Abstract
Intracellular poly[d-(−)-3-hydroxybutyrate] (PHB) depolymerases degrade PHB granules to oligomers and monomers of 3-hydroxybutyric acid. Recently an intracellular PHB depolymerase gene (phaZ1) from Ralstonia eutropha was identified. We now report identification of candidate PHB depolymerase genes from R. eutropha, namely, phaZ2 and phaZ3, and their characterization in vivo. phaZ1 was used to identify two candidate depolymerase genes in the genome of Ralstonia metallidurans. phaZ1 and these genes were then used to design degenerate primers. These primers and PCR methods on the R. eutropha genome were used to identify two new candidate depolymerase genes in R. eutropha: phaZ2 and phaZ3. Inverse PCR methods were used to obtain the complete sequence of phaZ3, and library screening was used to obtain the complete sequence of phaZ2. PhaZ1, PhaZ2, and PhaZ3 share ∼30% sequence identity. The function of PhaZ2 and PhaZ3 was examined by generating R. eutropha H16 deletion strains (ΔphaZ1, ΔphaZ2, ΔphaZ3, ΔphaZ1ΔphaZ2, ΔphaZ1ΔphaZ3, ΔphaZ2ΔphaZ3, and ΔphaZ1ΔphaZ2ΔphaZ3). These strains were analyzed for PHB production and utilization under two sets of conditions. When cells were grown in rich medium, PhaZ1 was sufficient to account for intracellular PHB degradation. When cells that had accumulated ∼80% (cell dry weight) PHB were subjected to PHB utilization conditions, PhaZ1 and PhaZ2 were sufficient to account for PHB degradation. PhaZ2 is thus suggested to be an intracellular depolymerase. The role of PhaZ3 remains to be established.
Polyhydroxyalkanoates (PHAs) are polyoxoesters produced by a wide range of bacteria when they find themselves in an environment with an available carbon source but limited in additional nutrient(s) required for growth (9). The short-chain-length PHAs, where R is a methyl or ethyl, have properties of thermoplastics and are biodegradable (Fig. 1). Much effort has focused on understanding the biology of PHA homeostasis for several reasons. First, this understanding could lead to expression of the appropriate gene set in heterologous systems to make PHA production economically competitive with oil-based polymers. Second, understanding PHA homeostasis serves as a paradigm for understanding the mechanism of homopolymerization reactions in which the product undergoes a phase transition during its formation, generating insoluble inclusions (granules). The intracellular PHAs can be degraded when the bacteria require carbon but are in otherwise nutrient-replete conditions, and the monomers and energy released can be reused to allow the bacteria to grow (17). The insoluble PHA granules must, therefore, be biosynthesized in a controlled fashion to facilitate enzymatic degradation. A variety of proteins associated with PHA homeostasis have been identified and are being characterized (3, 8, 10, 12, 20, 22). As part of our research to understand the mechanisms that control polymer size and reuse, we have been interested in identifying the intracellular depolymerases that degrade poly[d-(−)-3-hydroxybutyrate] (PHB) within the granules. The cloning, sequencing, and characterization in vivo of two new putative intracellular PHB depolymerases from Ralstonia eutropha are reported.
FIG. 1.
Thermoplastic short-chain-length PHAs are synthesized from monomers with short side chains.
Extracellular depolymerases, in contrast to the intracellular depolymerases, have been extensively characterized over the last decade (5). These enzymes are secreted into the environment to degrade PHA released from dead bacteria. These proteins in general contain a N-terminal signal peptide of 25 to 38 amino acids. The peptide is cleaved during passage of the protein out of the cytosol. These depolymerases contain a large N-terminal catalytic domain with a lipase box (GXSXG), a C-terminal PHB binding domain, and a linker region that connects the N- and C-terminal domains (for an excellent review, see reference 6). Several organisms contain extracellular depolymerase isozymes whose functions have not yet been clearly delineated.
The first sequence of an intracellular depolymerase was reported by Saegusa et al. (GenBank accession no. AB017612) (14) and was somewhat surprising, as the protein had no sequence similarity to the extracellular depolymerases. Recently this intracellular depolymerase, PhaZ (here designated PhaZ1), from R. eutropha was expressed, and its properties were examined in crude extracts. PhaZ1 is 47 kDa and has neither a lipase box nor an identifiable PHB binding domain. The depolymerase was shown to work best on amorphous PHB as found in the granules inside the cell. Furthermore, the enzyme was shown to convert the polymer into oligomers and monomers of hydroxybutyrate by the end of the degradation process (14). The striking differences between the sequences of the extracellular and intracellular depolymerases make the intracellular depolymerases interesting subjects for mechanistic and biological studies.
The function of PhaZ1 in vivo has recently been addressed by two groups independently (4, 14). Each group generated a ΔphaZ1 in R. eutropha H16 and then examined the fate of accumulated PHB under different growth conditions. The authors concluded from their studies that additional depolymerase(s) must be present to account for the observed phenotypes.
The availability of the R. eutropha phaZ1 sequence and the Ralstonia metallidurans genome sequence allowed us to successfully devise a strategy to find two additional candidate depolymerase genes in R. eutropha, designated phaZ2 and phaZ3. To understand the function of PhaZ2 and PhaZ3, a set of R. eutropha H16 phaZ gene deletion strains have been generated (ΔphaZ1, ΔphaZ2, ΔphaZ3, ΔphaZ1ΔphaZ2, ΔphaZ1ΔphaZ3, ΔphaZ2ΔphaZ3, and ΔphaZ1ΔphaZ2ΔphaZ3). The effect of these phaZ gene deletions on PHB accumulation and utilization when different R. eutropha strains were grown in rich medium and in PHB utilization medium are reported. These studies reveal that PhaZ2 is an intracellular depolymerase. The function of PhaZ3 remains to be established.
MATERIALS AND METHODS
Strains and plasmids.
All strains and plasmids used in this study are listed in Table 1. The oligonucleotides used in this study are listed in Table 2.
TABLE 1.
Strains and plasmids used in this studya
| Strain or plasmid | Descriptionb | Reference or source |
|---|---|---|
| R. eutropha strains | ||
| Ae H16 | Wild-type; Gm resistant | ATCC17699 |
| Re1097 | ΔphaZ1 strain, derived from Ae H16/pGY96 | This study |
| Re1107 | ΔphaZ3 strain, derived from Ae H16/pPK51 | This study |
| Re1108 | ΔphaZ1ΔphaZ3, strain, derived from Re1097/pPK51 | This study |
| Re1110 | ΔphaZ2 strain, derived from AeH16/pJOE39 | This study |
| Re1111 | ΔphaZ1ΔphaZ2ΔphaZ3 strain, derived from Re1108/pJOE39 | This study |
| Re1112 | ΔphaZ1ΔphaZ2 strain, derived from Re1097/pJOE39 | This study |
| Re1113 | ΔphaZ2ΔphaZ3 strain, derived from Re1107/pJOE39 | This study |
| E. coli strains | ||
| DH5α | Strain for ligation, cloning, and heterologous expression of PHA genes | New England Biolabs |
| DH5αF′ | F′/endA1 hsdR17 glnV44 thi-1 recA1 gyrA relA1DU169 deoR | 19 |
| S17-1 | recA pro hsdR RP4-2-Tc::Mu-Km::Tn7 | ATCC47055 |
| Plasmids | ||
| PBluescriptIIKS | Cloning vector; LacZa Ap resistance | Stratagene |
| pCR2.1-TOPO | High-copy-number plasmid used for cloning; confers Ap and Km resistance | Invitrogen |
| pJQ200mp 18Km | Derivative of pJQ200mp 18; Gm resistance gene disrupted; confers Km resistance | 21 |
| pGY96 | ΔphaZ1 gene replacement plasmid; confers Km resistance | This study |
| pJOE39 | ΔphaZ2 gene replacement plasmid; confers Km resistance | This study |
| pPK51 | ΔphaZ3 gene replacement plasmid; confers Km resistance | This study |
| pWt-250-2 | ∼240-bp PCR product corresponding to phaZ1; cloned in pCR2.1-TOPO | This study |
| pZ15 | ∼240-bp PCR product corresponding to phaZ2; cloned in pCR2.1-TOPO | This study |
| pZ31 | ∼240-bp PCR product corresponding to phaZ3; cloned in pCR2.1-TOPO | This study |
Plasmids constructed in this study that were used only as intermediates for construction of other plasmids are described only in Materials and Methods.
Abbreviations: Ap, ampicillin; Gm, gentamicin; Km, kanamycin.
TABLE 2.
Oligonucleotides used in this study
| Purpose and oligonucleotides | Sequencea | Location and orientationb |
|---|---|---|
| Probing intracellular depolymerases | ||
| phaZB-3 | GTSTAYRTBACXGAYTGG | Degenerate oligonucleotides (degeneracy: 192) |
| phaZB-4 | GCRTCGATBGGVCCXSCSAT | Degenerate oligonucleotides (degeneracy: 288) |
| Inverse PCR | ||
| phaZC1 | AAGTGCCCGGCGTCAAGCG | 5′ phaZ3 core sequence |
| phaZC2 | GCATATCGTGGCGGTTTGCC | 3′ phaZ3 core sequence |
| Library screening | ||
| phaZB6 | GCCGATCAGGTGCTGCACG | 5′ phaZ2 core sequence |
| phaZB10 | GCGGGATCCCAAATCCCAGGTCCGGTGG | 3′ phaZ2 core sequence |
| Generation of deletion strains | ||
| phaZ6 | CCGGAGGATCCCGCAACAGGTGGCGAC | 5′ end of region upstream of phaZ1 ORF (+) |
| phaZ7 | CGCAATCGCGGGCGTTTTCGCCTTTTCTGCCTGGGTCTA | Fusion upstream and downstream of phaZ1 ORF (−) |
| phaZ8 | TAGACCCAGGCAGAAAAGGCGAAAACGCCCGCGATTGCG | Fusion upstream and downstream of phaZ1 ORF (+) |
| phaZ9 | GCCGAGGATCCGCTGATCAACCCGGTGGTGG | 3′ end of region downstream of phaZ1 ORF (−) |
| phaZD1b | CGTGCCAGGCATAAACTGATGGCCCCGGCAGCCGCCAGC | Fusion upstream and downstream of phaZ2 ORF (−) |
| phaZD2c | AAAGGATCCCGAAGACAAAGGCAAAGGGGTAG | 3′ end of region downstream of phaZ2 ORF (−) |
| phaZD3b | GCTGGCGGCTGCCGGGGCCATCAGTTTATGCCTGGCACG | Fusion upstream and downstream of phaZ2 ORF (+) |
| phaZD4b | TTTGGATCCAGCCTTGGGGTGGATTTCATTC | 5′ end of region upstream of phaZ2 ORF (+) |
| phaZC8 | GCCAAGGCGACGGAGCGCTGCGATTCCCGCCTTTTTG | Fusion upstream and downstream of phaZ3 ORF (−) |
| phaZC9 | CAAAAAGGCGGGAATCGCAGCGCTCCGTCGCCTTGGC | Fusion upstream and downstream of phaZ3 ORF (+) |
| phaZC10 | GGCCGGATCCACTTGATTGCAAGCTGCTCC | 5′ end of region upstream of phaZ3 ORF (+) |
| phaZC11 | GGCCGGATCCTTATTCCGAGCACAAGTGCG | 3′ end of region downstream of phaZ3 ORF (−) |
| Deletion strain verification (additional oligonucleotides) | ||
| phaZ2as | AATGGCATGTTGATCGTTGGTG | Upstream of phaZ2 ORF (−) |
| phaZ2se | CCTCCTTTACTGCTTGTTGCCG | Downstream of phaZ2 ORF (+) |
Restriction site (BamHI) engineered into sequences is indicated in bold lettering. Degenerate oligonucleotide code: B = T, C, G; R = A, G; S = C, G; V = A, G, C; X = T, C, A, G; Y = T, C.
Forward (+) or reverse (−) orientation relative to ORF is indicated.
Media and growth conditions.
All Escherichia coli strains were grown aerobically at 37°C in Luria-Bertani (LB) medium or solid LB agar (1.2%). All R. eutropha strains were cultivated aerobically at 30°C in LB medium or Tryptic soy broth-dextrose free (TSB) medium (Becton Dickinson Microbiology Systems, Cockeysville, Md.). The mating of Escherichia coli S17-1 with R. eutropha was performed at 30°C on LB agar supplemented with thiamine (100 μg/liter), magnesium sulfate (1 mM), and calcium chloride (0.25 mM). The sacB gene selection was performed on sodium chloride-free LB agar supplemented with 5% sucrose and 0.2% fructose at 30°C. PHB production was examined in minimal medium (11) supplemented with 1% fructose and 0.01% ammonium chloride (PHB high); PHB utilization was examined in minimal medium supplemented with 0.5% ammonium chloride (PHB no carbon). Both processes were also studied in TSB rich medium. For E. coli strains, typical concentrations of antibiotics used were: ampicillin, 100 μg/ml; kanamycin, 25 μg/ml; or gentamicin, 10 μg/ml. For R. eutropha strains, the same concentrations were applied except for kanamycin, which was used at 270 μg/ml.
Chemicals and enzymes.
All restriction endonucleases, DNA markers, and phage T4 DNA ligase (EC 6.5.1.1) were obtained from New England Biolabs, Beverly, Mass. Thermus aquaticus (Taq) DNA polymerase (EC 2.7.7.7) and shrimp alkaline phosphatase (EC 3.1.3.1) were obtained from Roche Molecular Biochemicals, Indianapolis, Ind. All antibiotics and fine chemicals were obtained from Sigma Biochemicals, St. Louis, Mo. All DNA oligonucleotides were synthesized by MWG Biotech Inc., High Point, N.C.
DNA preparation and manipulation.
Standard approaches were used for DNA preparation and manipulation (1). Genomic DNA was extracted from R. eutropha with modifications previously described (23). PCR products were routinely cloned into pCR2.1-TOPO using a TOPO TA cloning kit (Invitrogen Corp., Carlsbad, Calif.).
Design of probes to identify intracellular depolymerase genes.
Candidate PHB depolymerase genes were identified by a BLAST comparison using phaZ1 and the recently sequenced R. metallidurans CH34 genome: http://jgi.doe.gov/JGI_microbial/html/ralstonia/ralston_homepage.html. Analysis yielded two open reading frames (ORF), phaZ1CH34 and phaZ2CH34, with high homology to R. eutropha phaZ1. A codon table was generated for R. eutropha based on the sequences of 17 R. eutropha H16 genes. The deduced amino acid sequences of phaZ1, phaZ1CH34, and phaZ2CH34 were aligned using the Clustal W algorithm (18). The alignment revealed two conserved regions of six and seven amino acids that were used for design of the degenerate oligonucleotides phaZB-3 and phaZB-4 (2). The oligonucleotides were used for amplification of ∼250-bp fragments of potential PHB depolymerase gene sequences from the Re1097 (ΔphaZ1) strain. Two candidate fragments with high homology to PHB depolymerase genes were identified and designated phaZ2 and phaZ3.
Construction of phaZ1 precise deletion gene replacement plasmid pGY96.
The 294- and 295-bp fragments of R. eutropha DNA corresponding to the regions immediately upstream and downstream of the phaZ1 ORF were amplified by PCR with the oligonucleotides phaZ6/phaZ7 and phaZ8/phaZ9. BamHI sites were introduced into both products by phaZ6 and phaZ9, and an overlapping segment of 39 nucleotides was introduced into the two products by phaZ7 and phaZ8. The two PCR products were combined and amplified with phaZ6 and phaZ9 to yield a 0.6-kb PCR product with sequence upstream and downstream of the phaZ1 ORF, flanked by BamHI sites, and lacking the phaZ1 gene. The PCR product was cloned into the AflII site of pCR2.1-TOPO to yield an intermediate plasmid. The 0.6-kb BamHI fragment of this intermediate plasmid was then cloned into the BamHI site of pJQ200mp18Km to yield pGY96.
Inverse PCR to obtain the complete phaZ3 sequence.
Genomic DNA from R. eutropha RE1097 (ΔphaZ1) was isolated and partially digested with Sau3AI (62 mU/μg of DNA, 80 min) in high dilution. The resulting DNA was self ligated. Inverse PCR (16) was performed on the resulting circular chromosomal fragments using the primer set phaZC1-phaZC2, designed within the known region of phaZ3 so that the primer 3′ ends faced towards the start codon and stop codon of the designated phaZ3 ORF. The unknown sequence portions of phaZ3 were amplified and flanked now by the inverted priming sites of phaZC1 and phaZC2. The resulting PCR products were cloned into pCR2.1-TOPO and sequenced.
Library generation and screening to obtain the complete phaZ2 sequence.
Inverse PCR was unsuccessful at obtaining the full sequence of phaZ2. A R. eutropha library was generated from the partially digested genomic DNA of Re1108. The digestion reaction (Sau3AI: 62 mU/μg of DNA; 80 min) was stopped with 0.1% sodium dodecyl sulfate (wt/vol). Fragments between 1 and 10 kb were cloned into the vector pBluescriptIIKS. Successfully transformed E. coli DH5αF′ clones were cultivated and stored (10% dimethyl sulfoxide; −80°C) in 96-well tissue culture plates (VWR International Inc., Buffalo Grove, Ill.). The library was screened by PCR in a 96-well multiblock PCR cycler (Primus 96 Plus, MWG Biotech Inc., High Point, N.C.). The presence of a 388-bp phaZ2 fragment was detected by the primer set phaZB6-phaZB10. Specificity was verified by restriction analysis of the PCR products with BglI. Positive candidates were sequenced, and the complete sequence was assembled using Contig Express (InforMax Inc., Annapolis, Md.).
Construction of phaZ3 and phaZ2 precise deletion gene replacement plasmids pPK51 and pJOE39, respectively.
The protocol was identical to that described above for pGY96. The 410- and 306-bp fragments immediately upstream and downstream of the phaZ3 ORF were generated using PhaZC8-PhaZC10 and PhaZC9-PhaZC11. The resulting PCR product was placed in pJQ200mp18Km to produce pPK51. Fragments upstream (455-bp) and downstream (487-bp) of the phaZ2 ORF were generated by using PhaZD3b-PhaZD4b and PhaZD1b-PhaZD2c. The resulting PCR product was used to generate pJOE39.
Construction of depolymerase deletion strains in R. eutropha H16.
The deletion strains in R. eutropha H16 were created by homologous recombination by a standard procedure (13, 21). The phaZ replacement plasmids pGY96, pJOE39, and pPK51 were introduced into the appropriate R. eutropha strains to generate ΔphaZ1, ΔphaZ2, ΔphaZ3, ΔphaZ1ΔphaZ2, ΔphaZ1ΔphaZ3, ΔphaZ2ΔphaZ3, and ΔphaZ1ΔphaZ2ΔphaZ3 by conjugation from donor strain E. coli S17-1. Construction of the desired deletions was confirmed by PCR with the oligonucleotides in Table 2. Oligonucleotides PhaZ6-PhaZ9 were used to confirm ΔphaZ1 strains; PhaZas-PhaZse were used to verify ΔphaZ2 strains; and PhaZC10-PhaZC11 were used to confirm ΔphaZ3 strains. Southern blot analyses were also carried out to verify the strain construction.
Cultivation conditions.
Wild-type (wt) R. eutropha and depolymerase deletion strains (ΔphaZ1, ΔphaZ2, ΔphaZ3, ΔphaZ1ΔphaZ2, ΔphaZ1ΔphaZ3, ΔphaZ2ΔphaZ3, and ΔphaZ1ΔphaZ2ΔphaZ3) were cultivated with aeration at 30°C. Gentamicin was included in all growth media, except when PHB utilization was being measured. Each procedure was done in duplicate. A single colony from a TSB plate was cultivated in 5 ml of TSB to saturation (∼40 h), at which time 2 ml was transferred into 100 ml of TSB in 500-ml baffled flasks and grown for 24 h. These cells were washed and transferred into 200 ml of TSB medium or 200 ml of PHB high medium in 1-liter baffled flasks to yield cultures with an initial optical density at 600 nanometers of 0.5. Cells (5 ml) were removed from the cultures at 0, 4, 8, 12, 24, 48, and 72 h. For PHB utilization, 100 ml of cells grown in PHB high medium for 72 h were washed with 0.85% saline and transferred into 200 ml of PHB no carbon medium. Cells (5 ml) were harvested at 4, 8, 12, 24, 48, and 72 h. In all cases, cells were analyzed for PHB content.
PHB quantification.
Aliquots of cells (above) were washed twice with ice-cold water and dried overnight at 80°C under vacuum. PHB was quantitated as crotonic acid by the sulfuric acid-high-pressure liquid chromatography method of Karr et al. (7). The samples were analyzed using an Aminex HPX-87H column (Bio-Rad, Hercules, Calif.) under the following conditions: column temperature, 50°C; gradient, isocratic; mobile phase, 5 mM sulfuric acid; flow rate, 0.6 ml/min. Crotonic acid was detected by a diode array detector at 210 nm.
Nucleotide sequence accession numbers.
Sequences of PhaZ2 and PhaZ3 have been submitted to NCBI database and are available under accession numbers AF549808 (PhaZ2) and AF549809 (PhaZ3).
RESULTS
Identification of phaZ2 and phaZ3.
Previous studies demonstrated the presence of the intracellular depolymerase PhaZ1 in R. eutropha (4, 14, 15). In vivo, the levels of PHB in wt and ΔphaZ1 R. eutropha strains were monitored. PHB depolymerase activity in crude extract was also measured (4, 14). The results from these studies suggested that at least one additional intracellular depolymerase was required to account for PHB utilization. We have used a PCR approach with degenerate oligonucleotide primers to identify candidate PHB depolymerase genes in R. eutropha H16. Specifically, phaZ1 was used to identify two genes in the R. metallidurans genome, phaZ1CH34 and phaZ2CH34. The deduced amino acid sequences of the proteins associated with these genes were generated and compared by Clustal W alignment. Two conserved regions of the proteins were identified (138VYVTDW143 and 210MGGPIDA216, using the numbering system of PhaZ1H16), and degenerate oligonucleotides were designed for use in PCR analysis. A R. eutropha H16 ΔphaZ1 strain, Re1097, was constructed, and its genomic DNA was used in conjunction with the degenerate primers to amplify DNA fragments that were then cloned. The clones of the expected size (∼250 bp) were sequenced. They revealed two distinct PCR products: phaZ2 and phaZ3.
These ∼250-bp internal fragments of putative phaZ2 and phaZ3 were used in conjunction with inverse PCR in an effort to identify the remaining portions of the phaZ2 and phaZ3 ORFs. Using primer set phaZC1-phaZC2, a 1.7-kb PCR fragment that harbored a 1,224-bp ORF containing the phaZ3 fragment was obtained and sequenced. Reamplification and sequencing of the phaZ3 ORF from the wt R. eutropha H16 verified the identification of a second depolymerase gene.
The same approach was unsuccessful in identifying the phaZ2 ORF, and therefore an alternative approach to obtain this sequence was pursued. A R. eutropha ΔphaZ1ΔphaZ3 strain (RE1108) was constructed. Its genomic DNA was isolated and used to create a genomic DNA library. The library was used to screen for the phaZ2 core fragment (388 bp) by PCR using the primer set phaZB6-phaZB10. Once the sequence of the fragment was verified, the entire sequence of candidate chromosome fragments was sequenced. One 3.3-kb fragment harbored a 1,260-bp ORF containing the phaZ2388 base pair fragment. The sequence was verified by reamplification and sequencing of the phaZ2 ORF from the wt H16. Figure 2 shows the deduced amino acid sequences of PhaZ1, PhaZ2, and PhaZ3 aligned by using the Clustal W algorithm. The alignment of PhaZ1 to PhaZ2, PhaZ1 to PhaZ3, and PhaZ2 to PhaZ3 shows 38, 46, and 43% sequence identity, respectively.
FIG. 2.
Alignment of PhaZ1, PhaZ2, and PhaZ3 from R. eutropha. Black, identical; gray, highly conserved.
PHB utilization in R. eutropha deletion stains.
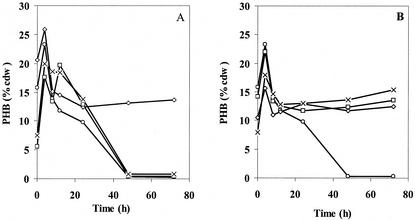
To determine if these newly identified genes actually function as PHA depolymerases, phaZ deletion strains of R. eutropha H16 were created: Re1097 (ΔphaZ1), Re1110 (ΔphaZ2), Re1107 (ΔphaZ3), Re1112 (ΔphaZ1ΔphaZ2), Re1108 (ΔphaZ1ΔphaZ3), Re1113 (ΔphaZ2ΔphaZ3), and Re1111 (ΔphaZ1ΔphaZ2ΔphaZ3). Each of these strains was examined for PHB when grown in rich medium (TSB) over 72 h and when grown in nutrient-limited medium (PHB high) for 72 h followed by transfer to PHB utilization medium (PHB no carbon) for 48 or 72 h. The results from the experiments in TSB are shown in Fig. 3A and B. They revealed a peak of PHB production between 8 and 12 h. In the wt, Re1110 (ΔphaZ2), and Re1107 (ΔphaZ3) strains, all of the PHB was removed by 48 h. In all of the other strains, each of which has phaZ1 deleted, the PHB levels remain unchanged after 12 h. At present, it is not clear if the differences in PHB observed for different strains during the first 12 h are significant. The results in TSB medium therefore provided no evidence that PhaZ2 or PhaZ3 can function as PHB depolymerases in vivo.
FIG. 3.
PHB production and utilization in TSB medium. (A) Results for wt (○), ΔphaZ1 (⋄), ΔphaZ2 (□), and ΔphaZ3 (×) R. eutropha strains. (B) Results for wt (○), ΔphaZ1ΔphaZ2 (⋄), ΔphaZ1ΔphaZ3 (□), and ΔphaZ1ΔphaZ2ΔphaZ3 (×) R. eutropha strains.
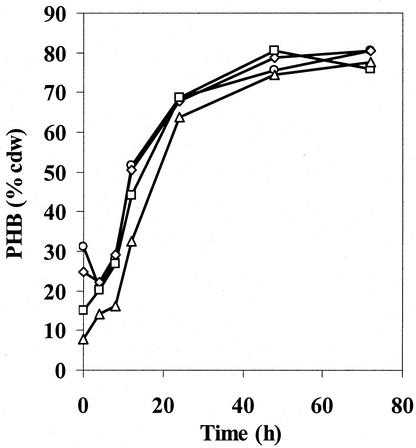
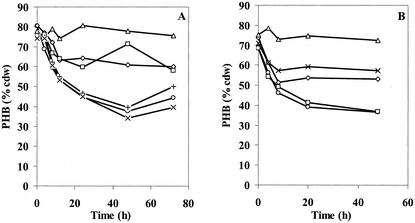
The results from experiments in which PHB was accumulated to ∼80% cell dry weight in PHB high medium and the cells were then switched into PHB no-carbon medium are shown in Fig. 4 and Fig. 5A and B, respectively. During the 72-h cultivation period in PHB (high), the PHB accumulation pattern of each phaZ deletion strain was very similar to that of the wt strain, all approaching 80% cell dry weight. When each strain was switched into PHB utilization medium, the wt strain had utilized half of its PHB content after 48 h. The ΔphaZ1ΔphaZ2ΔphaZ3 strain, on the other hand, did not lose any PHB content within the same period, suggesting that either PhaZ2 or PhaZ3 was involved in PHB degradation. The effect of single and double deletion of the three putative genes was further examined in an effort to understand the function of the newly identified genes. The ΔphaZ1 strain utilized only ∼20% of its PHB content, similar to previous results reported by Saito. The most interesting observations were that the ΔphaZ1ΔphaZ2 strain did not lose any significant amount of PHB, while the ΔphaZ2 strain behaved like the wt strain. The result for the ΔphaZ1ΔphaZ2 strain, in comparison to those for the ΔphaZ1 and ΔphaZ2 strains, suggested that PhaZ2 is an intracellular depolymerase and may act upon the product(s) generated by PhaZ1. The PHB degradation in ΔphaZ1ΔphaZ3 in several experiments appeared to be less than that in the ΔphaZ1 strain. However, at present, growth conditions have not yet been found to demonstrate unambiguously the function of PhaZ3 as a depolymerase.
FIG. 4.
PHB production in PHB(high) medium by wt (○), ΔphaZ1 (⋄), ΔphaZ1ΔphaZ3 (□), and ΔphaZ1ΔphaZ2 (▵) R. eutropha strains. The production pattern is the same in all deletion strains (additional data not shown).
FIG. 5.
PHB utilization in PHB (no carbon) medium. (A) Results for wt (○), ΔphaZ1(◊), ΔphaZ2(+), ΔphaZ1ΔphaZ3 (□), ΔphaZ2ΔphaZ3 (×), and ΔphaZ1ΔphaZ2 (▵) R. eutropha strains. (B) Results for wt (○), ΔphaZ1(◊), ΔphaZ3 (□), ΔphaZ1ΔphaZ3 (×), and ΔphaZ1ΔphaZ2ΔphaZ3 (▵) R. eutropha strains. These results are from an experiment independent of that corresponding to the results shown in panel A.
DISCUSSION
An understanding of PHA homeostasis requires identification of all the proteins involved in polymer biosynthesis, degradation, and their regulation. A number of proposals concerning the role of intracellular depolymerases have been advanced, with the most recent studies suggesting that there are multiple depolymerases in R. eutropha and that one might be constitutively active and a second might be inducible (4, 14). A test of the proposals requires identification of all of the proteins involved in PHB degradation. We now report the identification of two new candidate PHA depolymerase genes, phaZ2 and phaZ3, in R. eutropha. A sequence comparison (Fig. 2) reveals a high level of sequence identity. Given that PhaZ1 has been shown in vitro to degrade amorphous PHB granules to small oligomers, it is likely that PhaZ2 and PhaZ3 will possess similar activity. Thus far, no lab has purified the intracellular depolymerase to homogeneity or analyzed the products produced in the early stages of the depolymerase-catalyzed reactions. Thus differentiation between depolymerase functions and the requirement for isozymes requires further investigation.
To obtain evidence that PhaZ2 and PhaZ3 are actually PHB depolymerases, the role of these proteins in a variety of R. eutropha deletion strains has been examined under rich medium growth conditions and conditions for PHB utilization. Comparison of the results from the wt strain with those of various deletion strains after 72 h of cultivation under conditions in which PHB is utilized to provide a carbon source for growth establishes that PhaZ1 and PhaZ2 are involved in PHB degradation. PhaZ1, identified by Saito et al., was observed to have the largest effect. Our results provide the first direct evidence that PhaZ2 is an active PHB depolymerase.
Studies of the deletion strains relative to the wt strain when grown on rich TSB medium present a different picture. Under these conditions, the data exhibited a higher degree of variation between individual cultures. However, despite these variations, PhaZ1 is consistently the only protein involved in PHB degradation.
To understand the function of each of the depolymerases now requires analysis of mRNA levels, protein presence, and granule structure as a function of growth conditions. In addition, purification of the proteins and development of assays in the early stages of PHB degradation is essential to our understanding of and differentiation between the functions of the different depolymerases. These studies are in progress.
Acknowledgments
This work was supported by NIH grant GM49171 to JoAnne Stubbe and Anthony J. Sinskey, by a DAAD grant “Kurzstipendien für Abschlussarbeiten” for Joachim Lupberger, and by NIH training grant 5T32GM08334 to Jiamin Tian.
Plasmid pPK51 was constructed by Peter Kok.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 2.Compton, T. 1990. Degenerate primers for DNA amplification, p. 39-45. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, Calif.
- 3.Gerngross, T. U., K. D. Snell, O. P. Peoples, A. J. Sinskey, E. Cushai, S. Masamune, and J. Stubbe. 1994. Overexpression and purification of the soluble polyhydroxyalkanoate synthase from Alcaligenes eutrophus: evidence for a required posttranslational modification for catalytic activity. Biochemistry 33:9311-9320. [DOI] [PubMed] [Google Scholar]
- 4.Handrick, R., S. Reinhardt, and D. Jendrossek. 2000. Mobilization of poly(3-hydroxybutyrate) in Ralstonia eutropha. J. Bacteriol. 182:5916-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jendrossek, D., and R. Handrick. 2002. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 56:403-432. [DOI] [PubMed] [Google Scholar]
- 6.Jendrossek, D. 2001. Microbial degradation of polyesters. Adv. Biochem. Eng. Biotechnol. 71:293-325. [DOI] [PubMed] [Google Scholar]
- 7.Karr, D. B., J. K. Waters, and D. W. Emerich. 1983. Analysis of poly-beta-hydroxybutyrate in Rhizobium japonicum bacteroids by ion-exclusion high-pressure liquid-chromatography UV detection. Appl. Environ. Microbiol. 46:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebergesell, M., K. Sonomoto, M. Madkour, F. Mayer, and A. Steinbuchel. 1994. Purification and characterization of the poly(hydroxyalkanoic acid) synthase from Chromatium vinosum and localization of the enzyme at the surface of poly(hydroxyalkanoic acid) granules. Eur. J. Biochem. 226:71-80. [DOI] [PubMed] [Google Scholar]
- 9.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maehara, A., Y. Doi, T. Nishiyama, Y. Takagi, S. Ueda, H. Nakano, and T. Yamane. 2001. PhaR, a protein of unknown function conserved among short-chain-length polyhydroxyalkanoic acids producing bacteria, is a DNA-binding protein and represses Paracoccus denitrificans phaP expression in vitro. FEMS Microbiol. Lett. 200:9-15. [DOI] [PubMed] [Google Scholar]
- 11.Peoples, O. P., and A. J. Sinskey. 1989. Poly-β-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16: identification and characterization of the PHB polymerase gene (phbC). J. Biol. Chem. 264:15298-15303. [PubMed] [Google Scholar]
- 12.Pieper-Fürst, U., M. H. Madkour, F. Mayer, and A. Steinbüchel. 1994. Purification and characterization of a 14-kilodalton protein that is bound to the surface of polyhydroxyalkanoic acid granules in Rhodococcus ruber. J. Bacteriol. 176:4328-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 14.Saegusa, H., M. Shiraki, C. Kanai, and T. Saito. 2001. Cloning of an intracellular poly[d(−)-3-hydroxybutyrate] depolymerase gene from Ralstonia eutropha H16 and characterization of the gene product. J. Bacteriol. 183:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito, T., K. Takizawa, and H. Saegusa. 1995. Intracellular poly(3-hydroxybutyrate) depolymerase in Alcaligenes eutrophus. Can. J. Microbiol. 41(suppl. 1):187-191. [Google Scholar]
- 16.Silver, J. 1991. Inverse polymerase chain reaction, p. 137-146. In M. J. McPherson, P. Quirke, and G. R. Taylor (ed.), PCR: a practical approach. Oxford University Press, New York, N.Y..
- 17.Steinbuchel, A., and S. Hein. 2001. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv. Biochem. Eng. Biotechnol. 71:81-123. [DOI] [PubMed] [Google Scholar]
- 18.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittaker, P. A., A. J. Campbell, E. M. Southern, and N. E. Murray. 1988. Enhanced recovery and restriction mapping of DNA fragments cloned in a new lambda vector. Nucleic Acids Res. 16:6725-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieczorek, R., A. Pries, A. Steinbüchel, and F. Mayer. 1995. Analysis of a 24-kilodalton protein associated with the polyhydroxyalkanoic acid granules in Alcaligenes eutrophus. J. Bacteriol. 177:2425-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.York, G. M., J. Stubbe, and A. J. Sinskey. 2001. New insight into the role of the PhaP phasin of Ralstonia eutropha in polyhydroxybutyrate production. J. Bacteriol. 183:2394-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.York, G. M., J. Stubbe, and A. J. Sinskey. 2002. The Ralstonia eutropha PhaR protein couples synthesis of the PhaP phasin to the presence of polyhydroxybutyrate in cells and promotes polyhydroxybutyrate production. J. Bacteriol. 184:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.York, G. M., B. H. Junker, J. A. Stubbe, and A. J. Sinskey. 2001. Accumulation of the PhaP phasin of Ralstonia eutropha is dependent on production of polyhydroxybutyrate in cells. J. Bacteriol. 183:4217-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]