Abstract
Clostridium perfringens enterotoxin (CPE) induces cytolysis very rapidly through binding to its receptors, the tight junction proteins CLDN 3 and 4. In this study, we investigated CLDN 3 and 4 expression in breast cancer and tested the potential of CPE-mediated therapy. CLDN 3 and 4 proteins were detected in all primary breast carcinomas tested (n = 21) and, compared to normal mammary epithelium, were overexpressed in approximately 62% and 26%, respectively. Treatment of breast cancer cell lines in culture with CPE resulted in rapid and dose-dependent cytolysis exclusively in cells that expressed CLDN 3 and 4. Intratumoral CPE treatment of xenografts of T47D breast cancer cells in immunodeficient mice resulted in a significant reduction in tumor volume (P = 0.007), with accompanying necrosis. Necrotic reactions were also seen in three freshly resected primary breast carcinoma samples treated with CPE for 12 hours, while isolated primary breast carcinoma cells underwent rapid and complete cytolysis within 1 hour. Thus, expression of CLDN 3 and 4 sensitizes primary breast carcinomas to CPE-mediated cytolysis and emphasizes the potential of CPE in breast cancer therapy.
Breast cancer is the second leading cause of cancer death in women. While medical advances have significantly improved long-term survival of women diagnosed at the early stages, this has not been true for women with advanced breast cancer. Thus, innovative therapeutic modalities are sorely needed. The ability of Clostridium perfringens enterotoxin (CPE) to directly and rapidly lyse mammalian cells has been known for over 20 years1 and is responsible for the gastrointestinal symptoms associated with C. perfringens type A food poisoning.2 These symptoms are elicited following the release of CPE into the intestinal lumen, where it binds to its receptors on the surface of intestinal epithelial cells. This triggers the formation of a large multiprotein membrane pore complex and ultimately results in cell lysis.3 CPE-mediated cytolysis has been shown to occur extremely rapidly, requiring only 5 to 15 minutes.4 The process is very specific, since cells lacking expression of CPE receptors are completely unaffected by the toxin.5 Recently, Claudins (CLDN) 3 and 4 were identified as the receptors for CPE.6,7 The CLDN family of proteins, which function in the sealing of tight junctions, was discovered in 1998.8 Although more than 18 CLDN proteins have been identified, only CLDN 3 and 4 were found to sensitize cells to CPE-mediated cytolysis.9,10 Interestingly, CLDN 3 and 4 are highly expressed in several cancer types.11–13 The ability of CPE to rapidly and specifically lyses cells expressing CLDN 3 and/or 4, raised the possibility that the toxin may be useful in the treatment of these cancers.11–13 Here we show that CLDN 3 and 4 are consistently expressed in primary breast carcinomas and breast cancer cell lines. Additionally, CLDN 3 and 4 are overexpressed in approximately 62% and 26% of primary breast carcinomas, respectively, relative to normal mammary epithelium. The expression of CLDN 3 and 4 sensitizes them to CPE-mediated cytolysis, suggesting that this potently cytotoxic enterotoxin, when delivered locally, may be very useful in breast cancer therapy.
Materials and Methods
Cell Lines, Organoids, and Tumors
Most cell lines were obtained from American Type Culture Collection (Rockville, MD), and cultured according to conditions specified. Breast cancer cell lines 21PT and 21MT were kindly provided by Dr. Vimla Band (New England Medical Center, Boston, MA). Finite lifespan human mammary epithelial cells (HMEC) 286, 6–8, 11–24, 9–10, and 5–24 were gifts from Dr. Steven Ethier (University of Michigan, Ann Arbor. MI). Mammary epithelial organoid samples (N74, B31, and B54) were prepared from reduction mammoplasty specimens of normal women as described.14 Briefly, the specimens were enzymatically digested into duct-like structures (organoids), filtered, histologically confirmed to contain greater than 70% epithelial cells, and frozen at −70°C until use. Freshly resected primary breast carcinoma samples and paraffin blocks of primary breast carcinoma tissue were obtained from the Surgical Pathology Division of the Johns Hopkins Hospital, observing institutional guidelines for acquisition of such specimens.
Western Blotting
Primary breast carcinoma tissue containing greater than 70% epithelial cells as determined by H&E staining and normal mammary organoid tissue were homogenized. Total protein was extracted from homogenized breast tissues, HMEC, and breast cancer cell lines using lysis buffer consisting of 15% glycerol, 5% SDS, and 250 mmol/L Tris-HCl, pH 6.7. Equal amounts of protein from cell lysates were resolved using 12% SDS-PAGE (Invitrogen, Carlsbad, CA). Protein was transferred to ECL nitrocellulose membranes (Amersham, Arlington Heights, IL). Following Western transfer, membranes were probed with anti-human CLDN 3 (Zymed, San Francisco, CA), anti-human CLDN 4 (Zymed), or β-actin (Amersham) antibody diluted 1:1000 (CLDN 3 and 4) or 1:5000 (β-actin). Horseradish peroxidase-conjugated antibody against rabbit or mouse IgG (Amersham) was used at 1:1000 and binding was revealed using enhanced chemiluminescence (Amersham).
Immunohistochemistry
Paraffin-embedded sections were deparaffinized in xylene and rehydrated through graded ethanols. Antigen retrieval was performed by immersing sections in 0.01 mol/L sodium citrate, pH 6.0, and boiling by microwave for 20 minutes. Sections were then cooled to room temperature and endogenous peroxidase activity was quenched by immersing in 0.3% hydrogen peroxide for 30 minutes. Blocking was then performed by incubation in diluted normal goat (CLDN 3) or horse (CLDN 4) serum (Vectastain kit, Vector, Burlingame, MI) as per the manufacturer’s instructions. Sections were then incubated with rabbit polyclonal CLDN 3 or mouse monoclonal CLDN 4 at a 1:500 dilution for a period of 16 hours. Diluted biotinylated anti-rabbit or anti-mouse IgG (Vectastain kit) was added to the sections and incubated for 30 minutes. Vectastain ABC reagent was then added for 30 minutes. CLDN 3 and 4 protein was visualized using 3,3′-diaminobenzamidine (DAB) as per the manufacturer’s instructions (Vector). Sections were then counterstained in hematoxylin (Richard-Allan Scientific, Kalamazoo, MI) for 10 seconds. Lastly, sections were dehydrated through graded ethanols, cleared in xylene, mounted, and coverslipped. Images were acquired by light microscopy.
Cytotoxicity Assays
CPE was isolated and purified as previously described.13 The breast cancer cell lines MCF-7, SKBr3, T47D, HS578T, and MDA-MB-435 were plated in 6-well plates and grown to approximately 80% confluence in complete medium. Old medium was then removed and replaced with complete medium with or without CPE at concentrations ranging from 0.05 to 4 μg/ml. Cells were then incubated at 37°C for 60 minutes. Floating cells were collected and pooled with adherent cells removed by trypsinization. Total cells were then counted using a hemacytometer, and cell viability was determined by trypan blue dye (0.4%) exclusion.
Freshly resected primary breast tumor samples of approximately 5 to 10 mm3 were divided into two equal pieces and incubated with or without 10 μg of CPE in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) for a period of 12 hours. Tissues were subsequently fixed in 10% neutral buffered formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin.
A surgically resected high grade (Elston grade III) primary breast carcinoma was rapidly cut into small pieces under sterile conditions, placed in DMEM, and digested with collagenase A (Boehringer Mannheim, Mannheim, Germany) for a period of 2 hours at 37°C, rotated at 225 rpm. Digested tumor tissue was then filtered sequentially through 250-, 100-, and 40-μm filters creating a single cell suspension. Primary breast carcinoma cells were then isolated by incubation with the epithelia-specific antibody BerEP4 conjugated to magnetic beads (Dynal Biotech ASA, Oslo, Norway). Beads were washed 10 times in phosphate buffered saline with 0.5% bovine serum albumin (PBS/BSA). Primary breast carcinoma cells and attached beads were resuspended in PBS/BSA, divided equally into a 6-well plate, and incubated with or without 2 μg/ml CPE in DMEM supplemented with 10% FBS for a period of 60 minutes at 37°C. Percent cytotoxicity was determined by comparison of non-ballooned cell counts within a demarcated 1-cm2 grid at 0 and 60 minutes following the addition of CPE as previously described.6
Xenografts in Mice
SCID mice were kindly provided by Dr. Curt Civin (Johns Hopkins University School of Medicine, Baltimore, MD) and animals were maintained in a pathogen-free environment. Xenografts were generated in 6- to 8-week-old animals. Two days before injection of tumor cells, animals received one intramuscular injection of 1.5 mg/kg depo-estradiol (Florida Infusion Co., Palm Harbor, FL) diluted 1:3 in cottonseed oil. T47D human breast cancer cells were washed twice in serum-free DMEM and resuspended in Matrigel (BD, Franklin Lakes, NJ) at a concentration of 1 × 107 cells/0.1 ml. T47D cells (1 × 107) were injected subcutaneously in the left and right flank. Animals received an intramuscular injection of 0.5 mg/kg depo-estradiol diluted 1:3 in cottonseed oil at 1 and 2 weeks following initial estradiol administration. Tumor sizes were determined by measuring three diameters using calipers. On reaching a size of approximately 100 mm3, tumors were administered intratumoral injections of 2 μg of CPE, 10 μg of CPE, or PBS on days 1, 3, 5, 7, 9, 11, and 13. Tumor size was recorded on days 1, 7, and 14, after which animals were euthanized and tumors were removed. Tumors were fixed in 10% neutral buffered formalin for histological examination by hematoxylin and eosin (H&E) and Ki67 staining. Each experiment consisted of six treated tumors and six control tumors.
Results
Expression of CPE Receptors, CLDN 3 and 4, in Breast Cancer Cell Lines, Primary Breast Carcinoma, and Normal Mammary Epithelium
Recently, CLDN 3 and 4 were reported to be overexpressed in both ovarian and pancreatic carcinomas relative to normal epithelium as determined by serial analysis of gene expression (SAGE).11,15 Similarly, using SAGE analysis we found CLDN 3 and 4 mRNA to be overexpressed by two- to threefold in several breast cancer cell lines relative to finite life-span human mammary epithelial cells (HMEC).16 To validate these findings we performed Western blot analysis using a panel of breast cancer cell lines, primary breast carcinomas, and normal human mammary epithelial cells. CLDN 3 and 4 were consistently expressed in all primary breast carcinomas tested (15/15) and in 60% (CLDN 3) and 80% (CLDN 4) of breast cancer cell lines (n = 10) (Figure 1, A and B). In agreement with the results of our SAGE analysis,16 CLDN 3 and 4 proteins were overexpressed by more than twofold in 12/15 (P = 0.008) and 5/15 (P = 0.046) primary breast carcinomas, respectively, relative to HMEC and normal epithelial organoids obtained from reduction mammoplasty specimens as determined by densitometric scanning (Figure 1C).
Figure 1.
Expression of CLDN 3 and 4 proteins in breast cancer cell lines, primary breast carcinoma, and normal mammary epithelium. A: Human primary breast carcinoma, normal mammary organoid tissue, and finite life span human mammary epithelial cells (HMEC) were homogenized and total protein was extracted. Western analysis was performed on equal amounts of protein from cell lysates using CLDN 3, 4, and β-actin antibodies. Significant differences in CLDN 3 (P = 0.008) and CLDN 4 (P = 0.046) expression levels between primary breast carcinomas and normal mammary epithelial cells were determined by Student’s t-test. B: CLDN 3 and 4 protein expression in human breast cancer cell lines. Western analysis was performed on equal amounts of protein from total cell lysates using CLDN 3, 4, and β-actin antibodies. C: The level of CLDN 3 and 4 expression in primary breast carcinomas, normal mammary organoids, and HMEC normalized to actin were determined by densitometric scanning of radiographical film. D: Immunohistochemical analysis was performed on paraffin embedded sections of human primary breast carcinoma tissues identified by an asterisk in A using CLDN 3 and 4 antibodies. CLDN 3 and 4 proteins in primary breast carcinoma tissue and adjacent normal mammary epithelium were visualized using DAB. Sections were counterstained with hematoxylin and visualized by light microscopy (422, ×200; 126 and 973, ×400).
Although both the primary breast carcinoma and normal mammary organoid samples used were verified to contain greater than 70% epithelial cells, it is possible that the increase in CLDN 3 and 4 expression observed in primary breast carcinomas relative to normal mammary organoids was influenced by differences in the epithelial cell content of the tissue, since CLDN 3 and 4 are solely expressed by the epithelial cell component of human breast tissue. To address this possibility, we performed immunohistochemical (IHC) analysis of 10 primary breast carcinoma cases of varying histological grade, 4 of which were included in our Western blot analysis (identified by asterisk in Figure 1A). In each case the CLDN 3 and 4 staining patterns were compared to that in adjacent normal epithelium. Surrounding fibroblasts and adipocytes served as negative controls since these cells do not express CLDN proteins. Consistent with our Western blot analysis, CLDN 3 and 4 staining was detectable in all primary breast carcinomas tested and was higher in 5/10 and 3/10 primary breast carcinomas, respectively, compared to levels present in adjacent normal mammary epithelium (Figure 1D). In agreement with previous findings, CLDN 3 and 4 expression was restricted to epithelial cells and localized to the cell membrane, consistent with the biological role of CLDNs in the formation of tight junctions.
Efficacy of CPE-Mediated Cytolysis on Breast Cancer Cell Lines in Vitro
Although the cytolytic ability of CPE has been demonstrated in various cell types,4,12,13 the effect of CPE on breast cancer cells has not been characterized. To determine the effect of CPE on breast cancer cells, we treated three breast cancer cell lines that show expression of CLDN 3 and 4 (MCF-7, SKBr3, and T47D) and two breast cancer cell lines lacking detectable expression of CLDN 3 and 4 (HS578T and MDA-MB-435) with concentrations of CPE ranging from 0.05 to 4 μg/ml for a period of 60 minutes. Following CPE treatment, all of the cells in the culture dish were counted and percent cytotoxicity was determined by trypan blue dye exclusion. CPE treatment of breast cancer cell lines expressing CLDN 3 and 4 resulted in rapid and virtually complete cytolysis in a dose-dependent fashion (Figure 2A). Consistent with previous studies in cell lines from other carcinomas, breast cancer cell lines lacking CLDN 3 and 4 expression were completely resistant to the cytotoxic effects of CPE (Figure 2B). Thus, CPE treatment results in rapid and potent cytolysis specific to breast cancer cell lines expressing CLDN 3 and 4.
Figure 2.
Sensitivity of human breast cancer cell lines to CPE-mediated cytolysis in vitro. CLDN 3 and 4 expressing (MCF-7, SKBr3, and T47D; A) and non-expressing (HS578T and MDA-MB-435; B) breast cancer cell lines were incubated in complete media with or without CPE at concentrations ranging from 0.05 to 4 μg/ml for 60 minutes. Total cells were pooled and counted using a hemacytometer and cell viability was determined by trypan blue dye (0.4%) exclusion. Data from representative experiments performed in triplicate are expressed as % cytotoxicity ±SD.
Efficacy of CPE-Mediated Cytolysis of Breast Cancer Cell Line T47D in Vivo
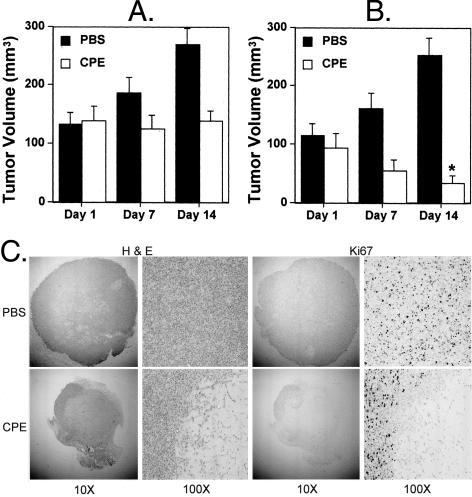
Next, we investigated whether CPE has cytolytic effects on breast cancer cells grown as tumors in vivo. Xenograft tumors were grown subcutaneously in SCID mice using the CLDN 3 and 4-expressing breast cancer cell line, T47D. Tumors were allowed to grow to a size of approximately 100 mm3 and then treated with a total of seven intratumoral injections of either 2 μg of CPE, 10 μg of CPE, or PBS alone administered every other day over the course of 14 days. Tumor volumes were measured on days 1, 7, and 14, after which all animals were euthanized and tumors were removed for histological examination. Animals receiving CPE did not exhibit any signs of systemic toxicity such as loss of weight, huddling, ruffled fur, etc. The effect of CPE on T47D xenograft tumors was dose-dependent. Tumors receiving intratumoral injections of 2 μg of CPE showed no increase in tumor volume between days 1 and 14, while the volume of tumors receiving 10 μg of CPE per injection was significantly reduced at day 14 relative to the volume on day 1 (P = 0.007) (Figure 3, A and B). Further, on histological examination, tumors treated with either dose of CPE showed large areas of necrosis as evidenced by H&E staining of tumor sections (Figure 3C). The extent of cell death was confirmed by staining for the proliferation marker Ki67, which showed the presence of very few viable tumor cells following CPE treatment. This observation directly correlates with the effects of CPE on tumor volume (Figure 3C). These experiments show that CPE treatment of T47D xenograft tumors in vivo results in cell necrosis, with a significant reduction in tumor volume.
Figure 3.
Sensitivity of human breast cancer cell line T47D to CPE-mediated cytolysis in vivo. Xenograft tumors were established in SCID mice using the human breast cancer cell line T47D. Tumors were allowed to grow to a size of approximately 100 mm3 and subsequently treated by intratumoral injection of 2 μg of CPE (A) or 10 μg of CPE (B) versus PBS alone on days 1, 3, 5, 7, 9, 11, and 13. Tumor volumes were measured on days 1, 7, and 14, after which animals were euthanized, tumors were removed, and tumor viability was determined by H&E and Ki67 staining (C). Each experiment is representative of 12 tumors. *P = 0.007 compared to volume on day 1 as determined by Student’s t-test.
Susceptibility of Primary Breast Carcinoma to CPE-Mediated Cytolysis
Although we have shown expression of CLDN 3 and 4 in all primary breast carcinomas tested (Figure 1A), the susceptibility of primary tumor tissue to CPE-mediated cytolysis remained to be tested. We subjected three freshly resected breast tumor tissues of various histological grades to CPE treatment in vitro. Tumor tissue samples of approximately 5 to 10 mm3 were divided into two equal pieces and incubated in complete media with or without 10 μg of CPE for a period of 12 hours to allow diffusion of CPE into the tissue. Tissues were subsequently fixed in 10% neutral buffered formalin, paraffin embedded, and sectioned. Subsequent histopathological analysis of H&E stained tumor sections revealed a consistently higher degree of tumor cell necrosis in tissues treated with CPE as compared to media alone (Figure 4A). No effect was seen on vascular endothelial or stromal cells, consistent with the absence of CLDN 3 and 4 expression in these cell types.
Figure 4.
Sensitivity of primary breast tumor to CPE-mediated cytolysis in vitro. A: Freshly resected primary breast tumor tissue samples of approximately 5 to 10 mm3 were divided into two equal pieces and incubated with or without 10 μg CPE in DMEM supplemented with 10% fetal bovine serum for a period of 12 hours. Tissues were subsequently fixed in 10% neutral buffered formalin, paraffin embedded, sectioned, and stained with H&E. Sections were visualized by light microscopy (×200). B: Primary breast carcinoma cells were isolated from freshly resected breast tumor using the epithelia-specific antibody BerEP4 conjugated to magnetic beads as described in Materials and Methods. Cells were aliquoted equally into the wells of a 6-well plate and treated with or without CPE at a final concentration of 2 μg/ml for 60 minutes. Percent cytotoxicity was determined by comparison of non-ballooned cell counts within a demarcated 1-cm2 grid at 0 and 60 minutes following the addition of CPE. The breast cancer cell lines MCF-7 and HS578T were included as positive and negative controls, respectively.
The susceptibility of primary breast carcinoma to CPE-mediated cytolysis was further explored by isolating carcinoma cells from a freshly resected Elston grade III breast tumor by immunopurification. One half of the carcinoma cells were treated with CPE (2 μg/ml) for 60 minutes, while the second half was placed in media alone. Percent cytotoxicity was then determined by non-ballooned cell counts as described previously.6 The breast cancer cell lines MCF-7 and HS578T were included as positive and negative controls, respectively. CPE treatment of epithelial cells isolated from the tumor resulted in nearly 100% cytotoxicity. This cytotoxic response was equivalent to the effect of CPE on CLDN 3 and 4-positive MCF-7 cells (Figure 4B). Consistent with previous results, no loss of viability was observed in HS578T cells that lack detectable CLDN 3 and 4 expression. These results indicate that primary breast cancer cells are also susceptible to the cytolytic effects of CPE, a response that is likely mediated through the binding of CPE to its receptors, CLDN 3 and 4, expressed at the cell membrane.
Discussion
CPE is a well-known virulence factor responsible for the gastrointestinal symptoms associated with C. perfringens type A food poisoning. However, its ability to rapidly and specifically lyse cells expressing its receptors, CLDN 3 and 4, could be effectively exploited in the treatment of cancers constitutively expressing these proteins. In this study we have shown overexpression of both CLDN 3 and 4 in approximately 62% and 26% of primary breast carcinomas, respectively, relative to normal mammary epithelium. CLDN 3 and 4-expressing breast cancer cell lines grown in cell culture and as xenograft tumors underwent rapid and dose-dependent cytolysis in response to CPE treatment. Consistent with our observations in cultured breast cancer cell lines, primary breast tumor samples as well as isolated carcinoma cells from freshly resected tumor specimens also underwent CPE-mediated cytolysis in vitro. These results raise the possibility that CPE treatment in vivo may be an effective targeted therapy for breast cancer.
Despite the ability of CPE to effectively lyse breast cancer cells, its clinical application faces obstacles similar to those of other protein-based therapeutics. A common problem facing most therapies is the development of a neutralizing antibody response preventing their repeated use. Although elevated titers of anti-enterotoxin antibodies developed following CPE ingestion have been found to provide no protection for human subjects against the effects of subsequent ingestion of CPE, careful studies need to be performed to accurately determine the presence, and nature of the immune response against CPE when administered by intratumoral, intraductal, and systemic routes.17
Another problem in the effective use of CPE as an anti-tumor agent is the inability to penetrate solid tumors. We found that intratumoral administration of CPE to the center of breast cancer xenografts resulted in significant necrosis, but left the boundaries of several tumors intact (data not shown). This suggests that CPE may have been largely restricted close to the point of injection and was unable to diffuse completely throughout the tumor. It is possible that alternative slow release formulations of CPE, such as liposomal preparations18 or polymers carrying the drug,19 would result in greater tumor penetration and improve therapeutic efficacy.
Systemic toxicity is an important concern for any new drug therapy. Although CPE-mediated cytolysis is specifically targeted against cells expressing CLDN 3 and 4, rendering many tissues immune to its effects, many other tissues such as prostate, lung, and the gastrointestinal tract express these proteins. Thus, systemic delivery of CPE would result in significant toxicity. This has been documented in mice where administration of doses as low as 0.1 mg/kg i.p. elicited symptoms associated with CPE-induced toxicosis such as immobility and loss of appetite.20 In our studies, intratumoral administration of 0.5 mg/kg resulted in a significant reduction in tumor volume without any signs of CPE-induced toxicosis (Figure 3B). Under the same conditions, administration of the same dose i.p. was toxic and had no effect on tumor volume (data not shown). Thus, local delivery methods may be most appropriate for the use of native CPE.
To deliver CPE locally we are currently exploring the use of intraductal administration. Administering therapeutics through the ductal network allows more direct access to both primary breast tumors and pre-neoplasias while greatly reducing systemic exposure. Although local delivery of CPE into the breast duct may circumvent systemic toxicity, it could also cause death of normal ductal cells, since they express low but detectable levels of CLDN 3 and 4 (Figure 1A). Our preliminary studies addressing this issue show that normal rat breast epithelium appears to be relatively resistant to the effects of CPE. On gross examination of stained whole mounts of untreated rat mammary glands and those treated with CPE, no differences were observed. More detailed examination by microscopic analysis of H&E stained sections did show evidence of prior damage in CPE-treated glands in the form of gland atrophy and hemosiderin deposition; however, the ductal architecture of the treated glands remained intact. The apparent reduced sensitivity of normal breast epithelium to CPE-mediated cytolysis was not due to reduced susceptibility of rat cells to CPE, since treatment of two rat mammary cancer cell lines established from N-nitroso,N′-methylurea-induced tumors expressing CLDN 3 and 4 resulted in rapid and virtually complete cytolysis in a dose-dependent fashion similar to that seen in human breast cancer cell lines (unpublished data). These preliminary studies suggest that normal mammary epithelial cells may be less susceptible to the effects of CPE when delivered by the intraductal route despite the expression of CLDN 3 and 4. The apparent reduced sensitivity of normal mammary epithelium to CPE could be due to the formation of more efficient tight junctions by normal mammary epithelium that limit CPE access to its receptors, and/or due to the lower expression of CLDN 3 and 4 in normal epithelium compared to tumor cells. This phenomenon has been previously documented in cell culture studies where CPE added to the apical domain of cells had a greatly reduced cytolytic effect than when added basolaterally.3 As carcinoma cells invade the breast duct, both the apical and basolateral domains of the cell become exposed to intraductally delivered agents. Further, these cells lose cell polarity, which also may result in an altered distribution of the receptors along the cell surface. In addition, cancerous lesions are known to have reduced cell cohesion.21,22 These factors would allow CPE to better contact its receptors and may explain the large differential in cytolytic effect of CPE on tumor cells compared to normal ductal epithelium, warranting detailed investigation into this delivery method.
Although the clinical application of CPE faces several challenges, it has several potential advantages as well. Currently, there are no known inhibitors of CPE. CPE-mediated cytolysis requires only the single step of CPE binding to its receptor. This is in contrast to other toxins and pro-drugs such as anthrax toxin and 5-fluorocytosine, which require additional enzymatic activation steps. Thus, the simplicity of CPE-mediated cytolysis may result in increased efficacy and reduced opportunity for the development of resistance. In addition, the documented ability of CPE to down-regulate the tight junction barrier through binding to CLDN 3 and 423 may enhance the anti-tumor effect of other treatment modalities. The local delivery of native CPE may be useful in the treatment of pre-neoplastic lesions such as ductal carcinoma in situ and in neo-adjuvant settings such as the loco-regional control of locally advanced breast carcinoma, as well as in tumor down-staging to allow breast conservation therapy. Taken together, these data provide evidence to suggest that CPE may have potential in the treatment of breast cancer.
Footnotes
Address reprint requests to Saraswati Sukumar, Ph.D., The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, BBCRB Room 410, 1650 Orleans St., Baltimore, MD 21231. E-mail: sukumsa@jhmi.edu.
Supported by PHHS Grant SPORE P50 CA88843 (to S.S), DAMD17–01-1–0285 (to S.S.), and DAMD17–02-1–0429 (to S.L.K.) from the U.S. Army Medical Research and Materiel Command.
References
- McClane BA, Hanna PC, Wnek AP. Clostridium perfringens enterotoxin. Microb Pathog. 1988;4:317–323. doi: 10.1016/0882-4010(88)90059-9. [DOI] [PubMed] [Google Scholar]
- Sarker MR, Carman RJ, McClane BA. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol Microbiol. 1999;33:946–958. doi: 10.1046/j.1365-2958.1999.01534.x. [DOI] [PubMed] [Google Scholar]
- McClane BA. The complex interactions between Clostridium perfringens enterotoxin and epithelial tight junctions. Toxicon. 2001;39:1781–1791. doi: 10.1016/s0041-0101(01)00164-7. [DOI] [PubMed] [Google Scholar]
- McClane BA, McDonel JL. The effects of Clostridium perfringens enterotoxin on morphology, viability, and macromolecular synthesis in Vero cells. J Cell Physiol. 1979;99:191–200. doi: 10.1002/jcp.1040990205. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y, Uemura T, Kozaki S, Sakaguchi G. The relationship between cytotoxic effects and binding to mammalian culture cells of Clostridium perfringens enterotoxin. FEMS Microbiol Lett. 1985;28:131–135. [Google Scholar]
- Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272:26652–26658. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol. 1997;136:1239–1247. doi: 10.1083/jcb.136.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000;476:258–261. doi: 10.1016/s0014-5793(00)01744-0. [DOI] [PubMed] [Google Scholar]
- Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–6287. [PubMed] [Google Scholar]
- Michl P, Buchholz M, Rolke M, Kunsch S, Lohr M, McClane B, Tsukita S, Leder G, Adler G, Gress TM. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology. 2001;121:678–684. doi: 10.1053/gast.2001.27124. [DOI] [PubMed] [Google Scholar]
- Long H, Crean CD, Lee WH, Cummings OW, Gabig TG. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res. 2001;61:7878–7881. [PubMed] [Google Scholar]
- Bergstraesser LM, Weitzman SA. Culture of normal and malignant primary human mammary epithelial cells in a physiological manner simulates in vivo growth patterns and allows discrimination of cell type. Cancer Res. 1993;53:2644–2654. [PubMed] [Google Scholar]
- Ryu B, Jones J, Blades NJ, Parmigiani G, Hollingsworth MA, Hruban RH, Kern SE. Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res. 2002;62:819–826. [PubMed] [Google Scholar]
- Nacht M, Ferguson AT, Zhang W, Petroziello JM, Cook BP, Gao YH, Maguire S, Riley D, Coppola G, Landes GM, Madden SL, Sukumar S. Combining serial analysis of gene expression and array technologies to identify genes differentially expressed in breast cancer. Cancer Res. 1999;59:5464–5470. [PubMed] [Google Scholar]
- Skjelkvale R, Uemura T. Experimental Diarrhoea in human volunteers following oral administration of Clostridium perfringens enterotoxin. J Appl Bacteriol. 1977;43:281–286. doi: 10.1111/j.1365-2672.1977.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Gabizon A, Chemla M, Tzemach D, Horowitz AT, Goren D. Liposome longevity and stability in circulation: effects on the in vivo delivery to tumors and therapeutic efficacy of encapsulated anthracyclines. J Drug Target. 1996;3:391–398. doi: 10.3109/10611869608996830. [DOI] [PubMed] [Google Scholar]
- Chiba M, Hanes J, Langer R. Controlled protein delivery from biodegradable tyrosine-containing poly(anhydride-co-imide) microspheres. Biomaterials. 1997;18:893–901. doi: 10.1016/s0142-9612(97)00027-6. [DOI] [PubMed] [Google Scholar]
- Wallace FM, Mach AS, Keller AM, Lindsay JA. Evidence for Clostridium perfringens enterotoxin (CPE) inducing a mitogenic and cytokine response in vitro and a cytokine response in vivo. Curr Microbiol. 1999;38:96–100. doi: 10.1007/s002849900410. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A. Ultrastructural modifications of intercellular junctions between tumor cells. In Vitro. 1970;6:15–20. doi: 10.1007/BF02616130. [DOI] [PubMed] [Google Scholar]
- Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]