Abstract
Inflammation-mediated endothelial cell (EC) dysfunction likely contributes to the pathogenesis of several vascular diseases including atherosclerosis. We found that stimulation of human umbilical vein ECs with lipopolysaccharide induced secretion of cyclophilin (CyPA) an intracellular protein belonging to the immunophilin family. We then found that when added exogenously CyPA has direct effects on ECs in vitro. At low concentrations (10 to 100 ng/ml) CyPA increased EC proliferation, migration, invasive capacity, and tubulogenesis. Gelatin zymography indicated increased secretion of active matrix metalloproteinase-2, a mediator of cell migration and angiogenesis. At high concentrations (eg, 2 μg/ml) CyPA had opposite effects, decreasing EC migration and viability, possibly in relation to induction of Toll-like receptor-4 expression, detected by immunocytochemistry and flow cytometry. In vivo CyPA expression was not detectable in the luminal ECs of normal mouse carotid arteries but was rapidly induced after systemic lipopolysaccharide injection. In an experimental mouse model of atherosclerosis, CyPA expression was detected in the ECs of neocapillaries of carotid artery lesions, supporting its association with pathological angiogenesis suggested by our in vitro results. In conclusion, we found that CyPA has a biphasic activity on ECs in vitro and is up-regulated in vivo in ECs under pathological states. Our results suggest that CyPA is a novel paracrine and autocrine modulator of EC functions in immune-mediated vascular disease.
Innate immune defense constitutes a rapid response, minutes to hours, and is associated with detection of pathogen-associated molecular patterns that evoke an inflammatory response. These pattern recognition receptors include various scavenger and Toll-like receptors (TLRs). Their ligands include pathogen-associated molecular patterns such as lipopolysaccharide (LPS), a gram-negative endotoxin.1 LPS is known to stimulate monocytes, macrophages, and neutrophils through the activation of transcription factors resulting in increased proinflammatory responses,2,3 associated with release of cytokines and other soluble mediators. As such, infectious agents that create a heightened state of the inflammatory response are likely to directly stimulate the endothelial lining of blood vessels.
Besides the well-known cytokines, other factors have become under recent investigation. Among these, cyclophilin A (CyPA), a soluble ubiquitously distributed intracellular protein belonging to the immunophilin family,4 was identified as a proinflammatory secretory product of LPS-activated macrophages5 and is known for its involvement in differentiation and proliferation of T cells, and was reported recently to be related to the growth and differentiation of other cells, such as human embryonic nerve cells.6 CyPA was detected in the serum of sepsis patients7 and the synovium of patients with rheumatoid arthritis.8 CyPA was reported to be an intracellular target for the potent immunosuppressive drug cyclosporin A,9 whose cytoprotective effect was recently suggested to be mediated by vascular endothelial growth factor (VEGF) receptor-2.10 However, the hypothesis of a direct stimulation of endothelial cell (EC) function by CyPA has not been previously investigated.
We hypothesized that LPS-stimulated ECs may secrete CyPA, and also that the secreted form of CyPA may directly stimulate ECs. We investigated potential CyPA secretion by ECs in vitro, as well as potential in vitro effects of exogenously added CyPA on cultured ECs. We also used an acute and a chronic mouse model to examine in situ endothelial CyPA expression in normal and diseased mouse carotid arteries.
Materials and Methods
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cord and grown in EBM-2 medium containing 5% fetal bovine serum, human fibroblast growth factor-B, heparin, VEGF, human epidermal growth factor, ascorbic acid, and hydrocortisone. Cells used in this study were between passages 4 and 8. For stimulation experiments, HUVECs were washed twice with serum-free medium and then incubated with serum-free medium EBM-2 containing the treatments, ie, 1 ng/ml to 2 μg/ml LPS (Sigma, St. Louis, MO), 1 ng/ml to 2 μg/ml human recombinant CyPA (BioMol, Plymouth Meeting, PA), and 0.5 μg/ml monensin (Sigma).
Proliferation and Cell Viability Assays
HUVECs (5 × 105 cells), seeded on sterile coverslips 24 hours before experimentation (80% confluency), were washed twice with serum-free EBM-2, and then incubated for 24 hours with serum-free medium containing CyPA (1 ng/ml to 2 μg/ml) and bromodeoxyuridine (BrdU, 20 μmol/L). Cell-seeded coverslips were fixed with 4% paraformaldehyde and stained with anti-BrdU antibody (Abcom, Cambridge, UK) and the general nuclear stain Hoechst 33258. We imaged the cells with a fluorescence microscope and quantified proliferation as the percent BrdU-positive cell nuclei (pink) versus total cell nuclei (blue). To ensure reproducibility, each experiment was independently performed three times. Cell viability after different treatments was determined using the Live Dead assay (Molecular Probes, Inc., Eugene, OR), following the manufacturer’s instructions.
In Vitro Assays
The potential ability of CyPA to mediate EC migration was tested in a scratch wound migration assay. HUVECs cultured on coverslips were wounded with a cell scrapper, and incubated for 24 hours in reduced serum (0.2% fetal bovine serum) media containing CyPA (1 ng/ml to 2 μg/ml). Cells were then fixed with 4% paraformaldehyde and imaged using phase contrast microscopy. Migration was quantified as the total cell number of cells migrated from the wound edge. Potential effects on EC invasion capacity were determined using a Transwell system with polycarbonate membranes (8.0-μm-pore size, 24-well chamber; Costar, Cambridge, MA).11 The membranes were coated with 0.1 mg/ml of gelatin in distilled water and allowed to dry at room temperature for 30 minutes. HUVECs suspended in serum-free EBM-2 were added to the upper chamber at 5 × 104 cells per well. The chemotactic stimuli, CyPA (1 ng/ml to 2 μg/ml) or VEGF (10 ng/ml) used as a positive control, added to serum-free EBM-2 were placed in the lower chambers, and HUVECs were allowed to migrate for 8 hours. The membranes were removed from the inserts and fixed with 4% paraformaldehyde for 10 minutes. Migrated cell nuclei were stained with Hoechst 33258 for 5 minutes and migrated cells were imaged with a fluorescence microscope and nuclei were counted.
The potential activity of CyPA in angiogenesis was determined using several in vitro assays. For the tube formation assay, HUVECs (1 × 104 cells) were seeded onto growth factor-reduced Matrigel in the presence or absence of CyPA and incubated at 37°C for 6 hours, after which cells were fixed with 4% paraformaldehyde. Changes in cell morphology were imaged by phase contrast and immunofluorescence microscopy. Tube formation was analyzed by counting the total number of lumens created in each microscopic field. Four randomly selected fields were examined for each condition.
Biochemical Analysis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis gelatin zymography was performed as previously to assess gelatinase production.12 Briefly, HUVECs seeded on 12-well plates in complete EBM-2 medium were cultured to 80% confluency, washed two times with serum-free media, then treated at indicated concentrations of CyPA in serum-free EBM-2. After incubation for 6 hours, the conditioned culture media were collected, mixed with sample buffer, and applied to 8% sodium dodecyl sulfate-polyacrylamide gel containing 1 mg/ml of gelatin. The lytic bands were imaged on gels after staining with Coomassie Brilliant Blue R250 and quantified using a gel analyzer (Gel Doc 1000; Bio-Rad, Hercules, CA). Expression of CyPA was detected by Western blotting (1:2000 rabbit anti-cyclophilin polyclonal antibody, BioMol)
Fluorescence-Activated Cell Sorting Analysis
Confluent HUVECs were stimulated with CyPA (1 ng/ml to 2 μg/ml) or LPS (1 μg/ml) for 24 hours, then washed with cold phosphate-buffered saline (PBS), harvested by enzyme-free cell dissociation buffer (Invitrogen, Carlsbad, CA), filtered with cell strainer, and washed with cold fluorescence-activated cell sorting staining buffer (0.5 mol/L Na2EDTA, fetal bovine serum, and Hanks’ balanced salt solution without phenol red) before being incubated (30 minutes, 4°C) with either buffer only, or with anti-human TLR-4 antibody (100 mg/ml; Santa Cruz Biotechnology, Santa Cruz, CA). The cells were washed once, then incubated (20 minutes, dark, 4°C) with Alexa Fluor 488 anti-mouse IgG (Molecular Probes), and analyzed with FACSort (Becton-Dickinson, San Jose, CA)
In Vivo Experimental Mouse Models
C57/BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were injected with LPS (100 μg/mouse, Sigma) diluted in PBS and sacrificed 12 hours after injection. Sections of experimental atherosclerotic lesions were obtained from ApoE knockout (−/−) mice (Jackson Laboratories) by a combination of flow cessation and high-fat diet as previously described.13 Fresh carotid arteries, harvested from normal mice or postoperatively from either LPS-injected wild-type mice or hypercholesterolemic ApoE−/− mice were collected and frozen embedded for immunohistochemistry or processed for protein extraction and biochemical analysis.
Immunofluorescence Analysis
Immunofluorescence was performed on cultured HUVECs or on 7-μm sections of mouse carotid artery. To detect CyPA, we used either a rabbit polyclonal anti-human CyPA or anti-mouse CyPA antibodies (BioMol) followed by Alexa Fluor 568 goat anti-rabbit IgG (Molecular Probes,). Human TLR-4 was detected using anti-human TLR-4 monoclonal antibody (Santa Cruz) followed by Alexa Fluor 488 anti-mouse IgG (Molecular Probes). Cell-specific staining was done using the rat anti-mouse CD31 antibody (Pharmingen, San Diego, CA) for ECs and the rat anti-mouse MAC3 (Pharmingen) for detection of macrophages, both followed by Alexa Fluor 488 goat anti-rat IgG (Molecular Probes). VCAM-1 was detected using the rat anti-mouse VCAM-1 (PharMingen) followed by Rhodamine Red X-conjugated goat anti-rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), whereas ICAM-1 was detected using biotin-conjugated hamster anti-mouse ICAM-1 (PharMingen), followed by AF488-conjugated streptavidin (Molecular Probes). Nuclei were counterstained with Hoechst 33258 (Sigma) and sections were imaged using a fluorescence microscope (Axioscope; Zeiss, Thornwood, NY).
Statistical Analysis
All data are presented as the mean ± SEM. Statistical analysis was performed using analysis of variance. Differences were judged statistically significant when P < 0.05.
Results
CyPA Is Secreted by LPS-Stimulated ECs and Can Act as a Mediator of EC Activation in Vitro
Secretion of CyPA, undetectable from HUVECs maintained under basal cell culture conditions, was found to increase with LPS treatment in a time- and dose-dependent manner (Figure 1). The highest secretion of CyPA was detected after 12 hours of LPS treatment after which levels decreased. Using the live-dead assay, we confirmed that at the concentrations tested LPS treatment did not decrease cell viability (data not shown).
Figure 1.
In vitro treatment with LPS induces secretion of CyPA by HUVECs. A: CyPA (18 kd) was detected in the culture medium of HUVECs by Western blotting. LPS is an effective stimulator of CyPA secretion by ECs (6 hours). B: Expression of CyPA was stimulated by LPS (1 μg/ml) in serum-free media in a time-dependent manner, with maximal stimulation detected at 12 hours. C: Detection of CyPA in HUVECs by immunofluorescence (red fluorescent signal, CyPA; blue fluorescent signal, nuclei counterstained with Hoechst). Addition of monensin, an inhibitor of protein secretion, increases intracellular accumulation of CyPA. Scale bar, 5 μm.
The ability of exogenously added CyPA to directly stimulate ECs was tested at different concentrations. We found that low concentrations (1 ng/ml to 100 ng/ml) of CyPA stimulated HUVEC proliferation in a dose-dependent manner (Table 1). The effect was statistically significant at 10 ng/ml of CyPA and comparable to that obtained through addition of a similar concentration of VEGF (Figure 2). However, at higher CyPA concentrations cell proliferation decreased with a concurrent increase in dead cells (data not shown), indicating a potential cytotoxic effect.
Table 1.
Cyclophilin (CyPA) Demonstrates Biphasic Effects upon Cultured Endothelial Cells: Effect of in Vitro CyPA Treatment of HUVECs upon Cell Proliferation and Migration
| CyPA (ng/ml)
|
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 10 | 100 | 1000 | 2000 | |
| Proliferating cells (%) | 18.6 (8.2) | 20.6 (0.9) | 32.7 (1.2)* | 19.7 (1.5) | 9.0 (1.0) | 7.0 (1.2) |
| Migrated cell number | 48 (7) | 95 (6)* | 181 (18)* | 114 (15) | 58 (7) | 36 (6) |
All values are mean (SEM).
P < 0.05 versus no treatment (basal conditions).
Figure 2.
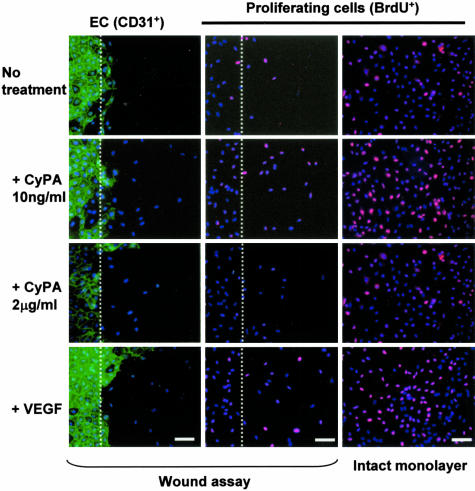
CyPA stimulates endothelial wound healing (left and middle rows) and proliferation in vitro. Scratch wound assay (dotted line indicates wound edge) showed that the low CyPA concentration tested (10 ng/ml) enhanced migration of ECs (detected with anti-CD31, green fluorescence). Nuclei are counterstained with Hoechst (blue). ECs migrating past the wound edge were quantified after 24 hours. Middle and right rows: EC proliferation, detected using incorporation of BrdU (red), in subconfluent wounded or intact monolayers under different conditions (immunofluorescence). Nuclei of proliferating cells appear as pink. CyPA (10 ng/ml) increased basal level (no treatment) of cell proliferation to a level comparable to that induced by VEGF, used as a positive control. A biphasic effect was observed, with high CyPA concentrations (2 mg/ml) decreasing cell proliferation.
CyPA Can Function as Angiogenic Stimulus in Vitro
To reveal the effect of CyPA on processes that enable angiogenesis we next tested in vitro the effects on the migratory and invasive activity of ECs. Addition of CyPA to the culture medium during the scratch wound migration assay again revealed its biphasic effect (Table 1). Monolayer wound closure was significantly enhanced (P < 0.05) at low doses of CyPA, peaking at 10 ng/ml, even compared to the full growth medium, but was inhibited at high doses (2 μg/ml) of CyPA. Some of the enhancing effect was likely mediated though stimulation of cell proliferation by CyPA, investigated and confirmed through addition of BrdU during the assay (Figure 2).
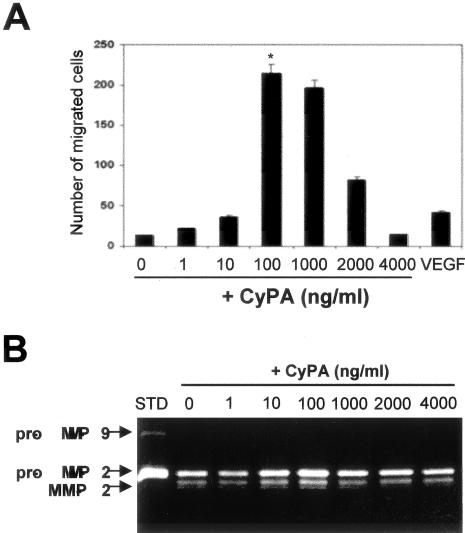
Next, the ability of CyPA to increase EC invasion through matrix was investigated using Transwell inserts precoated with gelatin (10 mg/ml). We found a dose response in HUVEC invasiveness to CyPA (Figure 3). The HUVEC invasiveness peaked at 100 ng/ml CyPA to ∼16.4-fold over the no treatment control (P = 0.0014), and then decreased at higher doses. Interestingly, at similar concentrations (10 ng/ml), CyPA and the positive control (VEGF) had comparable effects on endothelial invasiveness.
Figure 3.
A: CyPA increases HUVEC invasiveness through a gelatin matrix (Transwell assay, VEGF 10 ng/ml). B: CyPA increases secretion and activation of gelatinolytic activity associated with matrix metalloproteinase-2 (sodium dodecyl sulfate-polyacrylamide gel electrophoresis gelatin zymography). No effects on secretion of the related gelatinase matrix metalloproteinase-9 were detected, suggesting matrix metalloproteinase-2 as the likely enhancer of endothelial invasiveness.
As a potential mechanism for the increased cell migration through gelatin we assayed the gelatinolytic activity secreted by ECs. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis gelatin-zymography (Figure 3) indicated that lower concentrations of CyPA, which significantly increased the invasive capacity, also increased endothelial gelatinolytic activity associated with secreted matrix metalloproteinase-2. A statistically significant increase of gelatinolytic activity was measured at 100 ng/ml of CyPA (P < 0.05, n = 3 independent experiments).
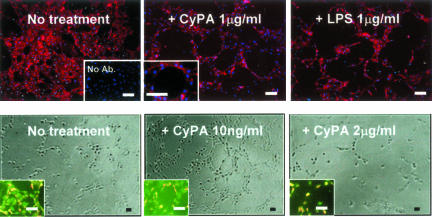
Finally, we tested the effect of CyPA using the in vitro endothelial tube formation assay (Figure 4). We found that low concentrations of CyPA dramatically increased formation of tubes over baseline levels. The total number of tubes was ∼10.5-fold increased at 10 ng/ml of CyPA compared with control (P = 0.0021, n = 6). At this concentration the effect was comparable to the VEGF stimulation used as positive control, but the effect diminished with increased CyPA concentrations, at which CyPA also decreased cell viability (Figure 4).
Figure 4.
In vitro endothelial tube formation assay. Top: ECs highlighted by immunofluorescence using anti-CD31 (red), nuclei counterstained with Hoechst (blue). LPS and CyPA treatments of HUVECs appear to have similar capacity to induce formation of EC tubes on Matrigel. Right bottom inset in no treatment section illustrates the negative control for immunocytochemistry (no Ab, no primary antibody). Left bottom inset in CyPA 1 mg/ml section illustrates at higher magnification the appearance of the lumen of a tube. Bottom (phase contrast): tube formation assay performed on growth factor-reduced Matrigel. The total number of tubes was increased ∼10.5-fold at 10 ng/ml of CyPA compared with the nontreated control (P = 0.0021), but decreased at high concentrations (2 μg/ml of CyPA). Insets (live-dead assay) illustrate effects on cell viability likely contributing to the differences—note the increased percentage of dead cells (red fluorescence) compared to live cells (green). Scale bars, 50 μm.
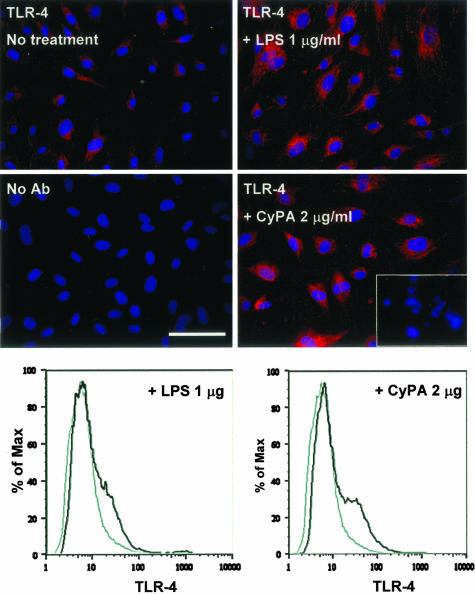
Because high concentrations of CyPA negatively affected cell viability, we asked the question whether exogenous CyPA may induce the endothelial expression of the TLR-4, implicated in the innate immune response and apoptosis.14 We found that, at low concentrations (1 to 100 ng/ml), CyPA did not change baseline expression of TLR-4, but the highest CyPA concentration tested (2 μg/ml) resulted in a significant increase of the TLR-4 expression in ECs (Figure 5), similarly to the effect of LPS treatment. Expression detected by immunocytochemistry was also confirmed by flow cytometry (Figure 5).
Figure 5.
Induction of TLR-4 expression in HUVECs by CyPA and LPS treatments. Top: Immunocytochemical detection of TLR-4 expression in HUVEC monolayers. High concentrations of CyPA (2 μg/ml) induce expression similar to LPS treatment (red fluorescence, TLR-4; blue fluorescence, nuclei). Bottom right inset illustrates detection of picnotic nuclei at these concentrations. No Ab section illustrates the negative control for immunocytochemistry, cells processed in the absence of primary antibody. Bottom graphs: Flow cytometric analysis of TLR-4 expression on HUVECs before and after stimulation with LPS or CyPA demonstrates induction of TLR-4 by high concentrations of CyPA, as indicated by the right shift of the black curve.
CyPA Expression Is Increased in Vivo in Vascular Pathological Situations
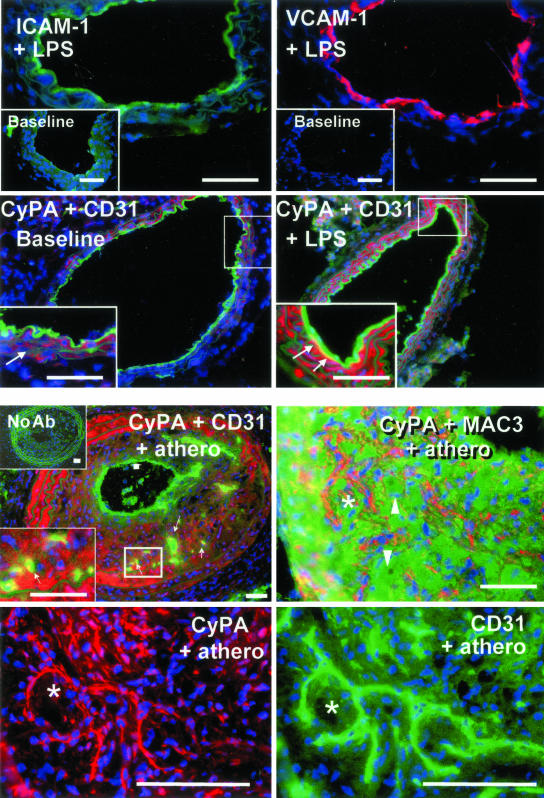
Although normal carotid arteries indicated low CyPA expression in the smooth muscle cells of the medial layer, CyPA was not detected in the luminal endothelial layer (Figure 6). To assess potential acute in vivo induction of CyPA by LPS, we injected LPS (100 μg/mouse) in normal mice and assessed effects on carotid artery 12 hours later. We first confirmed activation of the luminal ECs, as demonstrated by induction of VCAM-1 and ICAM-1 expression (Figure 6). Also, compared with the normal mouse carotid artery, injection of LPS greatly induced the expression of CyPA throughout the arterial wall. Our in vivo observations thus correlated well with the stimulatory effect of LPS on CyPA expression in vitro, specifically with the discovered induction in ECs.
Figure 6.
In vivo vascular expression of CyPA is associated with acute and chronic pathological conditions. Immunofluorescence analysis of mouse carotid artery cross-sections from healthy, untreated mice (baseline), mice injected with LPS (+LPS), or from mice with experimental atherosclerotic lesions (+ athero). Blue fluorescence, cell nuclei counterstained with Hoechst. Top: LPS injection induces acute endothelial activation, as indicated by induction of ICAM (green fluorescence) and VCAM-1 (red fluorescence) expression. Compare to nondetectable baseline expression (insets) of these molecules in normal endothelium. Second row: Luminal endothelial layer is detected using anti-C31 (green fluorescence). Low baseline (left) level of CyPA expression (red fluorescence) is detectable within the normal arterial wall in smooth muscle cells (inset, arrows) but not in luminal ECs. Right: Injection of LPS (+LPS) increased expression of CyPA throughout the wall and induced expression associated with the luminal endothelium (yellow fluorescence). Bottom left inset illustrates the higher magnification of the boxed area. Note the appearance of the yellow signal (arrows), indicating co-localization of CyPA (red) and luminal ECs (green). Bottom: Experimental atherosclerotic lesions induced in the carotid artery of ApoE−/− mice (+athero) a model for a chronic pathological condition of arteries. Top: CyPA (red) is detected diffusely within the intimal lesion and the medial layer. Left: CyPA did not appear associated with the luminal endothelium (thick arrow) but was co-localized (small arrows) with the ECs (green) of neocapillaries that develop within these lesions. Bottom left inset is a higher magnification of the boxed area illustrating the yellow signal in capillaries. Top inset illustrates the negative control for immunohistochemistry, consecutive section processed in the absence of primary antibodies (no Ab). Right: Simultaneous detection of CyPA (red) and macrophages (green), using anti-MAC3, suggesting little overlap. Note some obviously negative macrophages (arrowheads). Bottom: At higher magnification the staining patterns of consecutive sections (the asterisk indicates the same tissue area) for CyPA (red) and endothelium (CD31, green). Although CyPA expression is not restricted to the neocapillaries, these are clearly positive for CyPA. Scale bars, 50 μm.
To investigate whether arterial expression of CyPA in vivo could also be detected in a chronic inflammatory condition of the vasculature, we investigated expression in carotid arteries from a mouse model of atherosclerosis.13 We found that CyPA was highly expressed in the medial layer, likely by smooth muscle cells. In this situation, the luminal ECs did not appear positive, however the endothelium of the microvessels newly formed within the atherosclerotic lesion were all positive (Figure 6), consistent with a connection between CyPA and pathogenic angiogenesis in vivo. Western blotting of carotid artery lysates indicated that development of lesions was associated with an overall significant increase (P < 0.05) in the tissue levels of CyPA (data not shown).
Discussion
In this study we investigated the hypothesis that CyPA is a potential new inflammatory product and stimulator of ECs. We first showed that LPS, one of the bacterial cell wall components shown to activate inflammatory processes15 and to induce secretion of CyPA in macrophages,5 also induces relatively rapid (peak at 12 hours) secretion of CyPA by ECs in vitro. We confirmed that direct in vitro treatment with CyPA of cultured ECs stimulates multiple processes, including cell proliferation, migration, invasion through a matrix-coated membrane, and tube formation. Interestingly, we found that CyPA had a biphasic effect on ECs. At low concentration, CyPA behaved as a potentiator of angiogenesis. As such, we tested the hypothesis that the effects of CyPA were mediated via stimulation of VEGF expression by ECs. However our enzyme-linked immunosorbent assay for secreted VEGF levels by CyPA-stimulated HUVECs indicated no effect of CyPA treatment (data not shown), suggesting that the angiogenic activity at low CyPA concentrations was independent of VEGF, but may be a direct effect of CyPA. On the other hand, we found that CyPA increased EC gelatinolytic activity, another component of the angiogenic switch.16
The high concentration of CyPA tested (2 μg/ml) decreased EC viability and activated the expression of TLR-4, which functions as an LPS receptor. In our hands TLR-4 up-regulation in ECs was at least comparable to that induced by LPS stimulation, which was recently reported.17 Other similar endotoxins have been reported to activate TLR-4.18
Our results suggest that CyPA may act as a novel biphasic paracrine and autocrine stimulatory factor for ECs. Other biologically active factors such as transforming growth factor-β119 and estrogen20 were described to have a biphasic activity. The details of the signaling pathways engaged through stimulation by exogenous CyPA are awaiting future research.
In vivo expression of CyPA was confirmed in mouse models of immune-mediated vascular disease. In the normal mouse carotid artery, we found very low levels of CyPA restricted to medial layer within the smooth muscle cells, which may have been because of low-grade infection or presence of low levels of oxidative stress.21 However, after systemic LPS injection, luminal ECs of carotid artery became activated, as demonstrated by positive detection of VCAM-1 and ICAM-1 induction. This endothelium also expressed CyPA detectable by immunohistochemistry. CyPA also was detectable in the sera of LPS-injected mice (data not shown) consistent with the quick— hours to days—LPS-driven responses during innate immunity and with our observations of in vitro induction of CyPA by LPS stimulation. Interestingly previous reports indicated that CyPA was also detected in the serum of patients with sepsis.7 Thus under such conditions increased levels of circulating CyPA will likely directly affect endothelial and, respectively, vascular function.
On the other hand, in the chronic situation created by existence of atherosclerosis, a pathological vascular condition in which innate immunity was recently implicated,22 we did not find expression of CyPA on the luminal ECs. We showed previously decreased activation of luminal ECs under these conditions.23 However, in the same specimens the ECs forming the neocapillaries within the lesions, a pathological angiogenic response, were positive for CyPA. We suggest that such specific co-localization with the endothelium of neo-capillaries is potentially of great interest because of its pathological implications. In addition, this is consistent with our novel findings of the induction of CyPA expression by ECs in vitro under certain pathological conditions and its apparent angiogenic activity observed in the in vitro assays.
We propose that CyPA is a previously overlooked autocrine and paracrine modulator of EC behavior that may contribute to the pathogenesis of immune-mediated endothelial activation and dysfunction associated with vascular diseases.
Footnotes
Address reprint requests to Zorina S. Galis, Ph.D., Department of Medicine, Division of Cardiology, Emory University, 1639 Pierce Dr.-WMB, Atlanta, GA 30322. E-mail: zgalis@emory.edu.
Supported by the National Institutes of Health (RO1 HL64689 and RO1 HL71061), the American Heart Association (established investigator award no. 0040087N to Z.S.G.), and the National Research Service (postdoctoral award no. 1 F32 HL68449 to S.M.L.).
References
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- Ostos MA, Recalde D, Zakin MM, Scott-Algara D. Implication of natural killer T cells in atherosclerosis development during a LPS-induced chronic inflammation. FEBS Lett. 2002;519:23–29. doi: 10.1016/s0014-5793(02)02692-3. [DOI] [PubMed] [Google Scholar]
- Nakagomi A, Freedman SB, Geczy CL. Interferon-gamma and lipopolysaccharide potentiate monocyte tissue factor induction by C-reactive protein: relationship with age, sex, and hormone replacement treatment. Circulation. 2000;101:1785–1791. doi: 10.1161/01.cir.101.15.1785. [DOI] [PubMed] [Google Scholar]
- Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci USA. 1992;89:3511–3515. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahreini P, Hovland AR, Kumar B, Andreatta C, Edwards-Prasad J, Prasad KN. Effects of altered cyclophilin A expression on growth and differentiation of human and mouse neuronal cells. Cell Mol Neurobiol. 2001;21:65–79. doi: 10.1023/a:1007173329237. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Schumacher A, John S, Geiger H, Geisslinger G, Bang H, Brune K. Elevated serum cyclophilin levels in patients with severe sepsis. J Clin Immunol. 1997;17:380–386. doi: 10.1023/a:1027364207544. [DOI] [PubMed] [Google Scholar]
- Billich A, Winkler G, Aschauer H, Rot A, Peichl P. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J Exp Med. 1997;185:975–980. doi: 10.1084/jem.185.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Burakoff SJ, Bierer BE. Immunophilins in protein folding and immunosuppression. EMBO J. 1994;8:391–400. doi: 10.1096/fasebj.8.6.7513288. [DOI] [PubMed] [Google Scholar]
- Alvarez-Arroyo MV, Yague S, Wenger RM, Pereira DS, Jimenez S, Gonzalez-Pacheco FR, Castilla MA, Deudero JJ, Caramelo C. Cyclophilin-mediated pathways in the effect of cyclosporin A on endothelial cells: role of vascular endothelial growth factor. Circ Res. 2002;91:202–209. doi: 10.1161/01.res.0000027562.91075.56. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Schimmel P. Highly differentiated motifs responsible for two cytokine activities of a split human tRNA synthetase. J Biol Chem. 1999;274:23155–23159. doi: 10.1074/jbc.274.33.23155. [DOI] [PubMed] [Google Scholar]
- Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation. 2000;102:2861–2866. doi: 10.1161/01.cir.102.23.2861. [DOI] [PubMed] [Google Scholar]
- Ivan E, Khatri JJ, Johnson C, Magid R, Godin D, Nandi S, Lessner S, Galis ZS. Expansive arterial remodeling is associated with increased neointimal macrophage foam cell content: the murine model of macrophage-rich carotid artery lesions. Circulation. 2002;105:2686–2691. doi: 10.1161/01.cir.0000016825.17448.11. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- Rietschel ET, Brade H. Bacterial endotoxins. Sci Am. 1992;267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuke S, Ulmer AJ, Kusumoto S, Katus HA, Heine H. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res. 2002;56:126–134. doi: 10.1016/s0008-6363(02)00512-6. [DOI] [PubMed] [Google Scholar]
- Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. 2001;166:2018–2024. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Vassalli JD, Orci L, Montesano R. Biphasic effect of transforming growth factor-beta 1 on in vitro angiogenesis. Exp Cell Res. 1993;204:356–363. doi: 10.1006/excr.1993.1043. [DOI] [PubMed] [Google Scholar]
- Banerjee SK, Campbell DR, Weston AP, Banerjee DK. Biphasic estrogen response on bovine adrenal medulla capillary endothelial cell adhesion, proliferation and tube formation. Mol Cell Biochem. 1997;177:97–105. doi: 10.1023/a:1006888020596. [DOI] [PubMed] [Google Scholar]
- Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160:2145–2155. doi: 10.1016/S0002-9440(10)61163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]