Abstract
Spitz nevi are benign melanocytic nevi that overlap histopathologically with melanoma. We previously found copy number increases of chromosome 11p frequently paralleled by mutations in the HRAS oncogene mapping to this region. In this study, we explored mechanisms that inhibit proliferation in the presence of HRAS activation. We analyzed MAP-kinase activation using immunohistochemistry for phospho-ERK, cyclin D1, and microphthalmia transcription factor expression in 17 Spitz nevi with and 18 Spitz nevi without 11p copy number increase. We found relatively high levels of phospho-ERK and cyclin D1 expression suggesting MAP-kinase pathway activation in both groups of Spitz nevi. However, Spitz nevi with 11p copy number increases showed significantly higher levels of cyclin D1 expression and lower levels of microphthalmia transcription factor expression suggesting stronger MAP-kinase pathway activation in this group. Contrasting this apparent activation, the proliferation rate as assessed by Mib1 expression was low in both groups. An analysis of cell-cycle inhibitory proteins including p16, p21, and p27 showed that the majority of Spitz nevus cells expressed high levels of p16, with cells of the cases that had increased copy number of 11p expressing significantly higher levels than those of Spitz nevi with normal copy number of 11p. We propose that in benign nevi with constitutive activation of the MAP-kinase pathway, p16 functions as an essential mediator of oncogene-induced senescence preventing progression to melanoma.
Melanocytic nevi are benign melanocytic neoplasms that typically start developing on sun exposed skin during childhood and adolescence but occasionally can be present at birth. After a period of gradual expansion a nevus typically stays stable in size and can undergo changes of involution later in life. To form a clinically noticeable lesion, melanocytes have to undergo a number of cell divisions. However, different from melanoma, the proliferation of melanocytes in nevi eventually ceases. Recent studies indicate that activating mutations of genes within the RAS-RAF-MEK-ERK-MAP-kinase pathway occur in nevi as well as in melanoma. Mutations of NRAS have been described in congenital nevi1 and mutations of HRAS were found in Spitz nevi.2 Most recently a high frequency of mutations of BRAF, a kinase immediately downstream of RAS, has been described in melanoma and nevi.3,4 These findings indicate that activation of the MAP-kinase pathway may be required for transformation of melanocytes, but not sufficient for the development of melanoma. In vitro studies with primary human cells have shown that in the absence of cooperating factors single oncogenes are insufficient for malignant transformation and can lead to a permanent growth arrest or apoptosis.5 Senescence has been proposed as a mechanism of growth arrest, but its relevance in human neoplasia in vivo is unclear.
We have recently found copy number increases of chromosome 11p that go along with mutations of the HRAS on that chromosome in a subset of Spitz nevi.2 Spitz nevi are benign melanocytic nevi that are notorious among pathologists because they can closely mimic melanoma under the microscope. After a period of occasionally rapid growth they are thought to eventually stop growing and remain stable. They usually obtain a diameter of several millimeters to a centimeter. Transformation to melanoma has not been reported, although, the morphological overlap with melanoma would make such an occurrence difficult to recognize. Remarkably, some Spitz nevi have been reported to metastasize to the lymph node without evidence of further progression.6
Because the HRAS mutation was frequently accompanied by an increased copy number of the mutated allele (unpublished observation)2 a sustained and marked activation of the MAP-kinase-signaling cascade can be assumed to be present in these Spitz nevi. A previous study showed that amplification of the wild-type HRAS allele is sufficient to transform sensitive cells.7This suggests that 11p copy number gain alone without HRAS mutation may be sufficient in activating the MAP-kinase-signaling cascade.
Because of the small size of Spitz nevi, fresh tissue is almost impossible to obtain. We therefore used immunohistochemistry on paraffin sections to assess the status of components in the MAP-kinase-signaling pathway in these tumors. Activation of the MAP-kinase pathway leads to phosphorylation of ERK, as well as increased expression of cyclin D1. The microphthalmia transcription factor (MITF) is a master regulator gene in melanocytes and is a phosphorylation target of MAP-kinase cascade8 targeting it for degradation.9 Sustained activation of MAP-kinase also has been reported to mediate cell-cycle arrest by induction of p16, p21, p27, and p53.10–12 In other cell types ras-induced senescence goes along with increased expression of p16, p21, and p53. We therefore used antibodies for phospho-ERK, cyclin D1, and MITF to assess the activation status of the MAP-kinase pathway, and determine the expression of the cell-cycle inhibitors p16, p21, and p27 to study mechanisms of growth arrest in Spitz nevi with and without increased copy number of HRAS.
Materials and Methods
Selection of Cases
Thirty-five Spitz nevi from the Dermatopathology Section of the Department of Pathology of the University of California, San Francisco, in which the copy number status of the HRAS locus had been previously established were used in this study. Seventeen of these cases had increased copy number of chromosome 11p including the HRAS gene. The remaining 18 Spitz nevi had normal copy number of 11p.
Antibodies
The following antibodies were used in this study: phospho-ERK: 1/2 9101S (1:1000; Cell Signaling, Beverly, MA). The conditions for this antibody were determined using formalin-fixed, paraffin-embedded cell pellets from the human breast tumor cell line SKBR3 that were grown after stimulation or inhibition of MAP-kinase (Figure 1). Cyclin D1: monoclonal antibody ASM29 (dilution 1:200 overnight; Zymed, South San Francisco, CA) according to standard procedures. MITF: monoclonal antibody D5 (dilution 1:1) was a generous gift from David E. Fisher (Dana Farber Cancer Institute, Boston, MA). MIB1: dilution 1:500 overnight, according to the manufacturer’s instructions (DAKO, Carpinteria, CA). p16: 1:50 for overnight (Labvision, Fremont, CA). p21: monoclonal antibody (1:50 for 30 minutes) (Calbiochem, San Diego, CA). p27: monoclonal antibody K25020 (1:50 for 30 minutes) according to the manufacturer’s instructions (Transduction Laboratories, Lexington, KY). 3-Amino-9-ethylcarbazole and diaminobenzidine were used as chromagens.
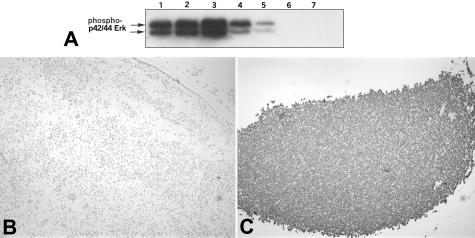
Figure 1.
Calibration of phospho-ERK immunohistochemistry. A: Western blot for phospho-ERK in whole cell lysates of SKBR3 cells under different conditions: lane 1, 10% fetal bovine serum (FBS); lane 2, 10% FBS and 10 ng/ml EGF; lane 3, 10% FBS and 20-minute exposure to EGF; lane 4, no serum; lane 5, no serum and 10 μmol/L MEK inhibitor UO126 (Promega); lane 6, no serum and 25 μmol/L UO126; lane 7, no serum and 75 μmol/L UO126. B: Negative control: SKBR3 cells were grown in the absence of serum and 10 μmol/L MEK inhibitor UO126 (Promega), formalin-fixed, paraffin-embedded, and stained with anti-phospho-ERK-1/2 antibody at 1:200. C: Positive control: SKBR3 cells were grown in 10% FBS and 20-minute exposure to EGF, formalin-fixed, paraffin-embedded, and stained with anti-phospho-ERK-1/2 antibody at 1:200.
Scoring of Immunohistochemistry
We recorded the staining intensity, pattern (membrane, cytoplasmic, both), and percentage of positive cells.13 Expression levels (0 to 5) and percentage of positive cells were determined using the Openlab 3.06 software (Improvision Inc., Lexington, MA). Image analysis was performed on two or three representative digital images obtained from each case using the ×40 objective, an Olympus BX51 camera (Olympus, Melville, NY), and the SPOT Advanced program (Diagnostic Instruments, Sterling Heights, MI). A positive cell was defined as a cell with an intensity level greater than or equal to 1. If the expression level was heterogeneous throughout the lesion, the level for the areas of highest intensity was recorded.
The proportion of positively labeled cells was assessed using Openlab’s color models to automatically recognize the number of positive cells. Objects with an area smaller than that of the smallest melanocyte on each image were excluded from the count. The average of two ×40 images was obtained. Each automated reading was checked manually by taking the average of the number of positive cells in two ×40 images. Discrepancies only arose in cases in which excess amounts of nonmelanocytic cells were present. In these situations the manual count was recorded. Mib-1 expression was assessed entirely manually because of the relatively low labeling rate in the melanocytic compartment compared to a relatively high labeling of nonmelanocytic cells such as lymphocytes and basal keratinocytes.
Statistical Analysis
To compare the proportions of positive cells in the groups with and without 11p amplification t-tests were used.
Results
Activation of the MAP-Kinase Pathway and Proliferation
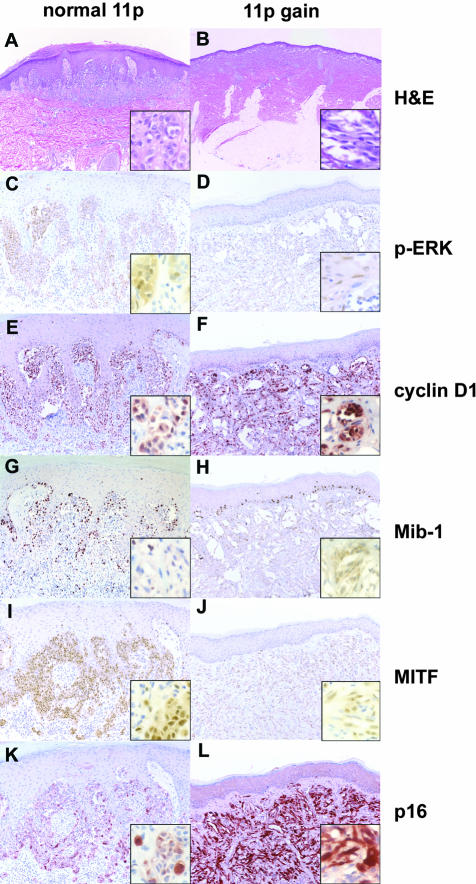
Our previous studies had shown that the majority (67%) of Spitz nevi with 11p copy number increase have mutations in the HRAS gene.2 In the present study, HRAS sequence information was available for 17 cases. Of these cases HRAS was found to be mutated in 8 of 11 (73%) cases with the copy number increase and 0 of 6 (0%) without the copy number increase. We therefore assume that HRAS is a major factor in the selection of increased copy number of chromosome 11p and that the gene dosage increase results in higher levels of MAP-kinase activation. We tested this hypothesis by determining the expression levels of downstream components of the MAP-kinase pathway. The phospho-ERK expression levels were carefully calibrated using paraffin-embedded cell pellets of cell lines in the presence or absence of a MEK inhibitor upstream the MAP-kinase pathway (Figure 1). As shown in Table 1 and Figure 2, C and D, phospho-ERK was expressed in the majority of cells at relatively high levels in both groups of Spitz nevi irrespective of the presence or absence of 11p copy number increase (Figure 2, E and F). By contrast, cyclin D1 was expressed at significantly higher levels and in a higher proportion of cells in cases with 11p amplification. MITF showed the opposite pattern and was expressed at significantly lower levels in the cases with 11p amplification consistent with the view that MAP-kinase targets it for degradation9 (Figure 2, I and J). The proliferation rate as assessed by Mib-1 labeling was low in both groups of Spitz nevi (Table 1 and Figure 2, G and H). We note that the case depicted in Figure 2G has an increased number of lymphocytes, and that virtually all Mib-1 labeling can be attributed to lymphocytes and basal keratinocytes.
Table 1.
Quantitative Immunohistochemical Findings in Spitz Nevi with and without 11p Copy Number Increase
| 11p copy number increase | No 11p copy number increase | P value | |
|---|---|---|---|
| Phospho-ERK | |||
| Avg. proportion of positive cells % (IRQ range) | 71.43 (70–90) | 77.41 (76.50–86.25) | 0.26 |
| Avg. intensity (IRQ range) | 2.68 (2.12–3.0) | 3.09 (2.5–3.62) | 0.12 |
| Cyclin D1 | |||
| Avg. proportion of positive cells % (IRQ range) | 79.25 (74.25–95) | 39.81 (29.25–50.75) | <0.00001 |
| Avg. intensity (IRQ range) | 3.59 (3.38–4) | 3.16 (2.88–3.62) | 0.05 |
| MITF | |||
| Avg. proportion of positive cells % (IRQ range) | 65.88 (60–83.25) | 65.00 (49.5–84.25) | 0.61 |
| Avg. intensity (IRQ range) | 2.41 (2–3) | 3.03 (2.75–3.5) | 0.02 |
| MIB1 | |||
| Avg. proportion of positive cells % (IRQ range) | 3.13 (1–4) | 2.15 (1–3) | 0.31 |
| Avg. intensity (IRQ range) | 3.57 (4–4) | 3.77 (4–4) | 0.55 |
| p16 | |||
| Avg. proportion of positive cells % (IRQ range) | 61.5 (42–85) | 46.13 (13.75–74.75) | 0.06 |
| Avg. intensity (IRQ range) | 3.68 (3–4) | 2.81 (2–3.62) | 0.007 |
| p21 | |||
| Avg. proportion of positive cells % (IRQ range) | 44.88 (27–66) | 38.53 (20–47.75) | 0.92 |
| Avg. intensity (IRQ range) | 2.85 (2.5–3) | 2.94 (2.88–3) | 0.65 |
| p27 | |||
| Avg. proportion of positive cells % (IRQ range) | 33.25 (5.25–57.5) | 25.47 (5–39.38) | 0.87 |
| Avg. intensity (IRQ range) | 2.25 (1.5–3.5) | 2.12 (1.88–3) | 0.87 |
Columns one and two show the average proportion of cells with an expression intensity greater than or equal to 1 and the average intensity of expression of 35 Spitz nevi cases (17 with 11p amplification and 18 without). A P value <0.05 was considered significant.
Figure 2.
Immunohistochemistry findings for two representative Spitz nevi with normal copy number of chromosome 11 (left column) or with increased copy number chromosome 11p (right column). A, C, E, G, I, and K show a Spitz nevus with normal copy number of chromosome 11p and B, D, F, H, J, and L show a Spitz nevus with increased copy number of chromosome 11p. A and B show the H&E stains, C and D show immunohistochemistry for phospho-ERK, E and F for cyclin D1, G and H for Mib-1, I and J for MITF, and K and L for p16. Note: Mib-1 stains basal keratinocytes and scattered lymphocytes in G.
Expression Status of Cell-Cycle Inhibitors
The above data suggests that the MAP-kinase pathway is activated in both groups of Spitz nevi and that Spitz nevi with increased 11p copy number show stronger activation. However, MAP-kinase pathway activation contrasts with the observed low proliferation rate. To analyze whether the expression of cell-cycle inhibitors prevented S phase entry, we analyzed the expression levels of the cell-cycle inhibitors p16, p21, and p27.
p21 and p27 were expressed in fewer cells and at lower levels than p16 in all lesions. There was no statistically significant difference in expression levels or in the proportion of labeled cells of p21 or p27 between Spitz nevi with or without 11p copy number increase. By contrast, Spitz nevi with 11p copy number increase expressed significantly higher levels of p16 protein than those with normal copy number (Table 1 and Figure 2, K and L). The proportion of p16-expressing cells was similar between the groups.
Discussion
In our study, all Spitz nevi showed increased expression levels of phospho-ERK and cyclin D1 indicating MAP-kinase pathway activation in both groups. For the Spitz nevi that had copy number increase of chromosome 11p, the increased copy number of the mutated HRAS allele as well as the wild-type HRAS allele7 is expected to lead to a sustained and marked activation of the MAP-kinase-signaling cascade. Our finding that Spitz nevi with normal copy number of chromosome 11p also expressed phospho-ERK and cyclin D1 at significant levels suggests that in these Spitz nevi the MAP-kinase pathway is activated by a mechanism different from HRAS. Mutations in the BRAF gene frequent in other types of nevi4 appear to be absent in Spitz nevi.14 Future studies are necessary to assess the mutation status of other components of the MAP-kinase pathway.
Although, we found no difference in the expression levels of phospho-ERK between the two groups of Spitz nevi, two observations suggest a stronger activation of the MAP-kinase pathway in nevi with increased copy number of chromosome 11p. First, Spitz nevi with increased copy number of 11p had significantly lower expression levels of MITF. MITF is a transcription factor that is essential for the regulation of pigmentation and the development and survival of melanocytes. It is expressed in the majority of melanocytic neoplasms including melanoma.15 MITF is phosphorylated by ERK, which increases its transcriptional activity,8 but also targets it for ubiquitin-mediated degradation.9 In the presence of increased MAP-kinase signaling, expression levels of MITF are therefore expected to be lower. Second, Spitz nevi with copy number increases of 11p showed significantly higher expression levels of cyclin D1. The map kinase pathway directly acts on the cyclin D1 promoter leading to increased expression of the cyclin D1 protein.16 However, the transcriptional regulation of cyclin D1 is complex and we cannot exclude the possibility that additional pathways such as the WNT17 or PI3 kinase pathway18 may account for the increased expression levels.
The presence of mutations and/or copy number increases of HRAS together with expression of phospho-ERK and cyclin D1 indicate constitutive activation of the MAP-kinase pathway in Spitz nevi. Despite these proliferative stimuli Spitz nevi undergo a benign course clinically, and the proportions of cells in S phase as assessed by Mib-1 labeling was low to absent in our and other studies.19 Of the negative cell-cycle regulators studied only p16 was expressed at high levels in the majority of Spitz nevus cells. P16 is a negative cell-cycle regulator that inhibits G1 cyclin-dependent kinases.20 The p16 gene has been shown to be deleted or mutated in a large percentage of malignant melanoma and germ line mutations of p16 lead to a melanoma-prone condition.21 Interestingly, p16 was expressed at significantly higher levels in Spitz nevi with increased copy number of chromosome 11p. In vitro, constitutive activation of the MAP-kinase pathway through activated oncogenes in normal cells leads to a p16-mediated permanent growth arrest.5 This condition has been termed premature senescence or oncogene-induced senescence.22 p16 has been shown to be essential for melanocyte senescence as well23,24 and found to be expressed in other types of melanocytic nevi.25 It is therefore tempting to speculate that Spitz nevi and possibly melanocytic nevi in general could represent an example of oncogene-induced senescence in vivo. In the presence of an intact p16 tumor suppressor pathway melanocytes could withstand full transformation by activated oncogenes. The observation that Spitz nevi with increased copy number of chromosome 11p expressed significantly higher levels of p16 suggests a direct link between MAP-kinase pathway activation and p16 expression levels in melanocytes. Future studies are necessary to establish the nature of this link and to examine potential candidate mediators such as the ETS transcription factors.26
Footnotes
Address reprint requests to Boris C. Bastian, M.D., Departments of Dermatology and Pathology, University of California, San Francisco, UCSF Box 0808, San Francisco CA 94143. E-mail bastian@cc.ucsf.edu.
Supported by the National Institutes of Health (R33 CA95300 to B.C.B.).
References
- Carr J, Mackie RM. Point mutations in the N-ras oncogene in malignant melanoma and congenital naevi. Br J Dermatol. 1994;131:72–77. doi: 10.1111/j.1365-2133.1994.tb08460.x. [DOI] [PubMed] [Google Scholar]
- Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increases of HRAS in Spitz nevi with distinctive histopathologic features. Am J Pathol. 2000;157:967–972. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS. High frequency of BRAF mutations in nevi. Nat Genet. 2002;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Barrett TL, Skelton HGD, Lupton GP, Graham JH. Spindle cell and epithelioid cell nevi with atypia and metastasis: malignant Spitz nevus. Am J Surg Pathol. 1989;13:931–939. doi: 10.1097/00000478-198911000-00003. [DOI] [PubMed] [Google Scholar]
- Pulciani S, Santos E, Long LK, Sorrentino V, Barbacid M. ras gene amplification and malignant transformation. Mol Cell Biol. 1985;5:2836–2841. doi: 10.1128/mcb.5.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000;14:301–312. [PMC free article] [PubMed] [Google Scholar]
- Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Fang L, Igarashi M, Ouchi T, Lu KP, Aaronson SA. Sustained activation of Ras/Raf/mitogen-activated protein kinase cascade by the tumor suppressor p53. Proc Natl Acad Sci USA. 2000;97:8302–8305. doi: 10.1073/pnas.150024397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle PA, Elenitsas R, Satyamoorthy K, Wolfe JT, Guerry DT, Schuchter L, Van Belle TJ, Albelda S, Tahin P, Herlyn M, Elder DE. Progression-related expression of beta3 integrin in melanomas and nevi. Hum Pathol. 1999;30:562–567. doi: 10.1016/s0046-8177(99)90202-2. [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Palmedo G, Flaig MJ, Puchta U, Reckwerth A, Rutten A, Mentzel T, Hugel H, Hantschke M, Schmid-Wendtner M-H, Kutzner H, Sander CA. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol. 2003;121:1160–1162. doi: 10.1046/j.1523-1747.2003.12559.x. [DOI] [PubMed] [Google Scholar]
- Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, Ballotti R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol. 1998;142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovec H, Sewing A, Lucibello FC, Muller R, Moroy T. Oncogenic activity of cyclin D1 revealed through cooperation with Ha-ras: link between cell cycle control and malignant transformation. Oncogene. 1994;9:323–326. [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman R, Malkin L, Sabo E, Kerner H. MIB-1 monoclonal antibody to determine proliferative activity of Ki-67 antigen as an adjunct to the histopathologic differential diagnosis of Spitz nevi. J Am Acad Dermatol. 2001;44:500–504. doi: 10.1067/mjd.2001.111635. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Kamb A, Shattuck-Eidens D, Eeles R, Liu Q, Gruis NA, Ding W, Hussey C, Tran T, Miki Y, Weaver-Feldhaus J, McClure M, Aitken JF, Anderson DE, Bergman W, Frants R, Goldgar DE, Green A, MacLennan R, Martin NG, Meyer LJ, Youl P, Zone JJ, Skolnick MH, Cannon-Albright LA. Analysis of the p16 gene CDKN2 as a candidate for the chromosome 9p melanoma susceptibility locus. Nat Genet. 1994;8:23–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- Mathon NF, Lloyd AC. Cell senescence and cancer. Nat Rev Cancer. 2001;1:203–213. doi: 10.1038/35106045. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D, Medrano EE. Melanin accumulation accelerates melanocyte senescence by a mechanism involving p16INK4a/CDK4/pRB and E2F1. Ann NY Acad Sci. 2000;908:71–84. doi: 10.1111/j.1749-6632.2000.tb06637.x. [DOI] [PubMed] [Google Scholar]
- Sviderskaya EV, Hill SP, Evans-Whipp TJ, Chin L, Orlow SJ, Easty DJ, Cheong SC, Beach D, DePinho RA, Bennett DC. p16(Ink4a) in melanocyte senescence and differentiation. J Natl Cancer Inst. 2002;94:446–454. doi: 10.1093/jnci/94.6.446. [DOI] [PubMed] [Google Scholar]
- Funk JO, Schiller PI, Barrett MT, Wong DJ, Kind P, Sander CA. p16INK4a expression is frequently decreased and associated with 9p21 loss of heterozygosity in sporadic melanoma. J Cutan Pathol. 1998;25:291–296. doi: 10.1111/j.1600-0560.1998.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, Sharrocks AD, Peters G, Hara E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]