Abstract
To understand mechanisms that may underlie the progression of a demyelinated lesion to a chronic state, we have used the cuprizone model of chronic demyelination. In this study, we investigated the fate of oligodendrocytes during the progression of a demyelinating lesion to a chronic state and determined whether transplanted adult oligodendrocyte progenitors could remyelinate the chronically demyelinated axons. Although there is rapid regeneration of the oligodendrocyte population following an acute lesion, most of these newly regenerated cells undergo apoptosis if mice remain on a cuprizone diet. Furthermore, the oligodendrocyte progenitors also become progressively depleted within the lesion, which appears to contribute to the chronic demyelination. Interestingly, even if the mice are returned to a normal diet following 12 weeks of exposure to cuprizone, remyelination and oligodendrocyte regeneration does not occur. However, if adult O4+ progenitors are transplanted into the chronically demyelinated lesion of mice treated with cuprizone for 12 weeks, mature oligodendrocyte regeneration and remyelination occurs after the mice are returned to a normal diet. Thus, the formation of chronically demyelinated lesions induced by cuprizone appears to be the result of oligodendrocyte depletion within the lesion and not due to the inability of the chronically demyelinated axons to be remyelinated.
Although most acutely demyelinated lesions in multiple sclerosis (MS) are remyelinated,1 the lesions eventually progress to a state of chronic demyelination, characterized by sparse remyelination, few oligodendrocytes, and axon degeneration.2–5 Although the central nervous system (CNS) has the ability to regenerate new oligodendrocytes that remyelinate demyelinated axons following acute demyelination,6–9 it has been suggested that mature oligodendrocytes are not regenerated within chronically demyelinated lesions due to: 1) the depletion of the oligodendrocyte progenitors;4 2) the inability of the progenitors to proliferate and differentiate within the lesion due to aging10 or a non-conducive environment;11–14 and/or 3) axon damage3,15,16 or the inability of chronically demyelinated axons to be remyelinated.17
To test these hypotheses, we used the cuprizone model of chronic demyelination.18 Previously, we reported acute demyelination in C57BL/6 mice18,19 and the apoptotic death of mature oligodendrocytes within the corpus callosum in mice exposed to cuprizone for a short duration.6 Once cuprizone is removed from the diet, the mature oligodendrocytes begin to repopulate the lesion and remyelinate the demyelinated axons. This regeneration of the mature oligodendrocyte population is presumably derived from the differentiation of accumulating progenitors within the demyelinating lesion.6 However, if mice are maintained on the cuprizone diet for a prolonged period of time, remyelination eventually fails.18 In addition, we report here that the newly regenerated mature oligodendrocytes, along with the resident progenitors, become progressively depleted within the chronically demyelinated corpus callosum. However, adult O4+ oligodendrocyte progenitors transplanted into the chronic lesions differentiate into mature oligodendrocytes and remyelinate a large number of the demyelinated axons if the mice are returned to a normal diet following 12 weeks of cuprizone intoxication. Thus, the formation of chronically demyelinated lesions induced by long-term cuprizone toxicity is the result of oligodendrocyte depletion within the lesion and not due to a non-conducive environment or the inability of the chronically demyelinated axons to be remyelinated.
Materials and Methods
Induction of Acute and Chronic Demyelination
Adult male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). At 8 weeks of age, the mice were fed a diet of milled Purina mouse chow containing 0.2% cuprizone (Sigma Chemical Co., St. Louis, MO) by weight for up to 16 weeks to induce chronic demyelination.18 Additional mice were fed cuprizone for 6 weeks and then returned to a normal diet to allow remyelination.6,19 Some mice were anesthetized and perfused through the heart with 4% paraformaldehyde (PFA). The brains were removed and cut mid-sagittally. Half of the brain was then fixed overnight in 4% PFA at 4°C and then embedded in paraffin. 5-μm sections were cut with a microtome at the fornix region of the corpus callosum, mounted onto gelatin-coated slides (Fisher Scientific Corp., Fairlawn, NJ) and stored at room temperature until use. The other half of the brain was fixed in 4% PFA for 1 hour at 4°C and then placed into a 20% sucrose for 48 hours at 4°C and then snap-frozen in isopentane. Transverse sections, 10 μm thick, were cut with a cryostat at the fornix region of the corpus callosum, mounted onto gelatin-coated slides, and stored at −70°C until staining. All comparative analyses were focused at the medial region of the corpus callosum that was above the fornix and between the ventricles (corresponding to sections 220–260 of the mouse brain atlas20). The forebrains from another set of mice were removed for RNA analysis. Mid-sagittal sections of the forebrain were frozen immediately on dry ice. All animal procedures were conducted in accordance with guidelines approved by the IACUC and both the University of North Carolina and Columbia University Division of Laboratory Animal Medicine.
Glutathione-S-Transferase, Pi Isoform Immunohistochemistry, and Apoptosis Assay
To identify apoptotic mature oligodendrocytes within the brain, the NeuroTACS assay kit (Trevigen, Gaithersburg, MD) was used to identify apoptotic cells and mature oligodendrocytes were identified by the immunohistochemical detection of the pi isoform of glutathione-S-transferase (GST-pi),21 which does not detect immature NG2+ oligodendrocyte (unpublished observations). Paraffin-embedded sections were rehydrated in a graded series of alcohol washes, blocked with 5% normal goat serum (NGS)/0.1% Triton for 20 minutes, incubated with 0.1% trypsin/0.5 mol/L Tris for 15 minutes at 37°C, and then incubated with the rabbit polyclonal anti-GST-pi antibody (1:1000; Biotrin, Newton, MA) overnight at 4°C. The tissue sections were then incubated with 1X labeling buffer (Trevigen) for 5 minutes and then incubated with a labeling reaction mixture of 1X labeling buffer, biotin-labeled dNTPs (Trevigen), and Klenow enzyme (Trevigen) for 1.5 hours at 37°C. The reaction was stopped by placing the tissue sections into a 1X stop reaction buffer (Trevigen) for 5 minutes. The tissue sections were then incubated with a fluorescein-conjugated goat anti-rabbit secondary antibody diluted 1:75 in 5% NGS/0.2% Triton/2% streptavidin-Texas red complex (Vector Laboratories, Burlingame, CA) for 1 hour, counterstained with DAPI (Molecular Probes, Eugene, OR), coverslipped, and examined using an Olympus BX60 microscope equipped with epifluorescence optics.
NG2 Immunohistochemistry
To identify oligodendrocyte progenitors, frozen tissue sections were dried, rehydrated, blocked with 5% NGS/0.2% Triton, and then incubated with rabbit anti-NG2 (gift from Dr. William Stallcup, San Diego, CA) diluted 1:400 in 5% NGS/0.2% Triton overnight at 4°C. The sections were then incubated with a fluorescein-conjugated goat anti-rabbit secondary antibody (1:75; Vector Laboratories) for 1 hour, counterstained with DAPI, coverslipped, and examined using an Olympus BX60 microscope equipped with epifluorescence optics.
RNA Analysis
Total RNA from entire half of a forebrain sectioned mid-sagittally along midline was isolated, separated, and transferred to a Zeta-probe nylon membrane as described previously.22 Membranes were hybridized with 32P-labeled cDNA probes, synthesized using double-stranded polymerase chain reaction (PCR) methodology23 for amplification of cDNA for myelin-associated glycoprotein (MAG).24 Filters were washed and radioactivity in RNA was quantitated using a Packard Instant Imager electronic autoradiography system. To control for variability in sampling handling, values obtained were normalized to the amount of rRNA in each lane as described previously.22
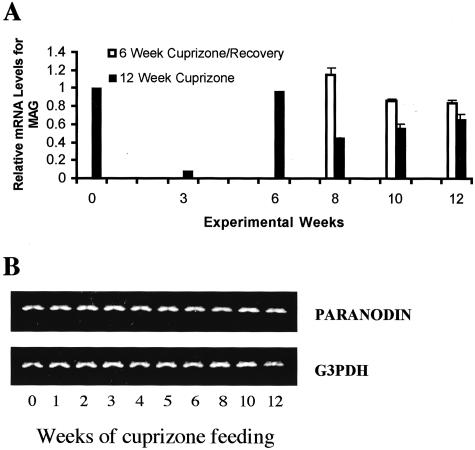
Additional RNA was reverse transcribed into cDNA and then amplified by PCR as described previously.6 The primer sets for paranodin were 5′-ACAAGGGAGTTACCTGCCATGAAC (sense) and 3′-GCACGGATTGGAGGGAAACG (antisense). The primer sets for G3PDH have been published elsewhere.25 Templates were denatured at 95°C for 5 minutes, followed by 30 cycles of denaturation (95°C for 1 minute) primer annealing (62°C for 1.5 minutes) and extension (72°C for 1 minute) with a final extension step at 72°C for 15 minutes. The amplified cDNA mixture of each sample was separated on a 2.5% agarose gel containing ethidium bromide and photographed. The amplified products for paranodin and G3PDH were 696 and 983 bp, respectively. Amplification of the housekeeping gene, G3PDH, confirmed RNA amplification and equal loading of each sample. The mRNA profiles were semiquantitated using a densitometer to normalize all samples for comparison.
Lectin Histochemistry
Microglia/macrophages were identified by binding biotin-conjugated Ricinus communis agglutin-1 (RCA-1; Sigma Chemical Co.) to paraffin-embedded sections and then visualized by the 3′3′-diaminobenzidine methodology (Vector Laboratories) as described previously.19
Isolation of O4+ Progenitors
Transgenic C57BL6 mice, in which green fluorescent protein expression is driven by the chicken β-actin promoter (GFP tg mice), were purchased from Jackson Laboratories. Progenitors were isolated from the corpus callosum in 8-week-old GFP tg mice as described previously.26,27 Briefly, the mice were anesthetized and the corpus callosum was carefully dissected and then mechanically and enzymatically dissociated with 0.125% trypsin (Sigma Chemical Co.) and 0.02% collagenase (Worthington Biochemical Co., Lakewood, NJ). The cellular suspension was filtered through sterile 70-μm mesh and the trypsin neutralized with 10% heat-inactivated serum. Cells were collected by centrifugation and then resuspended in 0.9 mol/L sucrose/MEM (Gibco, Carlsbad, CA). This cellular suspension was centrifuged and the progenitors (bottom layer) were collected and resuspended in serum-free medium (see below). Approximately 5 × 105 cells were isolated from the corpus callosum of each adult mouse with the cells being pooled from three corpora collosa.
O4+ progenitors were then purified from the total progenitors using immunopanning methods described previously.26 Briefly, immunopan plates were created by incubating goat anti-mouse IgM antibody (20 μg/ml; Sigma Chemical Co.), diluted in 50 mmol/L Tris, in Falcon 1007 petri dishes (Fisher) overnight at 37°C. The plates were then washed, incubated with O4 hybridoma supernatant (undiluted) at 37°C for 3 hours, and then blocked with 10% normal goat serum (NGS)/10% bovine serum albumin (BSA) at 37°C for 30 minutes. 5 × 105 cells were then incubated in each O4 immunoplate for 1 hour at 37°C. The plates were then washed and coated with trypsin/EDTA for 10 minutes at 37°C. The detached cells were collected in an equal volume of 10% heat-inactivated serum to neutralize the trypsin, centrifuged, and then resuspended in serum-free medium (1 × 104 O4+ cells/μl). Approximately 3.1 × 105 O4+ cells were isolated from the three corpora collosa.
Medium for Isolated Progenitors
Serum-free medium was composed of d-biotin (10 ng/ml; Sigma Chemical Co.), insulin (5 μg/ml; Sigma Chemical Co.), progesterone (20 nM; Sigma Chemical Co.), putrescine (100 μmol/L; Sigma Chemical Co.), selenium (5 ng/ml; Sigma Chemical Co.), transferrin (50 μg/ml; Sigma Chemical Co.), Hepes buffer (15 mmol/L; Sigma Chemical Co.), 3,3,5-triiodo-l-thyronine (15 nM; Sigma Chemical Co.), penicillin/streptomycin (100U/100 μg/ml; Gibco) and BSA (1 mg/ml; Sigma Chemical Co.) in DMEM/F12 (Gibco).
Transplantation of GFP+/O4+ Progenitors into the Chronically Demyelinated Corpus Callosum
At 8 weeks of age, wild-type male recipient mice (Jackson Laboratories) were fed a diet of milled Purina mouse chow containing 0.2% cuprizone by weight for 12 weeks to induce chronic demyelination within the corpus callosum. The mice were then anesthetized with ketamine (80 mg/kg body weight; Henry Schein Pharmaceutical, Port Washington, NY) and xylazine (10 mg/kg body weight; Henry Schein Pharmaceutical) and positioned in an immobilization apparatus designed for stereotactic surgery (David Kopf Instruments, Tujunga, CA). The scalp was opened and the O4+ oligodendrocyte progenitors isolated from the corpus callosum in GFP tg donor mice were injected (1 × 104 O4+ cells in 1 μl of serum-free medium) unilaterally into the chronically demyelinated corpus callosum in the wild-type recipient mice with a 10-μl Hamilton syringe using stereotactic coordinates of 0.7 mm posterior and 0.3 mm lateral to bregma at a depth of 1.7 mm.28 Additional control recipient mice were stereotactically injected with serum-free medium containing no progenitors. The opening in the skull was then filled with Gelfoam, the area swabbed with penicillin and streptomycin (Gibco) and the wound was sutured. Immediately after transplantation, the recipient mice were returned to a normal diet for 4 weeks allowing recovery. The mice were then anesthetized, perfused through the heart with either 4% PFA (light microscopy) or 2.5% glutaraldehyde (electron microscopy) and processed for analysis as described previously.6,18 All animal procedures were conducted in accordance with guidelines approved by the IACUC and the Columbia University Division of Laboratory Animal Medicine.
For light microscopy, frozen tissue sections were dried, rehydrated, blocked with 5% NGS/0.2% Triton and then incubated with the rabbit polyclonal anti-GST-pi antibody (1:1000; Biotrin) and the mouse monoclonal anti-GFP antibody (1:500; Chemicon, Temecula, CA) overnight at 4°C. The sections were then incubated with a biotin-conjugated goat anti-mouse secondary antibody (1:100; Vector Laboratories) and a rhodamine-conjugated goat anti-rabbit antibody (1:100; Southern Biotechnology Associates) for 1 hour. The sections were then incubated with a 2% streptavidin-fluorescein complex (Vector) in 5% NGS/0.2% Triton, counterstained with DAPI, coverslipped, and examined using an Olympus BX60 microscope equipped with epifluorescence optics.
For electron microscopy, glutaraldehyde-fixed brain samples were prepared as described previously.18 Coronal sections (1 μm) were stained with toluidine blue and the medial region of the corpus callosum was identified by light microscopy. The tissue was then trimmed and reoriented for thin sectioning so that cross sections of the corpus callosum could be examined by electron microscopy. Thin sections were cut, stained with uranyl acetate and lead citrate, photographed, and then the electron micrographs were analyzed as described previously.18 One thousand fibers (≥0.3 μm in diameter) from five different random sections of the corpus callosum were examined from each mouse (three mice at each time point).
Cell Number Quantification
Immunopositive cells were obtained by counting only those cells with an identified nucleus, observable by DAPI staining, within the medial region of the corpus callosum. Individual cell counts were conducted on both sides of midline corresponding to an area of 0.033 mm2. The cell counts from both areas were averaged to give a total for each tissue section. The cell counts (number of cells/mm2) are presented in the text as the mean and SEM from at least three mice for each time point.
Statistical Analysis
Statistical comparisons were made using a one-factor between-subjects analysis of variance. Multiple comparisons between groups were made using Tukey’s test.
Results
The Mature Oligodendrocyte Population Is Inadequately Regenerated within Chronically Demyelinated Lesion
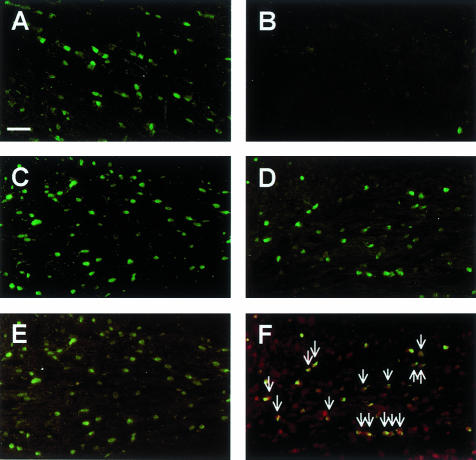
Cuprizone toxicity markedly depleted the mature oligodendrocyte population, as detected by immunocytochemistry using the anti-GST-pi antibody (Figures 1, A and B, and 2A).6 When mice were returned to a normal diet after 6 weeks of cuprizone feeding, the oligodendrocyte population returned to control numbers (Figures 1C and 2B).6 In contrast, in mice that were maintained on a cuprizone diet for up to 12 weeks, a large number of oligodendrocytes reappeared (Figure 1D), although numbers never fully returned to control (Figure 2A). Despite the presence of oligodendrocytes, however, there was little remyelination.18
Figure 1.
The long-term feeding of cuprizone has a profound effect on the mature oligodendrocyte population. Paraffin-embedded sections were immunostained with anti-GST-pi (green cells; A–F), while some sections were also examined for the presence of apoptotic cells (red nuclei; E–F). A: A large number of GST-pi+ oligodendrocytes were observed within the corpus callosum in untreated control mice. B: The GST-pi+ oligodendrocytes were almost completely absent at 5 weeks. The GST-pi+ oligodendrocytes reappeared 4 weeks after 6 weeks of cuprizone feeding (C) and after 12 weeks of continuous cuprizone feeding (D). No apoptotic GST-pi+ oligodendrocytes were identified 4 weeks after the mice were returned to a normal diet following 6 weeks of cuprizone feeding (E). A large number of apoptotic nuclei were co-localized with GST-pi+ oligodendrocytes after 10 weeks of cuprizone feeding (F). Scale bar, 25 μm.
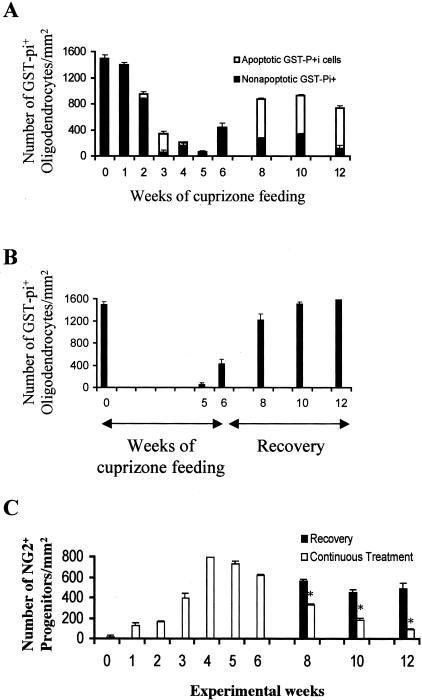
Figure 2.
Cuprizone induces the apoptotic death of mature oligodendrocytes and the progressive depletion of the oligodendrocyte progenitor population. The total number of mature oligodendrocytes, the number of non-apoptotic mature oligodendrocytes (black bars) and the number apoptotic mature oligodendrocytes (white bars) were quantitated and plotted as mean ± SEM for triplicate determinations (A, B). The number of GST-pi+ and apoptotic GST-pi+ cells/mm2 in mice fed cuprizone continuously for 12 weeks (A) and in mice fed cuprizone for 6 weeks and then returned to a normal diet for 6 weeks (B) are shown. C: The total number of NG2+ oligodendrocyte progenitors within the medial region of the corpus callosum were also quantitated and plotted as mean ± SEM for triplicate determinations in mice fed cuprizone continuously for 12 weeks (white bars) and in mice fed cuprizone for 6 weeks and then returned to a normal diet for 6 weeks (black bars). *P < 0.005.
The inability of the oligodendrocyte population to regenerate adequately and remyelinate the axons during long-term cuprizone treatment could be a consequence of the newly regenerated cells undergoing continued apoptosis as observed with the oligodendrocytes in acute lesions (Figure 2A).6 To address this issue, we used immunohistochemistry to determine whether apoptotic (TUNEL assay) GST-pi+ oligodendrocytes were present within the chronically demyelinated corpus callosum. Indeed, a large number of apoptotic GST-pi+ oligodendrocytes were identified in the corpus callosum in mice maintained on the prolonged cuprizone diet (Figures 1F and 2A). In contrast, no apoptotic GST-pi+ oligodendrocytes were observed in the remyelinating corpus callosum in mice returned to a normal diet after 6 weeks of cuprizone feeding (Figures 1E and 2B). Thus, there appears to be a number of newly generated GST-pi+ oligodendrocytes undergoing cell death during long-term cuprizone toxicity.
Oligodendrocyte Progenitors Become Progressively Depleted during the Formation of Chronically Demyelinated Lesions
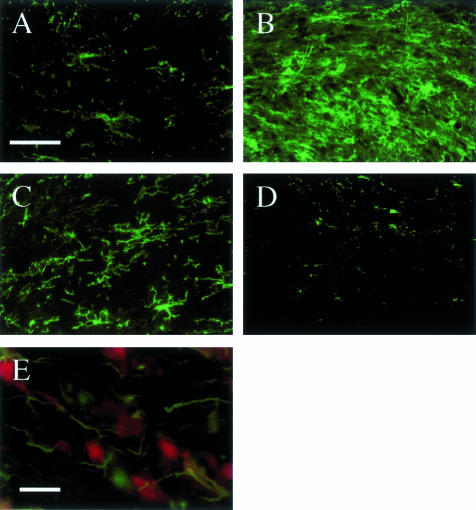
The large number of newly regenerated mature oligodendrocytes in the corpus callosum in mice fed cuprizone for 8 to 12 weeks (Figure 2A), many of which are undergoing apoptosis, suggests that these cells are being replenished by differentiating progenitors. Few NG2+ cells were observed in the corpus callosum in untreated control mice (Figures 2C and 3A). However, during the initial cuprizone-induced demyelination, NG2+ progenitors accumulated within the demyelinating corpus callosum (Figures 1C and 3B).6 A large number of these cells remained within the remyelinating corpus callosum after the mice were returned to a normal diet (Figures 1C and 3C). In contrast, there was a progressive loss of NG2+ progenitors within the chronically demyelinated corpus callosum in mice maintained on a cuprizone diet for up to 12 weeks (Figures 1C and 3D). This progressive depletion of progenitors in the face of increased death of newly generated oligodendrocytes may be a major reason for the failure of remyelination (see Discussion). Under our conditions, NG2 staining did not co-localize with F4/80+ microglia/macrophages as reported previously (Figure 3E).29
Figure 3.
The long-term feeding of cuprizone has a profound effect on the oligodendrocyte progenitor population. Frozen brain sections were immunostained with ant-NG2 (green cells). A: Very few NG2+ progenitors were present in the corpus callosum in untreated control mice. B: At 5 weeks, a large number of NG2+ progenitors accumulated within the demyelinated corpus callosum. C: A large number of NG2+ progenitors remained in a remyelinating corpus callosum 6 weeks after the mice were returned to a normal diet. D: Very few NG2+ progenitors were observed within a chronically demyelinated corpus callosum in mice fed cuprizone for 12 weeks. E: The NG2+ cells (green) that accumulated within the demyelinating corpus callosum at 3 weeks did not express the microglia/macrophage marker F4/80 (red). Scale bar, 25 μm for A–D and 10 μm for E.
The Presence of Microglia/Macrophages Coincides with Demyelination and Oligodendrocyte Death
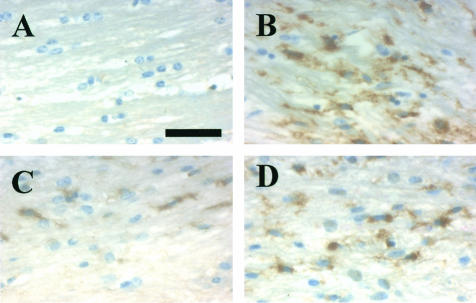
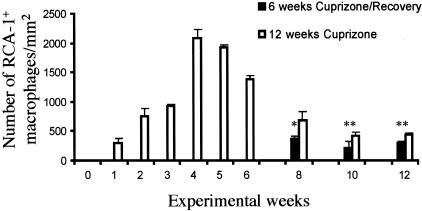
Since microglia/macrophage-derived factors have been implicated in oligodendrocyte death in vitro30–35 and in vivo,36 we examined whether these cells were present within lesions containing apoptotic oligodendrocytes. Few RCA-1+ microglia/macrophages were present within the corpus callosum in untreated adult mice (Figures 4A and 5). However, a large number of microglia/macrophages began to accumulate within the demyelinating corpus callosum by 4 weeks (Figures 4B and 5) and remained through 6 weeks (Figure 5) of cuprizone feeding. Thereafter, microglia/macrophage numbers (Figures 4C and 5) declined to control levels if mice were returned to a normal diet. In contrast, microglia/macrophage numbers within the chronically demyelinated corpus callosum remained elevated in mice fed cuprizone for up to 12 weeks (Figures 4D and 5). Thus, the presence of microglia/macrophages coincided with oligodendrocyte pathology within both acute and chronic lesions. However, it is unclear whether the microglia/macrophages have a direct role in the apoptotic death of the oligodendrocytes.
Figure 4.
The histological detection of microglia/macrophages within acute and chronically demyelinated lesions. Paraffin-embedded sections were stained with the RCA-1 lectin (brown) to detect the presence of microglia/macrophages. A: Few or no RCA-1+ microglia/macrophages were observed within the corpus callosum in untreated control mice. B: A large number of RCA-1+ microglia/macrophages were observed in a demyelinated corpus callosum at 4 weeks. A few RCA-1+ microglia/macrophages were observed in the remyelinated corpus callosum, 6 weeks after the mice were returned to a normal diet following 6 weeks of cuprizone feeding (C) or in the chronically demyelinated corpus callosum after 12 weeks of cuprizone feeding (D). Scale bar, 30 μm.
Figure 5.
The presence of RCA-1+ microglia/macrophages during acute and chronic demyelination. The total number of microglia/macrophages in the corpus callosum in cuprizone-fed mice (black bars) and mice returned to a normal diet following 6 weeks of cuprizone feeding (white bars) were quantitated and plotted as mean ± SEM for triplicate determinations. *P < 0.01, **P < 0.05.
Adult O4+ Progenitors Transplanted into the Chronic Lesions Remyelinate the Chronically Demyelinated Axons
We have previously shown that a dramatic reduction (>90%) in the mRNA levels for the myelin-specific gene, MAG,22 precedes acute demyelination and oligodendrocyte pathology during cuprizone intoxication (Figure 6A).6,19 During recovery after a 6-week exposure to cuprizone, there is sustained levels of MAG mRNA that coincides with remyelination. However, during continuous exposure to cuprizone, MAG mRNA diminishes at 8 weeks, far below MAG mRNA levels from mice recovering for 2 through 6 weeks from acute demyelination (Figure 6A). The perturbation in the oligodendrocyte-associated mRNA is in contrast to the lack of alteration in the steady-state mRNA (Figure 6B) and protein (Mason J, Dupree J, Girault J-A, Popko B, Matsushima G, unpublished data) levels for the neuron-associated protein, paranodin, during cuprizone intoxication. These findings suggest that neurons are unaffected by chronic cuprizone intoxication; yet, oligodendrocyte progenitors (Figure 1C) and GST-pi+ mature oligodendrocytes are diminished in number (Figure 2A). However, even though axon degeneration is also not observed within cuprizone-induced lesions,18 it is plausible that the eventual failure of remyelination during continuous cuprizone treatment is due to some sort of axon injury that prevents oligodendrocyte survival and/or interaction with demyelinated axons. To determine whether the chronically demyelinated axons retained the capacity to be remyelinated, we isolated O4+ progenitors from the corpus callosum in untreated adult GFP tg mice and transplanted them into the demyelinated corpus callosum in wild-type mice treated with cuprizone for 12 weeks. Control mice were injected with serum-free medium containing no progenitors. The mice were then returned to a normal diet for 4 weeks, allowing recovery. Only 8.1 ± 1.2% of the axons were myelinated within the corpus callosum in mice that were not transplanted (Figure 7, A and G). In contrast, there was a significant increase (P < 0.001) in the percentage of axons that were myelinated (42.2 ± 6.2%) within the corpus callosum in mice in to which progenitors were transplanted (Figure 7, B and G).
Figure 6.
Cuprizone-induced changes in mRNA levels for the myelin-specific protein, MAG, and the neuronal-specific protein, paranodin. Mice were fed cuprizone for 12 weeks (black bars) or they were returned to a normal diet following 6 weeks of cuprizone feeding (white bars). A: Steady-state mRNA levels were determined for MAG and the mean and SEM bars were plotted for triplicate determinations. B: RNA samples were analyzed for paranodin mRNA levels using RT-PCR. G3PDH was analyzed to assure that equal amounts of RNA and cDNA were amplified in each reaction.
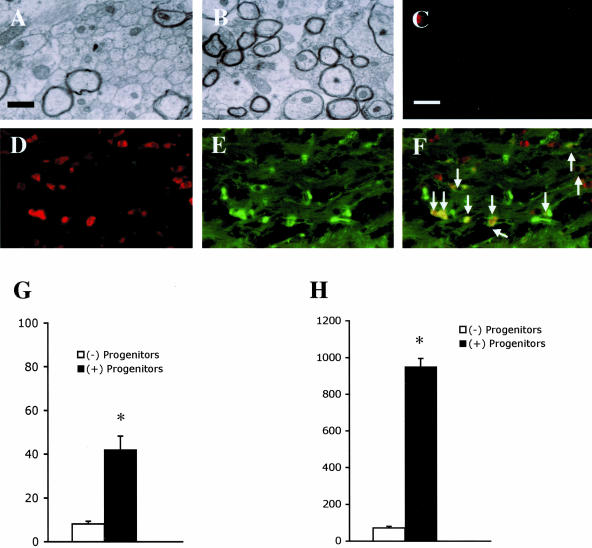
Figure 7.
Adult O4+ progenitors transplanted into the chronically demyelinated corpus callosum differentiate into mature oligodendrocytes and remyelinate the demyelinated axons. DMEM/F12 media containing either no progenitors (A, C) or O4+ progenitors isolated from the corpus callosum in adult GFP tg mice (B, D, E) were transplanted into the chronically demyelinated corpus callosum in mice treated with cuprizone for 12 weeks, at which time the mice were returned to a normal diet for 4 weeks to allow for recovery. Electron micrographs are of the corpus callosum in mice that did not have progenitors transplanted (A) or in mice that did have progenitors transplanted (B). Tissue sections from mice in which progenitors were either not transplanted (C) or were transplanted (D, E) were stained for the presence of GST-pi+ mature oligodendrocytes (red; C, D) and GFP+ cells (green; E). Arrows indicate the GFP+ cells that express GST-pi (F). The percentage of myelinated axons (G) and total number of GST-pi+ mature oligodendrocytes (H) were quantitated and plotted as mean ± SEM for triplicate determinations from mice that did not have progenitors transplanted (white bars) or from mice that did have progenitors transplanted (black bars). Scale bars: 1 μm in A and B and 10 μm in C–F. *P < 0.001
Examination of the mature oligodendrocyte population showed that only a few GST-pi+ mature oligodendrocytes (71 ± 9/mm2) were present within the chronically demyelinated corpus callosum in mice that were not transplanted (Figure 7, C and H), while a significantly (P < 0.001) larger number of GST-pi+ oligodendrocytes (950 ± 45/mm2) were present within the remyelinated corpus callosum in the mice into which progenitors were transplanted (Figure 7, D and H). Further immunohistochemical analysis showed that approximately 68.2 ± 6.4% of the GST-pi+ oligodendrocytes were also GFP+ (Figure 7, D–F), demonstrating that a large percentage of the newly regenerated oligodendrocytes were derived from the transplanted progenitors.
Discussion
The long-term feeding of cuprizone induces a series of demyelinating/remyelinating episodes leading to the formation of chronic lesions within the corpus callosum in C57BL/6J mice.18 During the first demyelinating episode, oligodendrocytes become depleted and then oligodendrocyte progenitors accumulate within the lesion and differentiate into mature oligodendrocytes leading to remyelination.6 In contrast, when a demyelinating insult persists, both oligodendrocytes and progenitors become progressively depleted, leading to the formation of chronically demyelinated lesions (Figure 2). The ability of transplanted adult O4+ progenitors to remyelinate the chronic lesions suggests that the axons therein retain the capacity to be remyelinated. In addition, the environment within these lesions is conducive for the survival and differentiation of oligodendrocyte progenitors once cuprizone toxicity is removed.
Oligodendrocytes Become Depleted within Demyelinating Lesions Progressing to a Chronic State
Our present work showing the depletion of mature oligodendrocytes within chronically demyelinated lesions in adult mice exposed to cuprizone for a prolonged period of time is consistent with chronic lesions in MS,37 experimental autoimmune encephalomyelitis (EAE),38 and the superior cerebellar peduncles in cuprizone intoxication.39 During cuprizone toxicity, mature oligodendrocytes become depleted, presumably via apoptosis, during acute demyelination.6 Similarly, most of the mature oligodendrocytes regenerated during recovery from acute demyelination also undergo apoptosis if the pathological insult is not removed, eventually leading to the depletion of these cells within the chronically demyelinated lesions (Figure 2, A and B). In contrast to the cuprizone model of demyelination, chronic demyelination and oligodendrocyte depletion is not observed within lesions that have undergone several demyelinating/remyelinating episodes if each regenerative response is allowed to occur in the absence of a demyelinating stimulus.40 Thus, the depletion of oligodendrocytes during cuprizone intoxication may be the result of a continuous insult on these cells in which they are not able to recover. This depletion of oligodendrocytes is primarily responsible for the inability of the chronic lesions to recover, even after the insult is removed.
Interestingly, microglia/macrophages persist within lesions progressing to a chronic state either during a prolonged demyelinating insult (Figures 4 and 5) or within lesions that are unable to recover from acute demyelination.41 It should be noted that only a few microglia/macrophages were present within the lesions being remyelinated by the transplanted O4+ progenitors in the absence of cuprizone intoxication (data not shown). Although microglia/macrophages secrete factors that induce the apoptotic death of mature oligodendrocytes in vitro,30–35 it is not clear whether these immune cells are responsible for inducing the death and depletion of oligodendrocytes within lesions progressing to a chronic state. However, in addition to microglia/macrophage-derived TNF-α being highly expressed within demyelinating lesions containing apoptotic oligodendrocytes, we have also previously reported that the apoptotic death of oligodendrocytes is delayed within TNF-α−/− mice.36 Thus, there is supporting evidence to the hypothesis that microglia/macrophages are responsible for oligodendrocyte death during demyelination.
We assume that the ability of the CNS to generate new oligodendrocytes following acute demyelination and also during the progression toward chronic demyelination is due to the differentiation of progenitors within the lesion. Although a large number of progenitors remain within the remyelinating lesion after the mice are returned to a normal diet,6 the progenitor population decreases dramatically during continuous cuprizone exposure (Figure 2C). This progressive depletion suggests that these cells are either dying, differentiating into oligodendrocytes, or both. However, since some of the progenitors appear to be differentiating into oligodendrocytes during the progression of the demyelinating lesion to a chronic state, it suggests that the depletion of the progenitor population is a consequence of the progenitors differentiating into mature oligodendrocytes. This assumption would suggest that a limited pool of oligodendrocyte progenitors exist within the adult CNS parenchyma and that the depletion of this pool following a long-term pathological insult may contribute to the formation of chronically demyelinated lesions.
In the adult CNS, oligodendrocyte progenitors residing within the parenchyma do not migrate into a demyelinating lesion in the surrounding area and contribute to the remyelination process.7,42 In contrast, others have speculated that progenitors residing in the subventricular zone (SVZ) appear to migrate into an acute demyelinating lesion, proliferate, and then differentiate into mature myelinating oligodendrocytes.6,43 The present work showing the depletion of progenitor cells within the chronically demyelinated lesion demonstrates the inability of progenitors, within or outside the lesion, to accumulate within chronically demyelinated lesions. This lack of accumulation appears to be the consequence of the local progenitor pool becoming depleted. Furthermore, we speculate that progenitors residing outside the lesion may not be able to migrate into the lesion due to either the absence of a chemo attractant signal required to recruit these progenitors into the chronic lesion and/or an inability of the progenitors to migrate within the parenchyma.
Chronically Demyelinated Axons Retain the Capacity to Be Remyelinated
A prolonged or repetitive demyelinating insult can induce a state of chronic demyelination within the adult CNS.18,38,39,44 It has been speculated that axon degeneration within demyelinating lesions in MS and EAE is due to long-term demyelination and/or cytotoxic factors derived from infiltrating immune cells.3,16 Although axon degeneration occurs within chronically demyelinated lesions in MS15 and EAE,3 this type of pathology does not occur within the chronically demyelinated corpus callosum induced by cuprizone.18 In addition, while demyelinating lesions induced by cuprizone contain microglia/macrophages and an array of macrophage-derived cytotoxic factors,36,45,46 these lesions contain few T lymphocytes (unpublished observations). Thus, axon degeneration may be the consequence of a direct attack against axons within demyelinated lesions in EAE mice or MS patients by T-lymphocyte-derived factors that are not present with cuprizone-induced lesions.
Even though oligodendrocyte progenitors have been identified within some chronic lesions in MS patients, it has been suggested that these progenitors are unable to differentiate into mature oligodendrocytes and remyelinate the demyelinated axons.12–14,17 The progenitors within these lesions may not be able to differentiate due to axon degeneration and/or the absence of an appropriate differentiation signal. The ability of transplanted progenitors to differentiate into mature oligodendrocytes and remyelinate chronically demyelinated axons within cuprizone-induced chronic lesions suggests that the appropriate differentiation signals are present within chronic lesions, at least under the conditions tested here. However, the environment within chronic MS lesions and chronic lesions induced by cuprizone may be dramatically different. Elucidating the environmental differences within MS chronic lesions and cuprizone-induced chronic lesions may provide insight into the signals required for oligodendrocyte progenitor differentiation within demyelinating lesions.
Adult O4+ Progenitors Are Able to Differentiate into Mature Oligodendrocytes and Remyelinate Chronically Demyelinated Axons in the Adult CNS
Although endogenous cycling progenitors have the capability to differentiate into mature oligodendrocytes and remyelinate demyelinated axons, the identity of these cells is unknown.7,42 We have recently isolated a population of O4+ progenitors from the corpus callosum in adult rodents that differentiates into mature oligodendrocytes in vitro.26 The work presented here further shows that these O4+ progenitors have the capability to differentiate into mature oligodendrocytes and remyelinate not only demyelinated axons, but chronically demyelinated axons in vivo. These O4+ progenitors do not express NG2 on isolation26,27 and it is not known whether they express NG2 at any time during their development. Therefore, the O4+ progenitors used in this study may either be at a different developmental stage compared to the NG2+ progenitors identified in situ or they may constitute a completely separate population of progenitors all together. Interestingly, some of the newly generated mature oligodendrocytes (approximately 30%) did not arise from the transplanted O4+ progenitors. This finding suggests that some of the transplanted progenitors down-regulate GFP or they may express a factor that either induces the proliferation and differentiation of the few remaining endogenous progenitors, promotes the survival of residual oligodendrocytes or recruits progenitors residing outside the lesion. Thus, it appears that these O4+ cells may constitute a primary source of oligodendrocyte progenitors within the adult CNS white matter. Further characterization of these cells may provide additional information regarding the factors and/or mechanisms that lead to the survival, migration, proliferation, and differentiation of oligodendrocyte progenitors during pathological conditions in vivo.
Acknowledgments
We thank Dr. Robert Bagnell, Victoria Madden, and Bernetta Abramson for technical assistance and Dr. William Stallcup for the generous gift of the NG2 antibody. We acknowledge Bill Hall for kind support of this research with a private gift.
Footnotes
Address reprint requests to Jeff Mason, Thomas Jefferson University, Department of Neurology, Farber Institute for the Neurosciences, 900 Walnut St., #400, JHN Building, Philadelphia, PA 19107. E-mail: jeffrey.mason@jefferson.edu.
Supported by grants from the Multiple Sclerosis Society (to J.L.M., G.K.M.) and the National Institutes of Health (to P.M., K.S., J.E.G., G.K.M.).
References
- Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. Brain. 1999;122:2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- Raine CS, Cross AH. Axonal dystrophy as a consequence of long-term demyelination. Lab Invest. 1989;60:714–725. [PubMed] [Google Scholar]
- Ludwin SK. Central nervous system remyelination: studies in chronically damaged tissue. Ann Neurol. 1994;6 (Suppl):S143–S145. doi: 10.1002/ana.410360735. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Andrews JM, Waltz JM, Terry RD. Ultrastructure studies of multiple sclerosis. Lab Invest. 1969;20:444–454. [PubMed] [Google Scholar]
- Mason JL, Jones JJ, Taniike M, Morell P, Matsushima GK. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res. 2000;61:251–262. doi: 10.1002/1097-4547(20000801)61:3<251::AID-JNR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Ludwin SK. Central nervous system demyelination and remyelination in the mouse. Lab Invest. 1978;39:597–612. [PubMed] [Google Scholar]
- Blakemore WF. Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci. 1973;20:73–83. doi: 10.1016/0022-510x(73)90119-6. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJM. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Shankar SL, Shaft-Zagardo B, Massimi A, Lee SC, Raine CS, Brosnan CF. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding N, Franklin R, Stevens S, Heldin C-H, Compston A, Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121:2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar C, Trapp BD. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr Opin Neurol. 2001;14:271–278. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Silber E, Sharief MK. Axonal degeneration in the pathogenesis of multiple sclerosis. J Neurol Sci. 1999;170:11–18. doi: 10.1016/s0022-510x(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Chang A, Wallace MD, Tourtellotte W, Rudick R, Trapp B. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Mason JL, Langaman C, Morell P, Suzuki K, Matsushima GK. Episodic demyelination and subsequent remyelination within the murine central nervous system: changes in axonal calibre. Neuropathol Appl Neurobiol. 2001;27:50–58. doi: 10.1046/j.0305-1846.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- Hiremath MM, Saito Y, Knapp GW, Ting JP-Y, Suzuki K, Matsushima GK. Microglia/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 1998;92:38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Abervine JB, Pierec ET. Atlas of the mouse brain and spinal cord. Cambridge: Harvard University Press; 1971 [Google Scholar]
- Tansey FA, Cammer WP. A pi form of glutathione-S-transferase is a myelin- and oligodendrocyte-associated enzyme in mouse brain. J Neurochem. 1991;57:95–102. doi: 10.1111/j.1471-4159.1991.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Morell P, Barrett CV, Mason JL, Toews AD, Hostettler JD, Knapp GW, Matsushima GK. Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol Cell Neurosci. 1998;12:220–227. doi: 10.1006/mcne.1998.0715. [DOI] [PubMed] [Google Scholar]
- Jansen R, Ledley FD. Production of high specific activity DNA probes using the polymerase chain reaction. Gene Anal Techn. 1989;6:79–83. doi: 10.1016/0735-0651(89)90020-4. [DOI] [PubMed] [Google Scholar]
- Lai C, Brow MA, Nave K-A, Noronha AB, Quarles RH, Bloom FE, Milner RJ, Sutcliffe JG. Two forms of 1B236/myelin-associated glycoprotein, a cell adhesion molecule for postnatal development, are produced by alternative splicing. Proc Natl Acad Sci USA. 1987;84:4337–4341. doi: 10.1073/pnas.84.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsik A, Malcus C, Akaoka H, Giraudon P, Belin M-F, Bernard A. Selective induction of cytokines in mouse brain infected with canine distemper virus: structural, cellular and temporal expression. J Neuroimmunol. 1996;65:1–9. doi: 10.1016/0165-5728(95)00173-5. [DOI] [PubMed] [Google Scholar]
- Mason JL, Goldman JE. A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2 and IGF-1. Mol Cell Neurosci. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Heterogeneity of cycling progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48:75–86. [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997 [Google Scholar]
- Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34:296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- Brogi A, Strazza M, Melli M. Induction of intracellular ceramide by interleukin-1β in oligodendrocytes. J Cell Biochem. 1997;66:532–541. doi: 10.1002/(sici)1097-4644(19970915)66:4<532::aid-jcb12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Hishahara S, Shoji S, Okano H, Miura M. ICE/CED-3 family executes oligodendrocyte apoptosis by tumor necrosis factor. J Neurochem. 1997;69:10–20. doi: 10.1046/j.1471-4159.1997.69010010.x. [DOI] [PubMed] [Google Scholar]
- McLaurin J, D’Souza S, Stewart J, Blain M, Beaudet A, Nalbantoglu J, Antel JP. Effect of tumor necrosis factor α and β on human oligodendrocytes and neurons in culture. Int J Dev Neurosci. 1995;13:369–381. doi: 10.1016/0736-5748(95)00012-6. [DOI] [PubMed] [Google Scholar]
- D’Souza S, Alinauskas K, McCrea E, Goodyer C, Antel JP. Differential susceptibility of human CNS-derived cell populations to TNF-dependent and independent immune-mediated injury. J Neurosci. 1995;15:7293–7300. doi: 10.1523/JNEUROSCI.15-11-07293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J-C, Magal E, Takayama S, Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Farooq M, Norton WT, Brosnan CF. Cytokine cytotoxicity against oligodendrocytes. J Immunol. 1991;147:1522–1529. [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF-α promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Raine CS. The Norton lecture: a review of the oligodendrocyte in the multiple sclerosis lesion. J Neuroimmunol. 1997;77:135–152. doi: 10.1016/s0165-5728(97)00073-8. [DOI] [PubMed] [Google Scholar]
- Linington C, Engelhardt B, Kapocs G, Lassman H. Induction of persistently demyelinated lesions in the rat following the repeated adoptive transfer of encephalitogenic T cells and demyelinating antibody. J Neuroimmunol. 1992;40:219–224. doi: 10.1016/0165-5728(92)90136-9. [DOI] [PubMed] [Google Scholar]
- Ludwin SK. Chronic demyelination inhibits remyelination in the central nervous system: an analysis of contributing factors. Lab Invest. 1980;43:382–387. [PubMed] [Google Scholar]
- Penderis J, Shields SA, Franklin JM. Impaired remyelination and depletion of oligodendrocyte progenitors does not occur following repeated episodes of focal demyelination in the rat central nervous system. Brain. 2003;126:1382–1391. doi: 10.1093/brain/awg126. [DOI] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. IGF Signaling through IGFR1 plays an important role in remyelination. J Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WW. Response of the oligodendrocyte progenitor cell population (defined by NG2 labeling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooran AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Johnson ES, Ludwin SK. The demonstration of recurrent demyelination and remyelination of axons in the central nervous system. Acta Neuropathol. 1981;53:93–98. doi: 10.1007/BF00689988. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Hellendall RP, Matsushima GK, Suzuki K, Laubach VE, Sherman P, Ting JP. The protective role of nitric oxide in a neurotoxicant-induced demyelinating model. J Immunol. 2002;168:427–433. doi: 10.4049/jimmunol.168.1.427. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1β promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]