Abstract
In the paracortex of lymph nodes, cellular immune responses are generated against antigens captured in peripheral tissues by dendritic cells (DCs). DC-SIGN (dendritic cell-specific ICAM-3 grabbing nonintegrin), a C-type lectin exclusively expressed by DCs, functions as an antigen receptor as well as an adhesion receptor. A functional homologue of DC-SIGN, L-SIGN (liver/lymph node-SIGN, also called DC-SIGN-related), is expressed by liver sinus endothelial cells. In lymph nodes, both DC-SIGN and L-SIGN are expressed. In this study, we analyzed the distribution of these two SIGN molecules in detail in both normal and immunoreactive lymph nodes. DC-SIGN is expressed by mature DCs in paracortical areas and in addition by DCs with an immature phenotype in the outer zones of the paracortex. L-SIGN expression was also detected in the outer zones on sinus endothelial cells characterized by their expression of the lymphatic endothelial markers LYVE-1 and CLEVER-1. During both cellular and humoral immune responses changes in the amount of DC-SIGN+ immature and mature DCs and L-SIGN+ endothelial cells were observed, indicating that the influx or proliferation of these cells is dynamically regulated.
Immune responses are initiated in secondary lymphoid organs by generating cellular and humoral effector mechanisms. In lymph nodes these responses are directed against antigens from peripheral tissues that have entered these organs through lymph either in solution or on dendritic cells (DCs). DCs in peripheral tissues serve as sentinels of the immune system, sampling incoming antigens and pathogens.1 These so-called immature DCs are triggered by inflammatory stimuli to migrate via afferent lymphatics to the paracortical areas of draining lymph nodes ferrying the locally acquired antigens. Concomitant with the induction of migration, DC maturation occurs to allow efficient antigen presentation to T lymphocytes.2 T cells continuously circulate through lymphoid organs, entering via the bloodstream through high-endothelial venules, until they meet a DC with the appropriate antigenic peptide. Engagement of the T-cell receptor together with secondary signals through co-stimulatory molecules leads to paracortical proliferation and activation of T cells. Via efferent lymph, activated T cells leave lymph nodes to perform their effector functions in the periphery.
This immune reaction against peripheral antigens through active transport by DCs to lymph nodes holds true for part of the antigens only. Many antigens from lymph and interstitial tissue fluids reach lymph nodes in soluble form.3 Similar to peripheral tissues, lymph nodes contain immature DCs; recent studies even postulate that the majority of DCs in lymph nodes exist in an immature state, acting at these sites to capture soluble antigens.4–7 Accordingly, immature lymph node DCs require activation signals to efficiently present antigenic peptides to T lymphocytes.6,8,9 These studies were performed in mice; in humans, the existence of lymphoid immature DCs remains unclear. DC-specific molecules could be useful to address this issue.
We have recently described that dendritic cell-specific ICAM-3 grabbing nonintegrin (DC-SIGN), a C-type lectin, is exclusively expressed by human DCs in peripheral tissues, such as skin and mucosa, and in lymphoid organs.10 In blood, we have characterized a subset of CD14+ DCs that express DC-SIGN.11 In the same study, plasmacytoid DCs did not express detectable levels of DC-SIGN, although lack of DC-SIGN expression on these interferon-producing DCs remains controversial.11–13 DC-SIGN has several properties contributing to the function of DCs. As has been described for other C-type lectins such as the mannose receptor,14 DC-SIGN can capture antigens for processing and subsequent presentation to T cells.15 A growing list of pathogens is bound by DC-SIGN,16 including human immunodeficiency virus,17 Mycobacterium tuberculosis,18 Leishmania amastigotes,19 and Dengue virus.20,21 Moreover, DC-SIGN regulates migration of DCs by binding its ligand ICAM-2 on endothelial cells and activation of resting T cells through ICAM-3 binding.22,23 In contrast to DC-SIGN, liver/lymph node-specific ICAM-3 grabbing nonintegrin (L-SIGN, also called DC-SIGNR), a functional homologue of DC-SIGN with similar binding activity, was not found to be expressed on DCs in peripheral tissues but on liver sinus endothelial cells, organ-resident antigen-presenting cells.24–26 On liver sinus endothelial cells, L-SIGN may function to facilitate interactions with lymphocytes as well as to bind antigens and pathogens. Interestingly, in lymph nodes, both SIGN family members are expressed, although the exact localization remains unclear.
In this study we have characterized the expression of DC-SIGN and L-SIGN in more detail in both normal and immunoreactive lymph nodes. We observed that in lymph nodes DC-SIGN is expressed by mature DCs present in the paracortex, where interactions with T lymphocytes take place, as well as on a large number of immature DCs in the outer zone of the paracortical areas, where antigen capture takes place. In the vicinity of these immature DC-SIGN+ DCs, L-SIGN+ cells are also detected. High-resolution staining demonstrated that cells expressing L-SIGN are specialized endothelial cells that co-express the recently described lymph endothelial markers LYVE-127 and CLEVER-1.28 During the induction of humoral and cellular responses, changes were observed in the number of mature DC-SIGN+ DCs in the paracortex and both immature DC-SIGN+ DCs and L-SIGN+ endothelial cells in the paracortical outer zones. Thus, DC-SIGN+ and L-SIGN+ cell populations are highly dynamic during immune activation and are potentially important players in lymph nodes. We propose that these immune cells use DC-SIGN and L-SIGN either for capturing antigens or for cellular interactions facilitating T-cell activation.
Materials and Methods
Antibodies
The following antibodies were used: AZN-D1, AZN-D2, AZN-D3 (anti-DC-SIGN monoclonal antibodies23,24), CSRD (polyclonal antiserum obtained after immunization of rabbits with the following peptide from DC-SIGN coupled to KLH: CSRDEEQFLSPAPATPNPPPA), PTTS (polyclonal antiserum obtained after immunization of rabbits with the following peptide from L-SIGN coupled to KLH: PTTSGIRLFPRDFQFQQIH), anti-S100 (Z0311, DAKO, Glostrup, Denmark), KP-1 (anti-CD68, DAKO), anti-CD31 (B&D, Oxnard, CA), 3.29B1 (anti-mannose receptor, kind gift of Dr. M. Cella, Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO, USA), anti-von Willebrand factor (clone F8/86, DAKO), anti-LYVE-1,27 3-372 (anti-CLEVER, kind gift of Dr. S. Jalkanen28, Medical Research Laboratory and Department of Medical Microbiology, Turku University, Turku, Finland), and anti-CD163 (described in van den Heuvel et al29).
Cells
K-562 cells were transfected with DC-SIGN or L-SIGN as described.23 Immature DCs were generated by culturing human blood monocytes in RPMI 1640/10% fetal calf serum containing interleukin-4 (500 U/ml, Schering-Plough) and GM-SCF (800 U/ml, Schering-Plough, Brussels, Belgium) for 5 to 8 days.
Human Tissues
Tissues were obtained from patients by surgical removal, following national ethical guidelines regarding the use of human tissues. One part was frozen for cryosections and one part was embedded in paraffin. Classification of immune reactive lymph nodes was performed on hematoxylin and eosin-stained paraffin sections. All lymph nodes showed mixed cellular and humoral responses using histology; in the classification the dominant response is indicated. The characteristics of the patients that underwent lymph node removal: patient 1: 9-year-old male, enlarged lymph node in neck because of skin allergy, classification: reactive lymph node, dermatopathic lymphadenopathy, and sinus histiocytosis, mild cellular response; patient 2: 25-year-old female, enlarged lymph node behind right ear because of eczema, histological classification: dermatopathic lymphadenopathy and sinus histiocytosis, humoral response; patient 3: 8-year-old male, enlarged lymph node in neck, histological classification: reactive paracortical hyperplasia and sinus histiocytosis, cellular response; patient 4: 85-year-old female, mammacarcinoma, enlarged lymph node in neck without tumor, histological classification: reactive paracortical hyperplasia, cellular response; patient 5: 28-year-old male, enlarged lymph node in neck without diagnosis, histological classification: reactive follicular hyperplasia, humoral response; patient 6: 33-year-old male, enlarged inguinal lymph node without diagnosis, histological classification: dermatopathic lymphadenopathy and sinus histiocytosis, cellular response.
Immunohistochemistry
Tissue cryosections (4 μm) were fixed in acetone for 10 minutes and incubated with primary and secondary antibodies (anti-mouse horseradish peroxidase and anti-rabbit biotin; Vector Laboratories, Burlingame, CA).23 Paraffin sections were rehydrated and subjected to antigen-retrieval by boiling in 0.01 mol/L citric acid (pH 6.0) for 10 minutes before incubation with antibodies. Staining was performed with the ABC-AP Vectastain kit (Vector Laboratories) or ABC-PO and diaminobenzidine tetrahydrochloride (0.5 mg/ml) and sections were counterstained with hematoxylin according to Pappanicolau. For immunofluorescence, Alexa 488 (Molecular Probes, Eugene, OR)- and Texas Red (Jackson, West Grove, PA)-conjugated secondary antibodies were used.
Results
Generation of DC-SIGN- and L-SIGN-Specific Antibodies
Expression of DC-SIGN, a DC-specific C-type lectin, can be detected using three different monoclonal antibodies that we have generated, AZN-D1, AZN-D2, and AZN-D3 (Figure 1A).22,23 Two of these antibodies, AZN-D2 and AZN-D3, cross-react with L-SIGN/DC-SIGNR, a structural and functional homologue of DC-SIGN (Figure 1A). To better distinguish between cells expressing DC-SIGN and/or L-SIGN, polyclonal antibodies were generated in rabbits by immunization with ∼20-mer peptides coupled to keyhole limpet hemocyanin (KLH). Based on amino acid differences, a peptide corresponding to the C-terminal part of DC-SIGN (CSRD) was used and an amino acid stretch in the cytoplasmic tail of L-SIGN (PTTS). Using K562 cells stably transfected with either DC-SIGN or L-SIGN, the specificity of these antibodies was confirmed (Figure 1B); CSRD specifically recognizes DC-SIGN whereas PTTS reacts with L-SIGN only. Moreover, confirming our previous results of Northern blot analysis,24 staining with these antibodies clearly demonstrates that monocyte-derived DCs express DC-SIGN but not L-SIGN (Figure 1B).
Figure 1.
The polyclonal antibodies CSRD and PTTS recognize DC-SIGN and L-SIGN, respectively. A: Monocyte-derived DCs and K562, mock transfected or transfected with DC-SIGN or L-SIGN, were stained with AZN-D1, AZN-D2, and AZN-D3 (5 μg/ml), followed by anti-mouse FITC antibodies, and analyzed by flow cytometry. An isotype control antibody was included. B: Alternatively, cytospin preparations of cells were fixed and stained with CSRD and PTTS (1:500), anti-rabbit biotin, and the ABC-AP Vectastain kit. Original magnifications, ×20.
Differential Tissue Expression of DC-SIGN and L-SIGN
Using these antibodies, cryosections from various tissues were screened for the expression of DC-SIGN and L-SIGN; results are summarized in Table 1. DC-SIGN expression was detected on DCs in peripheral tissues, such as placenta, skin, and mucosa and in T-cell areas of secondary lymphoid tissues, including lymph nodes and spleen, as described before.12,17,23 In contrast, L-SIGN expression was confined to endothelial cells in liver sinuses and in lymph nodes.24 Others have detected L-SIGN+ cells in placental villi;26 this discrepancy could be because of the developmental stage of the placenta. Thus, the SIGN family members have distinct expression patterns, except in lymph nodes, where both DC-SIGN and L-SIGN expression is found (see below).
Table 1.
Tissue Distribution of DC-SIGN- and L-SIGN-Expressing Cells*
| DC-SIGN | L-SIGN | |
|---|---|---|
| Skin | Dermal DC | - |
| Mucosa† | DC | - |
| Intestine | DC | - |
| Lung | DC | - |
| Liver | DC | Sinus endothelial cells |
| Placenta | DC | |
| Hofbauer cells | -‡ | |
| Spleen | DC in T-cell areas Ellipsoids | - |
| Lymph nodes | DC in T-cell areas cells in paracortex | Cells in paracortex |
Tissue cryosections of two patients or more were stained with CSRD or PTTS.
Mucosa of ileum, jejunum, rectum, and cervix.
Others have reported expression on placental villi.26
Expression of DC-SIGN and L-SIGN in Lymph Nodes
The distribution of DC-SIGN and L-SIGN was analyzed on cervical lymph nodes in more detail using immunohistochemistry. DC-SIGN is expressed on large cells with an irregular cell shape in the paracortex (Figure 2A). DC-SIGN-expressing cells are identified as DCs, based on co-expression of several markers on sequential sections, including MHC class II, the mannose receptor, CD11b and CD11c, and lack of surface expression of CD68 (Table 2). Moreover, DC-SIGN expression is also detected on cells in the outer zone of the paracortex, in proximity of paracortical and medullary sinuses (Figure 2A). In these areas, L-SIGN-expressing cells are also situated, although fewer in number as compared to DC-SIGN+ cells (Figure 2A). Similar expression patterns were observed in human mesenteric and inguinal lymph nodes (not shown). In comparison, the distribution of S100, an extensively used DC marker for paraffin-embedded lymphoid tissue,30 was studied. Expression of S100 was detected on a large population of DCs scattered throughout the paracortex and in B-cell follicles, whereas DC-SIGN expression was restricted to a subset of lymph node DCs (Figure 2B).
Figure 2.
DC-SIGN and L-SIGN are expressed on distinct cells in the outer zone of paracortical areas in lymph nodes. A: Tissue cryosections of human cervical lymph nodes (patient 1) were fixed in acetone and stained with CSRD and PTTS (1:500), followed by anti-rabbit biotin and ABC-AP Vectastain kit to detect expression of DC-SIGN and L-SIGN, respectively. B: Paraffin tissue sections (patient 1) were pretreated by boiling in citric acid and subsequently stained to detect DC-SIGN, L-SIGN, and S100 expression, using CSRD, PTTS, and anti-S100 antibodies (1:500), followed by anti-rabbit biotin, ABC-PO, and diaminobenzidine tetrahydrochloride. Arrows point to positive cells. S, sinus; OZ, outer zone of paracortex; P, paracortex; F, B-cell follicle. Original magnifications: ×10 (A and B, left); ×40 (A and B, right).
Table 2.
Characterization of DC-SIGN+ and L-SIGN+ Cells in the Peripheral Zone of the Paracortex*
| DC-SIGN+ T-cell area | Outer zone | L-SIGN+ outer zone | |
|---|---|---|---|
| DC markers | |||
| MHC class II | ++† | + | +/− |
| CD83 | − | − | − |
| C-type lectins | |||
| Mannose receptor | +/− | + | + |
| DEC-205 | − | − | − |
| Langerin | − | − | − |
| Adhesion molecules | |||
| CD11a | + | + | + |
| CD11b | − | +/− | +/− |
| CD11c | + | +/− | +/− |
| Lineage markers | |||
| CD1a | − | − | − |
| CD3 | − | − | − |
| CD14 | − | − | − |
| CD68 | +‡ | +‡ | − |
| CD163 | − | − | − |
| Acid phoshatase | +‡ | +‡ | − |
| CD31 | − | +/− | +§ |
| von Willebrand factor | − | − | + |
| Lymph endothelial markers | |||
| LYVE-1 | − | − | + |
| CLEVER-1 | − | − | + |
Lymph node sections were stained with CSRD or PTTS and double stained with antibodies against the listed molecules for immunofluorescence.
−, No expression detected; +/−, weak expression; +, expression; ++, high expression.
Expression in a perinuclear spot.
On luminal side only.
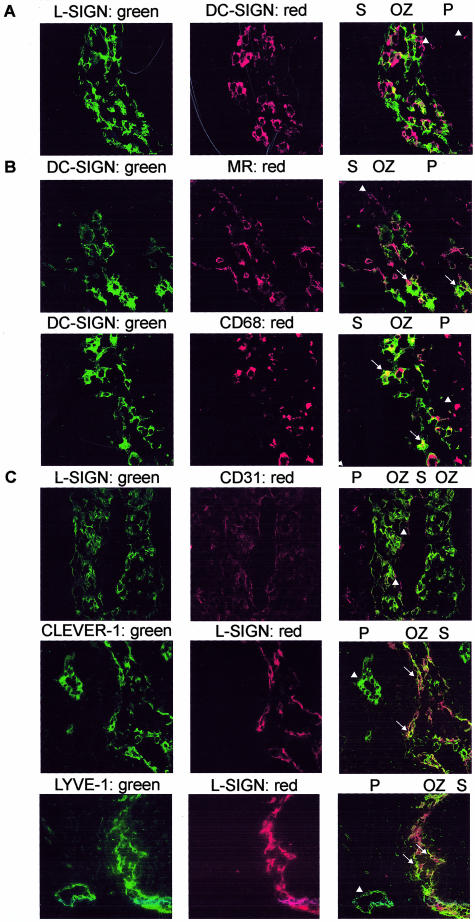
To determine whether DC-SIGN and L-SIGN are co-expressed on the same cell type present in the outer zones, double staining with the DC-SIGN-specific antibody AZN-D1 and L-SIGN-specific antibody PTTS was performed and analyzed by immunofluorescence. In paracortical T-cell areas, interdigitating cells with a typical DC morphology are DC-SIGN+ but lack expression of L-SIGN (Figure 3A). In the paracortical outer zone, most cells express either DC-SIGN or L-SIGN, and form a network of cells of which the cell extensions may overlap (Figure 3A). This is also demonstrated by overlapping of the extensions of DC-SIGN+ cells and adjacent L-SIGN+ cells because of close proximity. The fact that the adhesion molecules DC-SIGN and L-SIGN were expressed on adjacent cells prompted us to analyze interactions between these molecules. However, no binding of DC-SIGN to L-SIGN was observed (TBH Geijtenbeek, unpublished results). Because both ICAM-2 and ICAM-3 are present on both cell types (not shown), they may interact with each other using these cellular ligands. Because DC-SIGN and L-SIGN are expressed at the same site in lymph nodes but on distinct cell types, we set out to characterize these cells in more detail. Furthermore, we determined whether DC-SIGN- and L-SIGN-expressing cells are a subset of DC, macrophage, or endothelial cells.
Figure 3.
In the cortical outer zone, DC-SIGN+ cells express mannose receptor and CD68 intracellularly and L-SIGN+ cells co-express LYVE-1 and CLEVER-1. A: Tissues sections of lymph nodes (patient 1) double stained with AZN-D1 and PTTS, followed by anti-mouse Texas Red and anti-rabbit FITC, and analyzed by confocal microscopy. Arrowheads indicate DC-SIGN+L-SIGN− cells in the paracortex. B: Sections (patient 1) were double stained with CSRD, followed by anti-rabbit FITC and anti-CD68 or anti-mannose receptor, followed by anti-mouse Texas Red. C: Sections (patient 1) were double stained with PTTS, followed by anti-rabbit FITC and anti-CD31 and by anti-mouse Texas Red or with PTTS and anti-rabbit Texas Red in combination with anti-LYVE-1 or anti-CLEVER-1 and anti-mouse Alexa 488. Arrowheads indicate single-positive cells (CD68+, mannose receptor+, LYVE-1+, or CLEVER-1+), arrows point to double-positive cells. S, sinus; OZ, outer zone of paracortex; P, paracortex. Original magnifications, ×40.
DC-SIGN+ cells in the outer zone co-express the mannose receptor, a multilectin involved in antigen capture, and low levels of the β2 integrins CD11b and CD11c (Figure 3B and Table 2). These molecules are expressed by macrophages as well as DCs. No expression of the Langerhans’ cell-marker CD1a and a marker for mature DC, CD83, was found, but these DC-SIGN+ cells did express MHC class II molecules (Table 2). This prompted us to study expression of macrophage markers. Expression of CD68 was detected intracellularly in a spot in DC-SIGN+ cells (Figure 3B), a similar pattern as described before in immature DCs.31 This is in contrast to macrophages that exhibit an overall cytoplasmic and surface expression of CD68.31 A similar perinuclear staining pattern was observed for the lysosomal enzyme acid phosphatase in DC-SIGN-expressing cells (Table 2). In addition, neither CD163, a macrophage marker,29 nor CD14, a monocytic marker, were detected on DC-SIGN+ cells (Table 2). Taken together these results suggest that the DC-SIGN+ cells near the paracortical and medullary sinuses are not macrophages but represent DCs that have an immature phenotype.
In a previous study by Pohlmann and colleagues,26 it was postulated that lymph node endothelial cells express L-SIGN/DC-SIGNR. However, we observed L-SIGN expression in the proximity of the sinuses only and not on endothelium from small lymph vessels and high-endothelial venules. To further characterize L-SIGN+ cells, double stainings were performed using markers for DC, macrophage, and endothelial cells. The pan endothelial marker CD31 is highly expressed on the luminal side of the cell layer lining the sinuses, underneath which all cells express low amounts of CD31 (Figure 3C). L-SIGN expression is lacking on the most luminal part, but is expressed directly underneath on several layers of cells in a continuous lining. Another endothelial cell marker, von Willebrand factor, was expressed intracellularly in most L-SIGN+ cells (Table 2). In addition, expression of mannose receptor and low levels of CD11b and CD11c were detected on L-SIGN+ cells, but these cells lacked expression of the macrophage markers CD68, CD163, and acid phosphatase (Table 2). Because these results indicate that L-SIGN is expressed on certain cells of endothelial lineage, we analyzed expression of two markers for lymph endothelium, LYVE-127 and CLEVER-1.28 Whereas CLEVER-1 is expressed on both efferent and afferent lymph vessels and on high-endothelial venules,28 the hyaluronan receptor LYVE-1 is present on lymph endothelium in all tissues and on sinusoidal endothelium in liver and spleen.27,32 Interestingly, L-SIGN+ cells expressed both LYVE-1 and CLEVER-1 (Figure 3C, arrows). However, L-SIGN was not expressed by CLEVER-1+ high-endothelial venules and not on LYVE-1+ lymph endothelium (Figure 3C, arrowheads), but only on sinusoidal endothelium. Thus, L-SIGN expression is detected on a subset of endothelial cells near sinuses, that are present in several layers and co-express CD11b and CD11c and mannose receptor, that has been previously described on endothelial cells.33
Expression of DC-SIGN and L-SIGN in Immune Reactive Lymph Nodes
During an immune reaction, several changes occur in the various functional areas of lymph nodes in time, depending on the type and the intensity of the immune response. The tissue used for the detection of SIGN expression so far was, although taken from a patient with a dominant cellular reaction, relatively normal concerning cellular density and size (patient 1). We therefore performed immunohistochemical analyses using lymph nodes showing highly reactive humoral and cellular responses to assess the expression of DC-SIGN and L-SIGN in these circumstances.
During a dominant humoral response, hyperplasia of the cortex is observed in lymph nodes, with increased numbers of B-cell follicles and germinal centers (Figure 4A). None of the cells within the hyperplastic cortex were found to express either DC-SIGN or L-SIGN. The number of mature DC-SIGN+ DCs in the paracortex was normal in this lymph node (Figure 4A). However, high numbers of DC-SIGN+ immature DCs as well as L-SIGN+ endothelial cells were found in areas around the sinuses (compare Figure 4A with Figure 2A). Besides DCs and sinus endothelial cells, DC-SIGN and L-SIGN expression was not detected on other cells as determined by double stainings (not shown).
Figure 4.
Changes in the SIGN-expressing cell populations during immune responses. Tissue cryosections of human immunoreactive lymph nodes were fixed in acetone and stained with CSRD and PTTS (1:500), followed by anti-rabbit biotin and ABC-AP Vectastain kit to detect expression of DC-SIGN and L-SIGN, respectively. Lymph nodes were classified using H&E-stained paraffin sections and showed hyperplasia either in the cortex, containing numerous follicles (humoral response, patient 5; A), or in the paracortex (cellular response, patients 6 and 3; B and C). An overview is shown, as well as details from the medulla and paracortex. Arrows point to positive cells. S, sinus; F, B-cell follicle; P, paracortex. Similar results were obtained with patients 2 (humoral response) and 4 (cellular response). Original magnifications: ×5 (overview) and ×40 (others).
In dominant T-cell reactive lymph nodes, the cortical areas are normal whereas extensive proliferation (hyperplasia) of the paracortex is evident (Figure 4, B and C). The size of the lymph node can increase dramatically, depending on the intensity of the immune reaction (compare top panels of Figure 4; A, B, and C). In the paracortex, increased numbers of mature DC-SIGN+ DCs were present, in agreement with their T-cell stimulatory function (Figure 4B). A similar increase in the number of mature DC-SIGN+ DCs has been reported during acute Epstein-Barr virus infections.34 However, no changes in DC-SIGN- and L-SIGN-expressing cells in the paracortical outer zone were observed as determined by double stainings (not shown), although the amount of L-SIGN+ sinus endothelial cells increased dramatically in enlarged lymph nodes (Figure 4C, right).
Discussion
Early observations on the architecture of human lymph nodes using electron microscopy has revealed that the layer of sinus endothelial cells is not continuous, allowing cells and fluids to traverse.35–37 Indeed, T lymphocytes must cross this sinus endothelial layer to leave the paracortex en route to the efferent lymphatics for transport to the blood circulation. Interestingly, most lymph-borne antigens have been shown to reach the subcapsular, cortical, and medullary sinuses but to be excluded from the (para)cortex.3 This indicates that soluble antigens have to be captured and subsequently transported into the (para)cortex to ensure antigen presentation to T cells. However, it is currently unclear which cells perform this function. As we have shown in this study, immature DCs are found directly beneath the sinuses, in a location that is ideally suited for the capture of lymph-borne soluble antigens. These immature DCs express pathogen recognition receptors, namely DC-SIGN and mannose receptor, that function in capture of antigens and pathogenic components by recognizing unique carbohydrate determinants and subsequently processing for presentation to T cells.15,38,39 It is tempting to speculate that DC-SIGN+ immature DCs, on appropriate activation, migrate into the paracortex for antigen presentation to T lymphocytes. In agreement with this, the number of DC-SIGN+ mature DCs in the paracortex was increased during a cellular immune response (Figure 4). Thus, similarly to rodents,4,6,7 in human lymph nodes a large and dynamic population of resident immature DCs might exist for capturing lymph-borne antigens. In rodents, it is not clear at which site these immature DCs are located. Our observation that human lymph nodes contain immature DCs that are situated in proximity of the sinuses would position these cells optimally for incoming tissue antigens.
DC-SIGN and L-SIGN have distinct expression patterns, except in lymph nodes, where both are detected. Initial studies using reverse transcriptase-polymerase chain reaction indicated that co-expression of DC-SIGN and L-SIGN could occur on certain cell-types, including DCs.25 However, using highly specific antibodies, our studies have failed to detect simultaneous expression of L-SIGN on DC-SIGN+ DCs.24 In addition, using detailed immunohistochemical analysis, we here show that protein expression of DC-SIGN and L-SIGN does not coincide on the same cells in lymph nodes. Whereas DC-SIGN is restricted to lymph node DCs, L-SIGN is expressed on sinus endothelial cells. Thus, these two C-type lectins, that share binding to cellular ligands and pathogens, show a very restricted and distinct expression pattern.
L-SIGN+ endothelial cells are located exclusively to the outer zone of the paracortex and their number is increased during an immune response. Interestingly, L-SIGN+ cells co-express the lymph endothelial cell markers CLEVER-1 and LYVE-1. CLEVER-1 mediates binding of lymphocytes,28 indicating that these CLEVER-1+/L-SIGN+ endothelial cells may play a role in migration of lymphocytes leaving the paracortex. Remarkably, at certain sites multiple layers of L-SIGN+ sinus endothelial cells are detected forming a continuous lining that would have to be crossed by T lymphocytes leaving the lymph node (Figure 4). L-SIGN, by its ability to bind ICAM-3 on T cells,24 could assist in this process. The hyaluronan receptor LYVE-1 is co-expressed with L-SIGN on lymph sinuses as well as on LSEC.27,32 These specialized endothelial cells in liver can capture and present antigens locally to T cells, resulting not in activation of T cells, but in tolerance.40 The expression of both L-SIGN and mannose receptor on sinus endothelial cells would enable them to efficiently capture antigens.
Thus far, attempts to isolate L-SIGN+ lymph node endothelial cells for functional characterization have been unsuccessful. Recently, a murine homologue of DC- and L-SIGN, mSIGNR1, was cloned and was found to be expressed on specialized macrophages and liver sinusoidal endothelial cells.41,42 mSIGNR1 functions in efficient capture and internalization of soluble antigens,42 suggesting that L-SIGN in human lymph nodes might perform a similar function. In the murine system, more insight could be gained in the function of the dynamic population of L-SIGN-expressing cells in lymph nodes.
Acknowledgments
We thank A. van Schijndel for help with stainings, Dr. M. Cella for anti-mannose receptor antibodies, and Dr. S. Jalkanen for anti-CLEVER-1 antibodies and useful discussions.
Footnotes
Address reprint requests to Yvette Van Kooyk, Department of Molecular Cell Biology and Immunology, Vrije Universiteit Medical Center, van der Boechorststraat 7, 1081 BT Amsterdam, The Netherlands. E-mail: y.vankooyk@vumc.nl.
Supported by the Heart Foundation (grant 97.078 to A.E.) and the AIDS Foundation (grant 5008 to T.G.).
References
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickasingham SP, Reis e Sousa C. Mature T cell seeks antigen for meaningful relationship in lymph node. Immunology. 2001;102:381–386. doi: 10.1046/j.1365-2567.2001.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B, Cohen JL, Masurier C, Klatzmann D. Three populations of mouse lymph node dendritic cells with different origin and dynamics. J Immunol. 1998;160:708–717. [PubMed] [Google Scholar]
- Wilson NS, El-Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, Shortman K, Villadangos JA. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Manickasingham S, Reis e Sousa C. Microbial and T cell-derived stimuli regulate antigen presentation by dendritic cells in vivo. J Immunol. 2000;165:5027–5034. doi: 10.4049/jimmunol.165.9.5027. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C, Germain RN. Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J Immunol. 1999;162:6552–6561. [PubMed] [Google Scholar]
- Geijtenbeek TB, Engering A, Van Kooyk Y. DC-SIGN, a C-type lectin on dendritic cells that unveils many aspects of dendritic cell biology. J Leukoc Biol. 2002;71:921–931. [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Rushbrook S, Lee B, Coleman N. Expression of human immunodeficiency virus (HIV)-binding lectin DC-SIGNR: consequences for HIV infection and immunity. Hum Pathol. 2002;33:652–659. doi: 10.1053/hupa.2002.124036. [DOI] [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- Patterson S, Rae A, Hockey N, Gilmour J, Gotch F. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J Virol. 2001;75:6710–6713. doi: 10.1128/JVI.75.14.6710-6713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor C, van Kooyk Y, Adema G. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Immunol Rev. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- Appelmelk BJ, Van Die I, Van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, Van Kooyk Y. Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, Kewal-Ramani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares M, Puig-Kroger A, Muniz Pello O, Corbi AL, Rivas L. Dendritic-cell specific ICAM-3 grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human dendritic cells, is a receptor for Leishmania amastigotes. J Biol Chem. 2002;277:36766–36769. doi: 10.1074/jbc.M205270200. [DOI] [PubMed] [Google Scholar]
- Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Despres P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:1–6. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Krooshoop DJEB, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabosky V, Alon R, Figdor CG, van Kooyk Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Bashirova AA, Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ, Eilering JB, Martin MP, Wu L, Martin TD, Viebig N, Knolle PA, KewalRamani VN, van Kooyk Y, Carrington M. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J Exp Med. 2001;193:671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilleux EJ, Barten R, Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J Immunol. 2000;165:2937–2942. doi: 10.4049/jimmunol.165.6.2937. [DOI] [PubMed] [Google Scholar]
- Pohlmann S, Soilleux EJ, Baribaud F, Leslie GJ, Morris LS, Trowsdale J, Lee B, Coleman N, Doms RW. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci USA. 2001;98:2670–2675. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irjala H, Elima K, Johansson EL, Merinen M, Kontula K, Alanen K, Grenman R, Salmi M, Jalkanen S. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur J Immunol. 2003;33:815–824. doi: 10.1002/eji.200323859. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MM, Tensen CP, van As JH, Van den Berg TK, Fluitsma DM, Dijkstra CD, Dopp EA, Droste A, Van Gaalen FA, Sorg C, Hogger P, Beelen RH. Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858–866. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- Knight SC. Veiled cells—“dendritic cells” of the peripheral lymph. Immunobiology. 1984;168:349–361. doi: 10.1016/S0171-2985(84)80122-9. [DOI] [PubMed] [Google Scholar]
- Betjes MG, Haks MC, Tuk CW, Beelen RH. Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-term culture. Immunobiology. 1991;183:79–87. doi: 10.1016/S0171-2985(11)80187-7. [DOI] [PubMed] [Google Scholar]
- Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- Linehan SA, Martinez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore K, Sonnerborg A, Brostrom C, Goh LE, Perrin L, McDade H, Stellbrink HJ, Gazzard B, Weber R, Napolitano LA, van Kooyk Y, Andersson J. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 2002;16:683–692. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- Drinker CK, Wislocki GB, Field ME. The structure of the sinuses in the lymph nodes. Anat Rec. 1933;56:261–273. [Google Scholar]
- Moe RE. Fine structures of the reticulum and sinuses of lymph nodes. Am J Anat. 1963;112:311–335. [Google Scholar]
- Forkert PG, Thliveris JA, Bertalanffy FD. Structure of sinuses in the human lymph node. Cell Tissue Res. 1977;183:115–130. doi: 10.1007/BF00219996. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit ECM, Lanzavecchia A, Pieters J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22:432–437. doi: 10.1016/s1471-4906(01)01957-3. [DOI] [PubMed] [Google Scholar]
- Park CG, Takahara K, Umemoto E, Yashima Y, Matsubara K, Matsuda Y, Clausen BE, Inaba K, Steinman RM. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TBH, Groot PC, Nolte MA, van Vliet SJ, Gangaram-Panday ST, van Duijnhoven GCF, Kraal G, van Oosterhout AJM, van Kooyk Y. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-born antigens in vivo. Blood. 2002;100:2908–2916. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]