ligand to an iron atom of FB. The same pattern of resonances was present in the C14SPsaC-R62 pseudorevertant (data not shown).
FIG. 2.
Light-dark EPR spectra of PS I complexes isolated from the C14SPsaC-R18 pseudorevertant and from the wild-type strain. (A and C) Wild-type (A) and C14SPsaC-R18 (C) samples frozen to 15 K and illuminated for 5 min with white light to promote one electron to FA or FB. (B and D) Wild-type (B) and C14SPsaC-R18 (D) samples frozen under continuous illumination with white light to promote two electrons to FA and FB. The spectra in panels A and C are expanded twofold relative to the spectra in panels B and D. The g values of the resonances are depicted above the spectra. Experimental conditions were as follows: temperature, 15 K; microwave frequency, 9.4377 GHz (A and B) and 9.2756 GHz (C and D); modulation frequency, 100 KHz; modulation amplitude, 10 G. The differences in the magnetic field axis between panels A and C and panels B and D are due to the use of different resonators.
sll0088 gene and suppressor characterization.
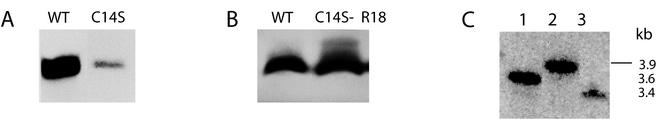
The two suppressor lines, C14SPsaC-R18 and C14SPsaC-R62, grow photoautotrophically at near-wild-type rates (Table 1) while the primary mutant C14SPsaC is incapable of photoautotrophic growth. Thylakoid membranes of the original C14SPsaC mutant contained decreased amounts of PsaC (46) (Fig. 3A). However, when thylakoid membranes from C14SPsaC-R62 were probed with the PsaC antibody, wild-type amounts of the mutant PsaC were found (Fig. 3B). This correlates well with the growth capabilities of this organism and is consistent with the idea that the original primary mutant failed to grow photoautotrophically because of problems in the stability-biogenesis of the PS I complex rather than because of a defect in the characteristics of PS I electron transfer due to the amino acid substitution. The PsaA and PsaB reaction center proteins were scanned by genetic complementation to determine if there were complementary mutations within the PS I complex, and none was found.
FIG. 3.
(A) Immunoblot of thylakoid membranes of C14SPsaC and wild-type cells probed with antibody to PsaC. (B) Immunoblot of thylakoid membranes of suppressor strain C14SPsaC-R18 probed with antibody to PsaC. (C) Southern blot of total genomic DNA of Synechocystis sp. strain PCC 6803 (50 ng; lane 1), Synechococcus sp. strain PCC 7002 (5 μg; lane 2), and C. reinhardtii (11 μg; lane 3). The Synechocystis sp. strain PCC 6803 sll0088 gene was used as the probe. WT, wild type.
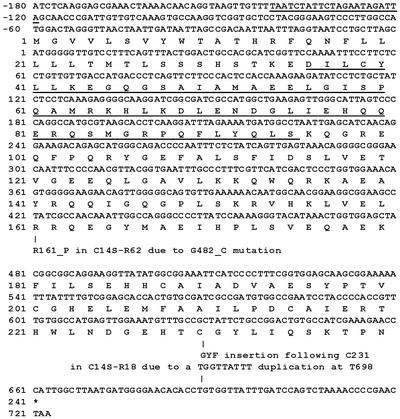
The dot transformation technique, however, identified a second-site mutation outside the PS I reaction center proteins in an open reading frame of the Synechocystis sp. strain PCC 6803 genome named sll0088. The nucleotide sequence of sll0088 and the deduced amino acid sequence, which are shown in Fig. 4, are available from the complete genome database of Synechocystis sp. strain PCC 6803 (http://www.Kazusa.or.jp/cyano/).
FIG. 4.
Nucleotide sequence of sll0088 and deduced amino acid sequence of the protein. The TTCnnGAA signature sequence is underlined in the promoter region. A putative HTH DNA-binding motif is underlined and was identified by alignment of amino acid sequences with several DNA regulatory proteins as shown in Fig. 5. The changes within sll0088 of the C14SPsaC-R62 and C14SPsaC-R18 pseudorevertants are also indicated.
The C14SPsaC-R62 pseudorevertant was found to substitute Pro for Arg at residue 161 as the result of a G482→C change in sll0088, and the C14SPsaC-R18 pseudorevertant was found to have a three-amino-acid insertion of Gly-Tyr-Phe following Cys231 as the result of a TGGTTATTT duplication at T690 in sll0088 (Fig. 4). These mutations apparently allow wild-type levels of PS I to be accumulated even in the presence of the primary mutation within psaC. This implies that sll0088 may repress the deleterious effect of these mutations, allowing high levels of PS I accumulation. This recognition could be at the gene or operon level, as a feedback from altered PS I complexes. It could be involved with overall cellular responses to increased oxidation levels within the mutant cell lines as predicted by decreased growth, oxygen evolution, and chlorophyll concentration, predictably leading to photoinhibition of PS I. Hence, the physiological and biochemical results suggest that the sll0088 gene influences the biogenesis and/or assembly of PS I.
sll0088 has homologs in other photosynthetic species.
Figure 3C shows genomic hybridization of sll0088 from Synechocystis sp. strain PCC 6803 with two other species, Synechococcus sp. strain PCC 7002 and the unicellular green alga Chlamydomonas reinhardtii. In these cases single genomic bands hybridized to a full-length probe for sll0088. The hybridization to a potential C. reinhardtii homolog is interesting in that it raises the possibility that regulation of PS I biogenesis and/or iron-sulfur cluster biogenesis in a eukaryote may similarly be influenced by an sll0088-like gene.
The protein encoded by sll0088 contains a typical helix-turn-helix (HTH) motif with similarity to transcriptional regulators.
Sequence analysis of the untranslated region in front of the sll0088 gene indicates that the promoter, underlined in Fig. 4, contains a TTCnnGAA signature sequence of a chloroplast heat shock transcription factor (6). To examine the structure of the protein encoded by sll0088 and its possible function in PS I assembly further, the amino acid sequence was used to search the GenBank database. As shown in Fig. 5, proteins with a high similarity, including those from Vibrio cholerae (18), Bacillus halodurans (40), Thermoplasma acidophilum (36), Streptomyces coelicolor (33), and Mycobacterium tuberculosis (9), are found to be hypothetical DNA-binding proteins. The greatest similarity is located at the N-terminal region. As demonstrated in Fig. 5, the N-terminal region of the protein encoded by sll0088 contains a predicted HTH DNA-binding structure similar to other gene-regulatory repressor proteins such as ArsR, the arsenic resistance operon repressor (28); SmtB, the cyanobacterial metallothionein repressor (10); LexA, a repressor in SOS response autocleavage; IolR, the DNA-binding protein iol operon repressor encoded by iolR (Bacillus subtilis) (44); GlpR, the glycerol-3-phosphate regulon repressor (14); AccR, the transcriptional repressor encoded by accR (3); DeoR, the deoxyribose operon repressor (42); LacR, a repressor of the lactose catabolism operon (34); MarR, the multiple antibiotic resistance repressor (1); HPR, a negative regulatory repressor of protease production (31); DtxR, an iron-dependent diphtheria toxin repressor (38); TroR, an iron-dependent repressor (Archaeoglobus fulgidus) (17); and SirR, a putative iron-dependent repressor (Staphylococcus epidermidis) (8). There are also similarities with the deoR family of gene-regulatory proteins (35) and the HTH region of the cyclic AMP regulatory protein (32). This comparative analysis suggests that the presence of the HTH DNA-binding domain correlates with a function as a transcriptional regulator. From protein sequence analysis, it is therefore demonstrated that the protein encoded by sll0088 contains a typical HTH structure in its DNA-binding domain, similar to other DNA-binding protein homologs from other organisms (shown in Fig. 5).
FIG. 5.
Alignment of the N-terminal region of the Synechocystis sp. strain PCC 6803 protein encoded by sll0088 (23, 24) with the DNA-binding domains of several regulatory repressor proteins from other organisms: ArsR, the arsenic resistance operon repressor (28); SmtB, the cyanobacterial metallothionein repressor (10); AccR, the transcriptional repressor (3); MarR, the multiple antibiotic resistance repressor (1); GlpR, the glycerol-3-phosphate regulon repressor (14); IolR, the DNA-binding protein iol operon repressor (44); DtxR, the iron-dependent diphtheria toxin repressor (38); and SirR, the putative iron-dependent repressor (S. epidermidis) (8). Alignment analysis was carried out by applying the MacVector Sequence Analysis program from Genetics Computer Group (Oxford Molecular Ltd., Accelrys, San Diego, Calif.).
To provide more insight into its function and regulation, the amino acid sequence was used for phylogenetic analysis with other typical HTH proteins that have been well characterized (reviewed in reference 22). It was found that the protein encoded by sll0088, together with DNA-binding homologs from several prokaryotes and the homolog protein from Arabidopsis thaliana, can be classified in the HTH protein group represented by Id (4), a typical negative regulator (data not shown).
Insertional inactivation of sll0088 in Synechocystis sp. strain PCC 6803.
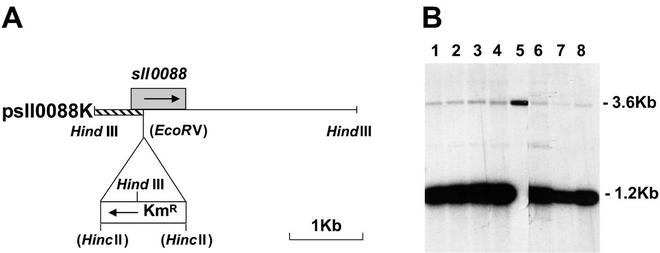
We attempted inactivation of sll0088 to verify that the hypothetical protein product encoded by sll0088 was important in assembling altered PsaC subunits. The single-copy wild-type sll0088 gene was inactivated by insertion of a kanamycin resistance gene cassette (Fig. 6A). This construct was designated psll0088K. This cassette was inserted into the wild-type gene at an EcoRV site, which was destroyed by the procedure, and the construct was transformed into the wild-type cells of Synechocystis sp. strain PCC 6803 and C14SPsaC cells (Fig. 4). The transformed C14SPsaC cells grew photoautotrophically (Table 1) and were kanamycin resistant. In effect, this created an “engineered” suppressor mutant of sll0088 that acts in a manner similar to C14SPsaC-R62, allowing near-normal growth (Table 1). Wild-type cells transformed with psll0088K were kanamycin resistant and were screened to determine if it was possible to delete wild-type copies completely. The transformed C14SPsaC and wild-type cells were taken through five serial platings under photoautotrophic and kanamycin selection conditions; however, it was not possible to delete the wild-type sll0088 gene completely (Fig. 6B). In comparison with the amount of wild-type DNA (Fig. 6B, lane 5), it was estimated that the amount of sll0088 DNA present was less than one genome copy per cell. It was not possible to completely remove the sll0088 gene even under nonphotosynthetic, heterotrophic growth conditions. This suggests that there might be some nonphotosynthetic process in Synechocystis sp. strain PCC 6803 that requires the protein product of sll0088, one possibility being cell division.
FIG. 6.
(A) Insertion of a kanamycin resistance cassette into an EcoRV site of sll0088 that had been cloned as a HindIII fragment. Arrows show the directions of transcription. (B) Southern blot of various transformants probed with the shaded area shown in panel A. All transformed lines were taken through five serial platings under photoautotrophic growth selection conditions. Lanes 1 to 4 represent four independent cell lines of C14SPsaC transformed with psll0088K. Lane 5 contains 50 ng of DNA from wild-type cells. Lanes 6 to 8 show wild-type cell lines transformed with psll0088K and grown under heterotrophic, mixotrophic, and photoautotrophic conditions (46), respectively. The hybridization at 1.2 kb represents the 0.7-kb HindIII-EcoRV fragment of Synechocystis sp. strain PCC 6803 DNA and the 0.5-kb HincII-HindIII fragment of the inserted kanamycin resistance gene. The hybridization at 3.6 kb represents the wild-type sll0088 gene.
Insertional inactivation of sll0088 in Synechococcus sp. strain PCC 7002.
We showed above (Fig. 3C) that Synechococcus sp. strain PCC 7002 contains a gene similar to sll0088. This cyanobacterium has a different physiology than Synechocystis sp. strain PCC 6803: it is adapted to saline rather than freshwater conditions, it is able to grow heterotrophically with glycerol in the dark rather than glucose under LAHG conditions, and it is capable of growing photoautotrophically under high illumination. We attempted to inactivate the sll0088 homolog in Synechococcus sp. strain PCC 7002.
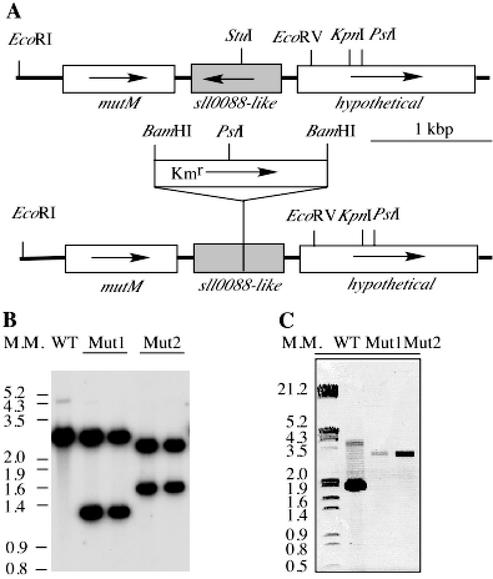
A 1.8-kbp KpnI-EcoRV DNA fragment containing the sll0088-like gene was cloned from the genome of Synechococcus sp. strain PCC 7002. A 1.3-kbp AphII gene conferring kanamycin resistance was inserted at the unique StuI site in the sll0088-like gene as shown in Fig. 7A. This construct was used to transform Synechococcus sp. strain PCC 7002 wild-type cells. To verify the complete segregation of the sll0088-like interruption in the mutant strains, genomic DNAs of the wild type and sll0088-like deletion mutants were fragmented by EcoRI/PstI digestions and the blot was probed with a 1.8-kb PCR fragment from the wild type (Fig. 7B). The probe hybridized to a 2.6-kb band, as predicted by sequence analysis. Two hybridization bands were detected in the mutant strains as a result of introducing the PstI site in the kanamycin resistance cartridge gene. PCR analysis verified that no DNA fragment with the same size as the wild type at 1.8 kbp could be identified in genomic DNAs of the sll0088-like insertion mutants (Fig. 7C). Only a 3.1-kbp fragment was amplified from the sll0088-like insertion mutant, which is evidence for the insertion of the 1.3-kbp kanamycin resistance cartridge. These results demonstrate complete segregation of the sll0088-like gene in Synechococcus sp. strain PCC 7002.
FIG. 7.
Mutagenesis of the sll0088-like gene in Synechococcus sp. strain PCC 7002. (A) Restriction maps of DNA fragments from the wild type and the sll0088-like mutant. (B) Southern blot hybridization of the genomic DNAs of the wild-type and the sll0088-like mutant strains. In Mut1 strains a Kmr gene is transcribed in the same direction as the sll0088-like gene, while in Mut2 strains it is transcribed in the opposite direction. Genomic DNAs were digested by EcoRI and PstI. The hybridization probe was prepared with a 1.8-kb PCR fragment containing the sll0088-like gene. (C) PCR analysis of segregation of the sll0088-like gene interruption. WT, wild type. Numbers to the left of panels B and C show molecular sizes in kilobase pairs.
Expression of the psaC gene in sll0088-like deletion mutants in Synechococcus sp. strain PCC 7002.
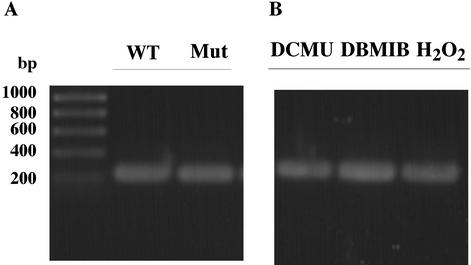
Our initial physiological characterization indicates that the sll0088-like deletion mutant in Synechococcus sp. strain PCC 7002 can grow photoautotrophically. No obvious difference could be observed in assembly-stability of the PS I complexes (data not shown). To determine if the sll0088 homolog is involved in the regulation of psaC expression, we measured the transcript level of psaC in the wild type and the sll0088 deletion mutant. As shown in Fig. 8A, no difference was found in the expression of the psaC gene in Synechocccus sp. strain PCC 7002 in the presence or in the absence of the sll0088 homolog. Also, the expression level of the psaC gene is not subject to the redox regulation when cells are grown under different oxidative stress conditions, as shown in Fig. 8B.
FIG. 8.
Results of reverse transcription-PCR analysis on expression of the psaC gene in Synechococcus sp. strain PCC 7002. Total RNAs were isolated from cells of Synechococcus sp. strain PCC 7002 wild type (WT) and the sll0088 gene mutant. A 1.2-ng quantity of RNA was used as template for reverse transcription-PCR with the One-Step reverse transcription-PCR kit from Qiagen. DBMIB, dibromomethyl-isopropyl p-benzoquinone.
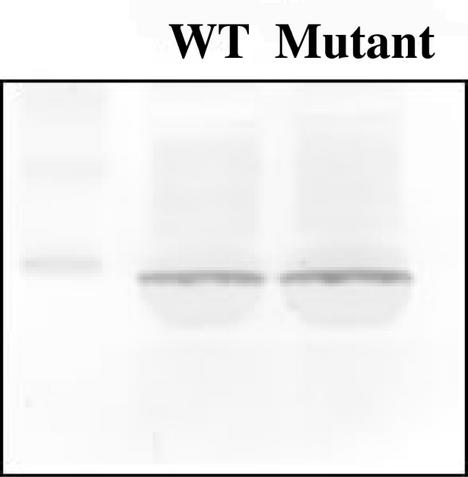
To examine whether the sll0088-like gene is involved in regulation of the translation of the PsaC protein, its assembly in thylakoid membrane was compared between the Synechococcus sp. strain PCC 7002 wild type and the sll0088-like deletion mutant. As shown in Fig. 9, no obvious difference can be seen in amounts of PsaC assembled in thylakoids of the wild type and of the mutant (similar to Synechocystis strain PCC 6803, Fig. 3B). These results indicate that the sll0088-like gene in Synechococcus sp. strain PCC 7002 is not directly involved in regulation of expression of the psaC gene on the transcriptional or translational levels.
FIG. 9.
Immunoblot analysis of the PsaC protein in thylakoid membranes of Synechococcus sp. strain PCC 7002 wild type (WT) and sll0088 (mutant). Thylakoid membranes were isolated from cells grown under normal growth conditions. Six micrograms of chlorophyll was loaded. Proteins were resolved by SDS-polyacrylamide gel electrophoresis, blotted onto a nitrocellulose membrane, and probed with antiserum against the PsaC protein from Synechococcus sp. strain PCC 7002. The left lane contains markers.
DISCUSSION
In this paper we have described primary mutants of a PS I subunit, PsaC, that were used to select for suppressor strains capable of photoautotrophic growth. We proposed that three classes of site-specific suppressors or pseudorevertants were to be expected from these experiments. Class I mutations affect electron transport into PS I via PS II and were not investigated but were inferred from earlier work employing PS II inhibitors (46). Class II mutations exist within a neighboring subunit that might complement site-specific mutations within PsaC. A number of these point-suppressor mutations were localized to the PsaB subunit (Fig. 1) but are not described here (Yu and McIntosh, unpublished). Class III mutations were predicted, by elimination, to be non-PS I proteins that might affect PS I regulation and/or biogenesis. More than 100 cell lines, termed suppressor mutations, were found, and the two characterized in detail were discovered to reside in a gene encoding a previously uncharacterized protein encoded by the open reading frame sll0088.
Mutations in the protein encoded by the sll0088 gene allow for the normal assembly of an active PS I complex, even with the presence of primary-site-specific mutations targeted to the iron-sulfur cluster cysteine ligands and affecting assembly-function (46). Two pseudorevertants to the primary mutation, C14SPsaC, were found; in C14SPsaC-R62 there is an Arg-to-Pro mutation in the protein encoded by sll0088, and in C14SPsaC-R18 there is a Gly-Tyr-Phe insertion at the carboxyl end of the protein encoded by sll0088. These suppressor lines allowed cells containing the C14SPsaC primary mutation to grow photoautotrophically, albeit with a slightly lowered chlorophyll a content and near-wild-type levels of PS I. The presumption is that these changes alter or prevent the function of the protein encoded by the sll0088 gene. This conclusion was supported by the engineering of an artificial suppressor mutant consisting of a kanamycin resistance cartridge inserted into the sll0088 gene. This construct allowed cells containing the C14SPsaC primary mutation to grow photoautotrophically and to accumulate wild-type levels of PS I. Primary mutations in PS I raise the issue of stress responses in the cell. This may be supported by the discovery of a class of mutations that we hypothesized could be impaired in PS II activity. These mutants would lower the electron flow from PS II to PS I, alleviating the toxicity caused by overreduction of PS I. This is possible in particular with cells with impaired PS I activity, giving rise to increased levels of reactive oxygen species due to inefficient electron transfer to NADP+.
The identical EPR spectra of FA and FB in PS I complexes isolated from the original mutant strain, C14SPsaC, and from the suppressor strain C14SPsaC-R18 show that the serine oxygen continues to provide a ligand to the FB iron-sulfur cluster. There is an important consequence to this finding, which is that the inability of the primary C14SPsaC strain to grow photoautotrophically is not due to an impairment of electron transfer as a result of the substitution of a sulfur ligand for an oxygen ligand; instead, it is due to a lower content of PS I in the cell, in which case the imbalance between PS II and PS I results in light-generated intermediates that are toxic to the cells. The inactivation of the sll0088 gene leads to higher levels of PS I, thereby restoring the proper balance between PS I and PS II. Indeed, the near-wild-type rates of electron transfer in PS I complexes isolated from the C14SPsaC mutant strain (21, 46) had initially suggested that the mixed-ligand (3•Cys 1•Ser) [4Fe-4S] cluster was fully competent in electron transfer; hence, the lesion was proposed to lie elsewhere.
The protein encoded by sll0088 contains a typical conserved domain, known as an HTH motif, following the proposed DNA-binding site (as shown in Fig. 4). The HTH is composed of two amphipathic α-helices and a loop region of variable length, which is proposed to be an important structural motif that mediates protein dimerization (homodimerization or heterodimerization) (for a review see reference 22). There are three major groups in the HTH family of DNA-binding proteins based on their structure and function: the bHTH family, the bHTH-Zip family, and the dnHTH family. The HTH protein family is represented by the Id protein (4), characterized by a lack of positive residues in the DNA-binding domain. As indicated in the phylogenetic analysis, the protein encoded by sll0088 can be classified in the dnHTH family. Based on its similarity in structural features with dnHTH proteins, it is possible to envision this protein as a repressor in control of gene expression. As indicated in Fig. 5, the HTH DNA-binding domain displays high similarity to several negative repressors, such as the arsenic resistance operon repressor ArsR (28); the cyanobacterial metallothionein repressor SmtB (10); the multiple antibiotic resistance repressor MarR (1); and the iron-dependent repressors DtxR (38), TroR (17), and SirR (8).
We did not observe any difference in psaC gene expression between the wild type and the sll0088 insertion mutants in Synechococcus strain PCC 7002. It therefore appears that the psaC gene is not under the regulation of sll0088. It is also shown that, in the sll0088 insertion mutant, the PsaC protein accumulates to wild-type levels (Fig. 3B and 9). Since the PsaC protein is allowed to accumulate in an sll0088 insertion background, we must assume that sll0088 does not act directly on the psaC gene or its protein product. These results indicate that inactivation of sll0088, presumably leading to the suppression of the wild-type protein, is involved in allowing (i) modified PsaC subunits to be assembled into the thylakoid membrane-embedded PS I complex or (ii) a stress response system responding to PS I lesions, one possibly involved in regulation of biogenesis or other proteins involved in a stress response. This was further shown by immunological characterization of the C14SPsaC protein in thylakoid membranes in which wild-type levels of the altered C14SPsaC protein were found in the photosynthetic membranes of the two suppressor lines. A corollary of this finding is that it should be possible to isolate additional second-site suppressors that would identify additional genes involved in the up-regulation of the amount of PS I in cyanobacterial cells. This should be a fruitful strategy for locating additional genes that are involved in iron-sulfur cluster biogenesis and/or the regulation of PS I reaction center proteins, Furthermore, a complete characterization of the sll0088 gene and its protein product should allow us to determine, as our data suggest, that sll0088 acts as a repressor of genes involved in PS I biogenesis.
Acknowledgments
This work is supported in part by USDA award 2001-35318-10125 to J.H.G., by grants from the NSF (MCB-0077586) NIH (GM-31625) to D.A.B., and by a DOE contract to L.M.
We thank Ramakrishnan Balasubramanian for proofreading the manuscript.
REFERENCES
- 1.Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat. Struct. Biol. 8:710-714. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S. L., and L. McIntosh. 1991. Partial conservation of the 5′ ndhE-psaC-ndhD 3′ gene arrangement of chloroplasts in the cyanobacterium Synechocystis sp. PCC 6803: implications for NDH-D function in cyanobacteria and chloroplasts. Plant Mol. Biol. 16:487-499. [DOI] [PubMed] [Google Scholar]
- 3.Beck von Bodman, S., G. T. Hayman, and S. K. Farrand. 1992. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc. Natl. Acad. Sci. USA 89:643-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benezra, R., R. L. Davis, D. Lockshon, D. L. Turner, and H. Weintraub. 1990. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61:49-59. [DOI] [PubMed] [Google Scholar]
- 5.Brasseur, G., J. P. Di Rago, P. P. Slonimski, and D. Lemesle-Meunier. 2001. Analysis of suppressor mutation reveals long distance interactions in the bc1 complex of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1506:89-102. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Q., K. Yu, and J. L. Stevens. 1992. Regulation of the cellular stress response by reactive electrophiles. The role of covalent binding and cellular thiols in transcriptional activation of the 70-kilodalton heat shock protein gene by nephrotoxic cysteine conjugates. J. Biol. Chem. 267:24322-24327. [PubMed] [Google Scholar]
- 7.Chitnis, P. R. 2001. Photosystem I: function and physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52:593-626. [DOI] [PubMed] [Google Scholar]
- 8.Cockayne, A., P. J. Hill, N. B. Powell, K. Bishop, C. Sims, and P. Williams. 1998. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect. Immun. 66:3767-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 10.Cook, W. J., S. R. Kar, K. B. Taylor, and L. M. Hall. 1998. Crystal structure of the cyanobacterial metallothionein repressor SmtB: a model for metalloregulatory proteins. J. Mol. Biol. 275:337-346. [DOI] [PubMed] [Google Scholar]
- 11.di Rago, J. P., S. Hermann-Le Denmat, F. Paques, F. P. Risler, P. Netter, and P. P. Slonimski. 1995. Genetic analysis of the folded structure of yeast mitochondrial cytochrome b by selection of intragenic second-site revertants. J. Mol. Biol. 248:804-811. [DOI] [PubMed] [Google Scholar]
- 12.Dzelzkalns, V. A., and L. Bogorad. 1988. Molecular analysis of a mutant defective in photosynthetic oxygen evolution and isolation of a complementing clone by a novel screening procedure. EMBO J. 7:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ermakova-Gerdes, S., and W. Vermaas. 1999. Inactivation of the open reading frame slr0399 in Synechocystis sp. PCC 6803 functionally complements mutations near the Q(A) niche of photosystem II. A possible role of Slr0399 as a chaperone for quinone binding. J. Biol. Chem. 274:30540-30549. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 15.Golbeck, J. H. 1999. A comparative analysis of the spin state distribution of in vivo and in vitro mutants of PsaC. A biochemical argument for the sequence of electron transfer in photosystem I as FX → FA → FB → ferredoxin/flavodoxin. Photosynth. Res. 61:107-149. [Google Scholar]
- 16.Golbeck, J. H. 1993. The structure of photosystem I. Curr. Opin. Struct. Biol. 3:508-514. [Google Scholar]
- 17.Hardham, J. M., L. V. Stamm, S. F. Porcella, J. G. Frye, N. Y. Barnes, J. K. Howell, S. L. Mueller, J. D. Radolf, G. M. Weinstock, and S. J. Norris. 1997. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transport system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene 197:47-64. [DOI] [PubMed] [Google Scholar]
- 18.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleischmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman, P. L., K. Adiwilaga, J. H. Golbeck, and D. P. Weeks. 1994. Sequence of a psaC gene from the cyanobacterium Synechococcus sp. PCC 6301. Plant Physiol. 104:1459-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung, K. H., and J. L. Spudich. 1998. Suppressor mutation analysis of the sensory rhodopsin I-transducer complex: insights into the color-sensing mechanism. J. Bacteriol. 180:2033-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung, Y. S., I. R. Vassiliev, J. P. Yu, L. McIntosh, and J. H. Golbeck. 1997. Strains of Synechocystis sp. PCC 6803 with altered PsaC. II. EPR and optical spectroscopic properties of FA and FB in aspartate, serine, and alanine replacements of cysteines 14 and 51. J. Biol. Chem. 272:8040-8049. [DOI] [PubMed] [Google Scholar]
- 22.Kadesch, T. 1993. Consequences of heteromeric interactions among helix-loop-helix proteins. Cell Growth Differ. 4:49-55. [PubMed] [Google Scholar]
- 23.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109-136. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions (supplement). DNA Res. 3:185-209. [DOI] [PubMed] [Google Scholar]
- 25.Li, N., P. Warren, J. Golbeck, G. Frank, H. Zuber, and D. Bryant. 1991. Polypeptide composition of the photosystem I complex and the photosystem I core protein from Synechococcus sp. PCC-6301. Biochim. Biophys. Acta 1059:215-225. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenthaler, H. K. 1987. Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods Enzymol. 148:350-382. [Google Scholar]
- 27.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 28.Maeder, D. L., R. B. Weiss, D. M. Dunn, J. L. Cherry, J. M. Gonzalez, J. DiRuggiero, and F. T. Robb. 1999. Divergence of the hyperthermophilic archaea Pyrococcus furiosus and P. horikoshii inferred from complete genomic sequences. Genetics 152:1299-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohad, N., and J. Hirschberg. 1992. Mutations in the D1 subunit of photosystem II distinguish between quinone and herbicide binding sites. Plant Cell 4:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pakrasi, H. B., J. G. Williams, and C. J. Arntzen. 1988. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 7:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perego, M., and J. A. Hoch. 1988. Sequence analysis and regulation of the hpr locus, a regulatory gene for protease production and sporulation in Bacillus subtilis. J. Bacteriol. 170:2560-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen, S., and G. M. Young. 2002. Essential role for cyclic AMP and its receptor protein in Yersinia enterocolitica virulence. Infect. Immun. 70:3665-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77-96. [DOI] [PubMed] [Google Scholar]
- 34.Rosey, E. L., and G. C. Stewart. 1992. Nucleotide and deduced amino acid sequences of the lacR, lacABCD, and lacFE genes encoding the repressor, tagatose 6-phosphate gene cluster, and sugar-specific phosphotransferase system components of the lactose operon of Streptococcus mutans. J. Bacteriol. 174:6159-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy, S., A. Sahu, and S. Adhya. 2002. Evolution of DNA binding motifs and operators. Gene 285:169-173. [DOI] [PubMed] [Google Scholar]
- 36.Ruepp, A., W. Graml, M. L. Santos-Martinez, K. K. Koretke, C. Volker, H. W. Mewes, D. Frishman, S. Stocker, A. N. Lupas, and W. Baumeister. 2000. The genome sequence of the thermoacidophilic scavenger Thermoplasma acidophilum. Nature 407:508-513. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., and M. J. Gething. 1989. Protein structure. Chaperones, paperones. Nature 342:224-225. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt, M. P., E. M. Twiddy, and R. K. Holmes. 1992. Purification and characterization of the diphtheria toxin repressor. Proc. Natl. Acad. Sci. USA 89:7576-7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smart, L., S. Anderson, and L. McIntosh. 1991. Targeted genetic inactivation of the photosystem I reaction center in the cyanobacterium Synechocystis sp. PCC-6803. EMBO J. 10:3289-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takami, H., K. Nakasone, Y. Takaki, G. Maeno, R. Sasaki, N. Masui, F. Fuji, C. Hirama, Y. Nakamura, N. Ogasawara, S. Kuhara, and K. Horikoshi. 2000. Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res. 28:4317-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tichy, M., and W. Vermaas. 2000. Combinatorial mutagenesis and pseudorevertant analysis to characterize regions in loop E of the CP47 protein in Synechocystis sp. PCC 6803. Eur. J. Biochem. 267:6296-6301. [DOI] [PubMed] [Google Scholar]
- 42.Valentin-Hansen, P., P. Hojrup, and S. Short. 1985. The primary structure of the DeoR repressor from Escherichia coli K-12. Nucleic Acids Res. 13:5927-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassiliev, I. R., M. L. Antonkine, and J. H. Golbeck. 2001. Iron-sulfur clusters in type I reaction centers. Biochim. Biophys. Acta 1507:139-160. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida, K., S. Seki, M. Fujimura, Y. Miwa, and Y. Fujita. 1995. Cloning and sequencing of a 36-kb region of the Bacillus subtilis genome between the gnt and iol operons. DNA Res. 2:61-69. [DOI] [PubMed] [Google Scholar]
- 45.Yu, J. P., and L. McIntosh. 1998. Isolation and genetic characterization of pseudorevertants from site-directed PSI mutants in Synechocystis 6803. Methods Enzymol. 297:18-26. [Google Scholar]
- 46.Yu, J. P., I. R. Vassiliev, Y. S. Jung, J. H. Golbeck, and L. McIntosh. 1997. Strains of Synechocystis sp. PCC 6803 with altered PsaC. I. Mutations incorporated in the cysteine ligands of the two [4Fe-4S] clusters FA and FB of photosystem I. J. Biol. Chem. 272:8032-8039. [DOI] [PubMed] [Google Scholar]