Abstract
Olig2 is a recently identified transcription factor involved in the phenotype definition of cells in the oligodendroglial lineage. The expression of Olig2 transcript has been demonstrated in human oligodendroglial tumors, although the protein expression has not been studied extensively. We developed a polyclonal antibody to human Olig2 and analyzed it immunohistochemically. The antibody depicted a single distinct band of predicted molecular weight by Western blotting, and did not cross-react with human Olig1. In normal human brain tissue, the nuclei of oligodendrocytes of interfascicular, perivascular, and perineuronal disposition were clearly labeled by the antibody. Similarly, the nuclei of oligodendroglial tumors were labeled. There was no apparent correlation between the staining intensity and histological grade. Astrocytic components within the tumors were generally less or not stained. Astrocytic tumors were also positive with the Olig2 antiserum to a lesser extent, and the difference between oligodendroglial and astrocytic tumors was demonstrated by a statistical analysis. Olig2 and glial fibrillary acidic protein were expressed in a mutually exclusive manner, and Olig2 expression was cell-cycle related. Neither central neurocytoma nor schwannoma cases were stained. Our antibody was demonstrated to be useful in recognizing normal oligodendrocytes on paraffin sections, and applicable in diagnosis of some brain tumors.
Oligodendorocytes are defined as the cells that make and maintain central nervous system myelin. A panel of cellular markers has been developed to identify cellular components of the central nervous system, but they can also be light microscopically discriminated by nuclear morphology, because each cell type generally has a distinct nuclear appearance. The nuclei of oligodendrocytes are round to oval, relatively dark, and generally smaller than those of neurons and astrocytes. In addition, three kinds of disposition of oligodendrocytes have been noted: alignment of closely apposed cells in rows along the nerve fascicles, juxtaposing neuronal somata, and abutting on blood vessels. Accordingly, they are classified into interfascicular, perineuronal, and perivascular oligodendrocytes, respectively.1 These morphological approaches are well-accepted, however, reliable and objective methods to recognize each oligodendrocyte on tissue sections have not been established. Myelin is a distinct structure that can be an ultrastructural and immunocytochemical hallmark of oligodendrocytes. To date, antibodies targeted at oligodendrocytes are mostly myelin-specific, such as myelin basic protein, galactocerebroside, and myelin-associated glycoprotein. They are suitable especially under cultured conditions, however, they are usually of no use for tissue sections because the direct continuity of the oligodendroglial cell bodies and processes is hardly demonstrated by conventional histological methods.1
Oligodendroglial tumors are well-characterized clinicopathological entities. Genetic alterations in oligodendrogliomas differ significantly from those commonly found in diffuse astrocytomas.2–4 Moreover, oligodendrogliomas can respond to certain types of chemotherapy.5,6 Thus, there is prognostic and therapeutic value in the accurate diagnosis of oligodendroglial tumors. However, their origin from differentiated oligodendrocytes or progenitor cells committed to oligodendroglial differentiation is difficult to prove, owing to a lack of reliable immunohistochemical markers. There is an urgent need to develop markers specific for both oligodendrocytes and their neoplasms.4,7,8
Studies on developmental neurobiology both in vivo and in vitro depend considerably on cell lineage-specific differentiation markers. Indeed, the establishment of specific markers itself has been a core of investigation. Recently, great progress has been made by analyzing transcription factors that regulate neural development. Among them, Olig1 and Olig2 are the first identified transcription factors that regulate oligodendroglial development.9,10 The expression of Olig2 persists in migratory oligodendrocyte precursors from late embryonic stage to adulthood.9–11 To date, studies on Olig genes have mostly been performed using chickens and rodents. They displayed corresponding results, however, the role of human Olig2 has not been analyzed extensively. In humans, Olig2 mRNA expression was demonstrated in oligodendroglial tumors by in situ hybridization.12,13 Because transcription factors function in the nucleus, we postulated that human Olig2 would also be localized intranuclearly, and that it would be quite advantageous for detecting oligodendrocytes immunohistochemically. In this study, we established an anti-human Olig2 antibody suitable for use with formalin-fixed, paraffin-embedded tissue sections.
Materials and Methods
Tissue Samples
Autopsied normal adult human brain tissue (3 cases) and systemic tissue (3 cases), surgically resected oligodendroglial tumors (40 cases), astrocytic tumors (35 cases), central neurocytoma (6 cases), and vestibular schwannoma (11 cases) were analyzed. All cases of central neurocytoma were composed predominantly of neurocytic cells with minimal glial elements. Details are summarized in Table 1. They were excised primarily for diagnostic and/or therapeutic purposes, and the rest were used for this study according to the ethical rules of Gunma University and the Japanese Society of Pathology. All of the tissue samples were fixed with 10% formalin for up to 2 weeks, and embedded in paraffin by a conventional method. The sections were cut at 3 μm thick, and stained with hematoxylin and eosin (H&E). Additional sections were prepared for the following immunohistochemical studies.
Table 1.
Immunohistochemical Results of Olig2-C in Tumor Cases
| Histology | Total | − | + | ++ | +++ |
|---|---|---|---|---|---|
| Oligodendroglial tumors* | 40 | 0 | 8 | 13 | 19 |
| Oligodendroglioma | 16 | 0 | 3 | 6 | 7 |
| Oligoastrocytoma | 3 | 0 | 0 | 1 | 2 |
| Anaplastic oligodendroglioma | 16 | 0 | 3 | 5 | 8 |
| Anaplastic oligoastrocytoma | 5 | 0 | 2 | 1 | 2 |
| Astrocytic tumors† | 35 | 6 | 17 | 6 | 6 |
| Pilocytic astrocytoma | 4 | 0 | 2 | 1 | 1 |
| Fibrillary astrocytoma | 10 | 0 | 7 | 2 | 1 |
| Anaplastic astrocytoma | 8 | 1 | 3 | 1 | 3 |
| Glioblastoma | 13 | 5 | 5 | 2 | 1 |
| Central neurocytoma | 6 | 6 | 0 | 0 | 0 |
| Schwannoma | 11 | 11 | 0 | 0 | 0 |
Olg2-C staining results are evaluated by intensity and numbers, and tumor samples are classified into a four-tiered scale (−, +, ++, +++); (see Materials and Methods). A significant difference (P = 0.001) is detected between oligodendroglial (*) and astrocytic (†) groups by Mann-Whitney’s U test.
Antibody
The amino acid sequence of the immunogens was designed based on genetic data of the human Olig2 gene (National Center for Biotechnology Information, accession no. NM005806). Two synthetic peptides composed of VSSRPSSPEPDDLFLC (amino acids 8 to 22 plus cysteine at the C-terminal) and CMGAGSLPRLTSDAK (amino acids 310 to 323 plus cysteine at the N-terminal) were conjugated with keyhole limpet hemocyanin (Sawady Technology, Tokyo, Japan) and used for immunogens separately. Japanese white rabbits weighing 2.5 kg were purchased from an animal breeding company. The animal experiment was permitted by the Animal Care and Experimentation Committee, Gunma University, Showa Campus. A solution of 0.5 ml containing 1.25 mg of immunogen was emulsified with an equal amount of Titer Max Gold (Funakoshi, Tokyo, Japan), and injected subcutaneously to the rabbits. Two, 4, 6, and 8 weeks later, the immunization was repeated in essentially the same way with the adjuvant being replaced with Freund’s incomplete adjuvant (Calbiochem, La Jolla, CA). Two weeks after the fifth administration, the antisera were obtained from the peripheral blood of each rabbit.
The crude antisera were affinity-purified by chromatography. Briefly, CNBr-activated Sepharose 4B gel (Amersham Pharmacia, Tokyo, Japan) was coupled with native immunogen peptides according to the manufacturer’s instructions, and the gel was packed in the column. The fraction bound to the ligated epitope was collected by a conventional method. Those targeted for amino acids 8 to 22 and 310 to 323 were named Olig2-N and Olig2-C, respectively.
For negative control experiments, a fraction without immunoreactive element of Olig2-C was prepared. The optimal dilution for immunostaining was determined by preliminary experiments. The suitably diluted antibody was mixed with 1.25 μg/μl of immunogen peptide, and incubated for 24 hours at 37°C. The sample was centrifuged at 10,000 × g for 60 minutes, and the supernatant was used for both immunohistochemistry and Western blotting.
Analyses of Specificity
The electrophoresis method is essentially the same as that described by Laemmli.14 Briefly, a portion of unfixed anaplastic oligodendroglioma tissue (this case was included in immunohistochemical analyses) was homogenized with sample buffer (1% sodium dodecyl sulfate, 1% 2-mercaptoethanol, 20% glycerol, 0.02% bromophenol blue, and 10 mmol/L Tris-HCl, pH 6.8), and boiled for 5 minutes. A 10% polyacrylamide gel layered with a 3% stacking gel was prepared, and the sample was electrophoresed with a prestained molecular marker (Bio-Rad, Hercules, CA). Afterward, the gel was transferred to Immobilon Transfer Membrane (Millipore, Bedford, MA) using a semidry blotting apparatus (Bio-Rad). The membrane was immunostained by standard procedure. Briefly, it was incubated with 10% normal goat serum for a few hours, followed by reaction with Olig2-C overnight at 4°C. After rinsing with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T), the membrane was reacted with a horseradish peroxidase-conjugated secondary antibody (Nichirei, Tokyo, Japan) for 60 minutes. After rinsing again with PBS-T, the membrane was floated in 3,3′-diaminobenzidine solution for a few minutes to visualize the signals. The immunoreactivity-absorbed Olig2-C was also applied to confirm its specificity.
To rule out cross-reactivity to Olig1, the human Olig1 cDNA clone was transfected to the cultured NIH3T3 cell line and immunostained by Olig2-C. Briefly, NIH3T3 cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s minimal essential medium supplemented with 10% fetal bovine serum. Human Olig1 cDNA was purchased as an EST clone (GenBank accession number BI552104) and subcloned to pcDNA3 (Invitrogen, Carlsbad, CA). Enhanced green fluorescent protein (EGFP) cDNA (from pEGFP1 vector; Clontech, Palo Alto, CA) was subcloned to pCAG GS.15 Expression vectors for human Olig1 (pcDNA3/hOlig1) and EGFP (pCAG GS/EGFP) were co-transfected into NIH3T3 cells using lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Transfected cells were identified with green fluorescence from GFP. After transfection, immunostaining was performed using affinity-purified antibodies, anti-Olig1 antibody (1:500),16 and Olig2-C (1:20). Primary antibodies were detected with goat-anti-rabbit-IgG-Alexa 594 (1/2000; Molecular Probes, Eugene, OR). Nuclei were stained with 4′,6-diamidino-2-phenylindole. The images were collected by a digital camera system (DP70; Olympus, Tokyo Japan) using microscopy (Olympus BX51).
Immunohistochemistry
Before extensive immunohistochemical studies with anti-Olig2 antisera, the effect of antigen retrieval by autoclaving was investigated. Three normal brain tissue and several oligodendroglial tumor samples were used for this pilot study. Dewaxed and rehydrated tissue sections were soaked in PBS or citrate buffer (10 mmol/L, pH 6.0), and heated to 120°C for 10 minutes. After cooling, the sections were covered with 10% normal goat serum, followed by reaction with primary antibodies overnight at 4°C. An immunoperoxidase staining kit (Nichirei) and 3,3′-diaminobenzidine solutions were used for visualization. For comparison, the sections skipping autoclaving were prepared. This preliminary study also compared Olig2-N with Olig2-C. Consequently, a remarkable effect of autoclaving (especially with citrate buffer) and superiority of Olig2-C was demonstrated, then further studies were made using Olig2-C with autoclaving pretreatment.
Next, all of the tissue samples including tumors as well as systemic tissue were immunostained by Olig2-C as described above. The number of Olig2-positive tumor cells were estimated visually as follows: 0, none; 1, less than one fourth; 2, less than half; 3, more than half. The staining intensity was also expressed using a four-tiered scale; 0, unstained; 1, stained less intensely than normal oligodendrocytes within the same sections; 2, stained similarly to normal oligodendrocytes; 3, stained more intensely than normal oligodendrocytes. Both parameters were evaluated separately in each case, and the multiplied values (0, 1, 2, 3, 4, 6, 9) were symbolized as − (0), + (1, 2), ++ (3, 4), and +++ (6, 9). The staining results between oligodendroglial and astrocytic groups were statistically analyzed by Mann-Whitney’s U-test.
To compare the cellular localization of glial fibrillary acidic protein (GFAP) and Olig2, anti-GFAP antibody17 was doubly immunostained with Olig2-C in normal brain tissue. A combination of anti-myelin basic protein (MBP) antibody (DAKO Cytomation, Glostrup, Denmark) and Olig2-C was also attempted using a method described elsewhere.18 Briefly, slides were immunostained with Olig2-C as described above, then treated in 0.1 mol/L glycine (pH 2.0) for 1 hour at room temperature to remove the Olig2-C-secondary antibody complex, and subsequently incubated with anti-GFAP or -MBP antibodies overnight at 4°C. They were colored by 4-chloro-1-naphthol using the same labeling kit. After washing in PBS, the slides were coverslipped without counterstaining and dehydrating procedures. Immunostaining with Ki-M1P (microglia/macrophage marker; Seikagaku, Tokyo, Japan) and anti-neurofilament (NF) antibodies19 was performed with sections adjacent to the Olig2-C-stained specimens.
As described in the results, oligodendrogliomas contained both Olig2-C-positive and -negative neoplastic cells. To disclose the reasons, double staining of Olig2-C with H&E was performed in two oligoastrocytomas and two anaplastic oligodendrogliomas. Furthermore, indirect dual-immunofluorescent staining of MIB-1 (Immunotech, Marseilles, France) and Olig2-C was also performed using two anaplastic oligodendrogliomas. Briefly, a mixture of both antibodies was incubated overnight at 4°C. After washing three times with PBS, slides were treated with a mixture of Alexa 488-conjugated anti-mouse immunoglobulin and Alexa 594-conjugated anti-rabbit immunoglobulin (Molecular Probes). After rinsing, slides were mounted with PBS/glycerol (1:9) containing 5% 1,4-diazabicyclooctane, and observed under a fluorescent microscope.
Results
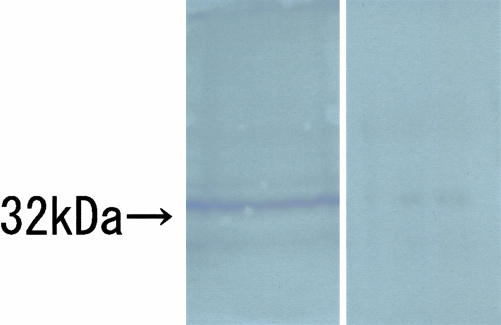
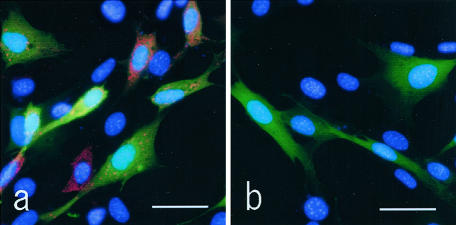
By Western blotting with Olig2-C, a distinct single band was recognized at approximately predicted molecular weight (32 kd), which was calculated by human Olig2 from the amino acid sequence, and the signal was lost when replaced with preabsorbed Olig2-C as the primary antibody (Figure 1). Immunoblotting by Olig2-N displayed several extra bands (data not shown). NIH3T3 cells transfected with human Olig1 cDNA clone was immunostained by anti-Olig1 antibody (Figure 2a), but not by Olig2-C (Figure 2b).
Figure 1.
Western blotting of Olig2-C. A distinct band of predicted molecular size (32 kd) is identified in the left lane. In the right lane, the band is not illustrated when the primary antibody is replaced with preabsorbed Olig2-C.
Figure 2.
NIH3T3 cells co-transfected with human Olig1 and EGFP are immunostained (red) either by anti-Olig1 antiserum (a) or Olig2-C (b). Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (blue), and EGFP emits green fluorescence. Positive red signals are recognized in a, while negative in b, demonstrating that transfection is successful, and Olig2-C does not react with Olig1. Similarly, cytoplasmic Olig1 signal was noted at the murine Olig1 transfection (data not shown). Scale bars, 25 μm.
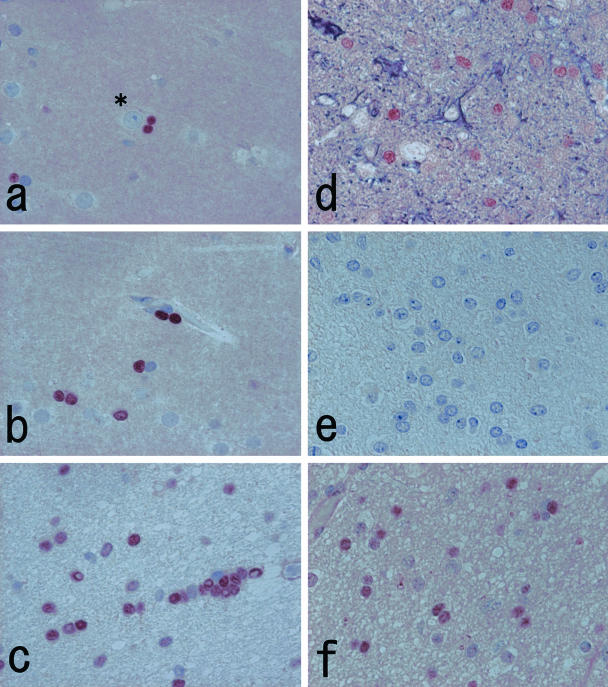
By immunohistochemistry with Olig2-C on normal human brain tissue, definite nuclear staining was noted. Labeled nuclei were round to oval, smaller than those of neurons and astrocytes, and often showed perineuronal (Figure 3a), perivascular (Figure 3b), and interfascicular disposition (Figure 3c). These characteristics corresponded exactly with those of oligodendrocytes. By double immunostaining with Olig2-C and anti-GFAP antibody, the nuclear staining with Olig2-C did not overlap on cells with cytoplasmic GFAP positivity (Figure 3d). Anti-MBP antibody immunolabeled cerebral white matter diffusely, whereas the cerebral cortex displayed randomly oriented linear immunostaining, illustrating myelin profiles. Olig2-C-labeled nuclei were surrounded by MBP-positive area, however, a definite continuity between them was hardly seen (data not shown). Comparative immunostaining of Olig2-C with Ki-M1P or anti-NF antibodies using adjacent sections also demonstrated mutually exclusive signals (data not shown). Ependymal cells, leptomeninges, and blood vessels were also negative with Olig2-C. Using preabsorbed Olig2-C (Figure 3e) as well as omission of autoclave pretreatment resulted in very weak staining. Olig2-N showed diminished staining of the oligodendroglial nuclei accompanied by diffuse background noise (Figure 3f).
Figure 3.
a to c: Immunohistochemistry with Olig2-C labels nuclei of the cells of perineuronal (a), perivascular (b), and interfascicular (c) dispositions. The size of immunolabeled nuclei is smaller than that of neurons (asterisk) and astrocytes. These characteristics are compatible with oligodendrocytes. d: Double immunostaining of Olig2-C (brown) and anti-glial fibrillary acidic protein antiserum (deep purple) demonstrates a nonoverlapping positive reaction. e: The nuclear staining is remarkably reduced when the primary antibody is replaced with a preabsorbed fraction of Olig2-C. f: Immunohistochemistry with Olig2-N displays weaker nuclear staining with diffuse background noise. Original magnifications, ×500 (a–f).
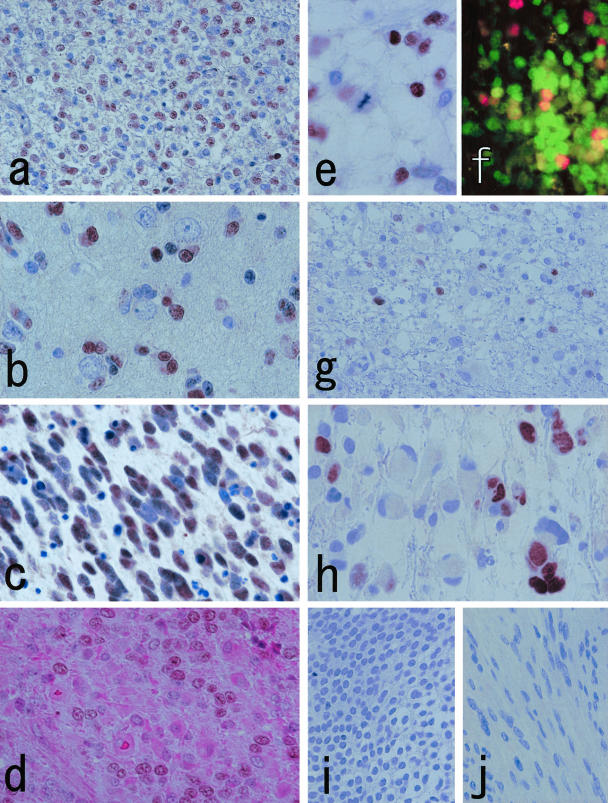
Therefore, further studies were made with Olig2-C. The overall staining results of tumor samples are summarized in Table 1. Immunohistochemistry on oligodendroglial tumors showed similar nuclear staining (Figure 4; a to c). All oligodendroglial tumor cases showed positivity to Olig2-C, whereas preabsorbed Olig2-C illustrated markedly reduced labeling (data not shown). Although the staining intensity differed among cases, neoplastic oligodendroglial cells were, in general, stained equally or more strongly than normal-looking oligodendrocytes, and there was no apparent correlation between the staining intensity and histological grade. It was of note that a few Olig2-C-negative neoplastic cells were admixed within positive areas in every case. Such heterogeneous staining did not seem to be an artifact, because unstained cells were evenly distributed in each specimen. To determine the mechanisms involved, double staining of Olig2-C with H&E was performed in four oligodendroglial tumors, and demonstrated that the astrocytic components with eosinophilic cytoplasm were weakly or not stained by Olig2-C (Figure 4d). We also noted that tumor nuclei in the mitotic phase were almost always Olig2-C-negative (Figure 4e). Double immunostaining with MIB-1 and Olig2-C demonstrated the existence of MIB-1-positive/Olig2-C-negative cells (Figure 4f).
Figure 4.
a and b: Oligodendroglioma cells with perinuclear haloes (a) and those showing perineuronal satellitosis (b) are stained by Olig2-C. Note that not all nuclei are immunostained. c: Olig2-C reaction is noted in anaplastic oligodendroglioma. This specimen is highly mitotic and accompanied by abundant apoptotic bodies. The apoptotic nuclei are Olig2-C-negative. d: Double staining of Olig2-C and H&E on anaplastic oligodendroglioma illustrates that cells with astrocytic differentiation are weakly or not stained by Olig2-C. e: A nucleus under mitotic phase is Olig2-C-negative (anaplastic oligodendroglioma). f: Dual-immunofluorescent staining with MIB-1 (red) and Olig2-C (green) illustrates MIB-1-positive/Olig2-C-negative cells in addition to some double-positive cells (anaplastic oligodendroglioma). g and h: Representative cases of astrocytic tumors (g, fibrillary astrocytoma; h, glioblastoma). Scattered Olig2-C-positive nuclei are noted. The cell bodies possessing labeled nuclei appear vacant or inconspicuous, whereas truly astrocytic cells with rich cytoplasm tend to be Olig2-C-negative. i and j: Central neurocytoma (i) and schwannoma (j) are negative with Olig2-C. Original magnifications: ×250 (a, f, g, i, j); ×500 (b–e, h).
On the other hand, the Olig2 expression in astrocytic tumors varied widely. The signal was typically weak and/or restricted (Figure 4, g and h), and 6 high-grade cases were negative and 12 cases displayed unequivocal (++, +++) nuclear staining. Except pilocytic astrocytomas, truly astrocytic cells with distinct cytoplasm and/or long processes were likely to be less or not stained with Olig2-C (Figure 4, g and h). Significance with regard to staining results was detected between oligodendroglial and astrocytic groups by Mann-Whitney’s U-test (P = 0.001). Neither case of central neurocytoma (Figure 4i) nor schwannoma (Figure 4j) was labeled by Olig2-C. Among systemic tissues, cytoplasmic granular staining was noted in a population of macrophages, renal tubules, and a localized part of the pancreatic ducts (data not shown).
Discussion
Oligodendroglial tumors are well-characterized clinicopathological entities. Their origin is assumed to be mature oligodendrocytes or progenitor cells committed to oligodendroglial differentiation, but this assumption is based solely on circumstantial evidence, namely, the morphological similarity of the neoplasms with their putative origin. To date, there has been no reliable immunohistochemical marker available for the specific and sensitive recognition of human oligodendroglial tumor cells.4,7,8 A number of antigens that are specifically expressed by normal oligodendrocytes have been identified, and applied to oligodendroglial tumors. These include myelin basic protein,20 proteolipid protein,21 myelin-associated glycoprotein,20 and galactocerebroside,21,22 as well as several enzymes such as carbonic anhydrase C.23 However, so far, none of these antigens have been approved as diagnostically useful markers for oligodendroglial tumors. They are either no longer expressed by neoplastic oligodendrocytes, or they are expressed only in a minority of cases, or their expression is not restricted to oligodendroglial tumor cells.4 The question of whether oligodendroglioma is indeed a derivative of oligodendroglia is still a matter of some controversy.
Considerable knowledge on oligodendrocyte development has been accumulated throughout the years about the origins and markers at various developmental stages. Motoneuron and oligodendrocyte lineages are thought to be closely related, and are sequentially generated in the ventral ventricular zone of the embryonic spinal cord during development.24 Olig1 and Olig2 were first identified as basic helix-loop-helix transcription factors that regulate oligodendroglial development.9,10 The expression of Olig1 and Olig2 mostly overlaps, however, Olig2 expression in the early spinal cord is higher than that of Olig1,9,10,25 and Olig2 has a broader expression domain in the embryonic forebrain.25 Olig2-knockout mice cannot survive beyond the neonatal period because of lack of motoneurons and oligodendrocytes,26,27 whereas Olig1-null mice show delayed myelination, otherwise no significant changes, indicating that Olig1 functions in oligodendrocyte maturation.26 Olig1 and Olig2 are also expressed in motoneuron precursors, while the expression is restricted in oligodendrocytes in adults,11 yet the reason is uncertain. To understand the molecular mechanisms, it must be identified as a downstream gene whose promoters are regulated by Olig1/2, and upstream factors that regulate Olig1/2 expression. Apart from these essential problems, we postulated that Olig families would probably be expressed in oligodendrocytes in a different way from previous oligodendroglial markers, and be good immunohistochemical targets. Studies on Olig genes have mostly been performed with chickens and rodents. In recent reports, the expression of human Olig1 and Olig2 was demonstrated in normal and/or neoplastic oligodendrocytes by in situ hybridization,12,13 reverse transcriptase-polymerase chain reaction,28 and immunohistochemistry.29 In our preliminary study by reverse transcriptase-polymerase chain reaction, the Olig2 transcript seemed to be more specific than that of Olig1 in oligodendroglial tumors (data not shown), thus we chose Olig2 as an initial target. Olig2 and Olig1 are functionally similar, and share a highly related basic helix-loop-helix motif. We chose the peptide sequences of N- and C-terminals as Olig2 peptide antigen because the basic helix-loop-helix domain exists in the middle of the protein and these are essentially dissimilar between human Olig1 and Olig2. Furthermore, we performed genome-wide homology analyses of the Olig2 peptide sequences, and confirmed that these sequences were unique. We also demonstrated that Olig2-C did not cross-react with cloned human Olig1 by immunocytochemistry.
Here, we established a new specific antibody targeted to human Olig2, and accomplished successful application to immunoblotting and immunohistochemistry on routinely processed paraffin sections. According to our unpublished data, immunohistochemistry of Olig2 and reverse transcriptase-polymerase chain reaction of Olig2 using RNA extracted from the adjacent paraffin sections showed concordant results. Olig2-C immunostained the nuclei of oligodendrocytes. Nonspecific cytoplasmic staining was observed in a small population of systemic cells, although no cross-reactivity was noted in neural cells. Olig2-N, which was targeted to a different sequence of Olig2, also immunostained oligodendroglial nuclei, although the specificity was not sufficient. We used 40 cases of oligodendroglial tumors for Olig2-C immunohistochemistry, and obtained essentially favorable results. Although some samples had been stored for more than 20 years, they were still suitable for Olig2-C immunostaining, suggesting the reliability and wide application of the antibody. We also obtained fine immunostaining results using frozen sections (data not shown) as well as formalin-fixed paraffin sections shown in this study. Olig2-C heterogeneously immunostained gliomas and further investigation uncovered that it was not because of an artifact (Figure 4; d to f). At least two mechanisms are involved in the phenomenon: one is that tumor cells with astrocytic phenotypes are likely to be less or not immunolabeled by Olig2-C, and the other is that Olig2 expression seems to be cell cycle-dependent. Because Olig2 is a transcription factor involved in oligodendrocyte differentiation, it is possible that the expression is linked to cell cycle exit and/or suppression of astrocytic phenotype. A mutually exclusive pattern of Olig2 and GFAP is also attractive, and the molecular mechanism has been begun to be elucidated.30
Schwann cells are defined as the cells that make and maintain the peripheral nerve sheath and myelin. A transcription factor, Sox10, another regulator of oligodendroglial development,31 is also expressed in Schwann cells,32 implying a shared regulatory pathway of central and peripheral myelination. Here, we demonstrated that schwannoma as well as normal Schwann cells did not express Olig2. Central neurocytoma should primarily be differentiated from oligodendroglial tumors histopathologically. A previous term, “intraventricular oligodendroglioma,” rightly indicates the histological similarity of both.33 In this study, the Olig2 protein expression was not demonstrated, suggesting combined usage of Olig2-C with various neuronal markers is helpful for the diagnosis of central neurocytoma. However, it is possible that those harboring substantial glial elements may give rise to different results, and should be examined further. Combined usage of Olig2-C with various neuronal markers is helpful for the diagnosis of central neurocytoma. Clear cell ependymoma, hemangioblastoma, clear cell meningioma, and a portion of pilocytic astrocytoma can also be listed as differential diagnoses of oligodendroglioma, and will be evaluated in the future.
In this study, a reduced Olig2 expression was demonstrated in astrocytic elements of oligodendroglial tumors. A previous study by in situ hybridization demonstrated that the astrocytic components of oligoastrocytoma did not express the Olig2 transcript,12 and no or very low expression of Olig2 mRNA could be detected in the astrocytic tumors.12,13 We also showed that considerable numbers of astrocytic tumors expressed Olig2, although less intensely than oligodendroglial tumors. Bouvier and colleagues28 described that Olig2 mRNA of pilocytic astrocytomas and glioblastomas was detected 10 of 10 (100%) and 12 of 19 (62%) cases. According to Ohnishi and colleagues,29 the Olig2 expression level in glioblastoma was the lowest among glial tumors examined, These results correspond well with ours. Anyhow, Olig2 staining does not completely prove oligodendroglial character in neoplastic settings, and the reason why astrocytic tumors may express Olig2 remains to be clarified. Previously, we were not able to regard a given cell as a neoplastic oligodendrocyte based solely on individual cellular features. Thus, we always required further structural and/or environmental evidence (perineuronal or perivascular satellitosis, chicken-wire vascular pattern, calcification, and so forth) to diagnose oligodendroglial tumors. Meanwhile, GFAP, an excellent astrocytic marker, allows identification of even an individual astrocyte. This imbalance may cause an underestimation of oligodendroglial components in gliomas. In this study, Olig2-C-positive cells in astrocytic tumors were likely to be devoid of definite cytoplasm, seemingly close to oligodendroglial morphology. Olig2-C may thus uncover oligodendroglial components that are hidden in astrocytic tumors. However, it is undeniable that Olig2 may be expressed aberrantly irrelevant to oligodendroglial differentiation. Pilocytic astrocytomas and a subset of glioblastomas are known to share some markers with oligodendrocyte progenitors such as NG2, PEN5, platelet-derived growth factor receptor α (PDGFRα).34,35 Therefore, comparative study of Olig1/2 with these markers on gliomas is very interesting, and remains to be elucidated. Recently, a new concept, glioblastoma with oligodendroglial components, has been proposed based on accumulated evidence that a subset of glioblastoma includes areas with oligodendroglial features, or a subset of oligodendroglioma acquires the morphological features of glioblastoma during malignant progression.36,37 Steady histopathological analyses may present a clue.
On the other hand, Marie and colleagues12 pointed out that the transcription level of Olig2 was up-regulated in oligodendroglial tumors. This previous finding, together with our results of emphasized Olig2-C immunostaining in neoplastic oligodendrocytes, may suggest that Olig2 participates in the oncogenesis of oligodendrogliomas.38 PDGFRα gene, that acts downstream from Olig genes, is amplified in a subset of anaplastic oligodendrogliomas.39 It may be possible that the overexpression of Olig genes in neoplastic oligodendrocytes up-regulates the proto-oncogene, that eventually contributes to the development of the tumors. In addition to identification of the oligodendrocytes, Olig2-C may be applied for quantification of the Olig2 expression to uncover how and what extent the molecule involves in glial oncogenesis. The staining intensity of each cell could be comparable within the same section, so we concluded that the oligodendroglial tumor cells expressed Olig2 stronger than normal oligodendrocytes or neoplastic astrocytes in each case. Our results are basically in accord with the previous findings,12,13,28,29 however, the relative comparison between different cases is difficult because the intensity can be influenced not only by net signal expression, but also fixation or storage conditions. Thus, it is desirable to use more precise methods to quantify Olig2 expression. One possibility is that tissue microarray technology will contribute to this purpose.
Further basic analyses are still required, however, the establishment of a novel antibody to identify oligodendrocytes is undoubtedly significant. In addition to neoplasms, a number of other diseases that involve oligodendrocytes are also known, such as neurodegenerative and metabolic disorders. Immunohistochemical detection of oligodendrocytes with Olig2-C is expected to contribute significantly to neuropathology.
Acknowledgments
We thank Dr. Kenji F. Tanaka, Division of Neurobiology and Bioinformatics, National Institute for Physiological Sciences, for technical assistance in transfection experiments.
Footnotes
Address reprint requests to Hideaki Yokoo, M.D., Department of Pathology, Gunma University Graduate School of Medicine, 3-39-22 Showa-machi, Maebashi, Gunma, 371-8511, Japan. E-mail: yokoo@med.gunma-u.ac.jp.
Supported in part by the Japanese Ministry of Education, Culture, Sports, Science, and Technology [grant-in-aid for scientific research (B) no.15300113].
References
- Szuchet S. The morphology and ultrastructure of oligodendrocytes and their functional implications. Kettenmann H, Ransom BR, editors. New York: Oxford University Press; Neuroglia. 1995:pp 23–43. [Google Scholar]
- Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol. 1994;145:1175–1190. [PMC free article] [PubMed] [Google Scholar]
- Kraus JA, Koopmann J, Kaskel P, Maintz D, Brandner S, Schramm J, Louis DN, Wiestler OD, von Deimling A. Shared allelic losses on chromosomes 1p and 19q suggest a common origin of oligodendroglioma and oligoastrocytoma. J Neuropathol Exp Neurol. 1995;54:91–95. doi: 10.1097/00005072-199501000-00011. [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Kros JM, Burger PC, Louis DN, Collins VP. Oligodendroglial tumours. Pathology and genetics. Kleihues P, Cavenee WK, editors. Lyon: IARC Press; Tumours of the Nervous System. 2000:pp 55–70. [Google Scholar]
- Cairncross JG, Macdonald DR. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann Neurol. 1988;23:360–364. doi: 10.1002/ana.410230408. [DOI] [PubMed] [Google Scholar]
- Cairncross JG, Macdonald DR, Ramsay DA. Aggressive oligodendroglioma: a chemosensitive tumor. Neurosurgery. 1992;31:78–82. doi: 10.1227/00006123-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Lantos PL, VandenBerg SR, Kleihues P. Oligodendroglial tumours. Graham DI, Lantos PL, editors. London: Arnold; 1997:pp 627–636. [Google Scholar]
- McLendon RE, Enterline DS, Tien RD, Thorstad WL, Bruner JM. Oligodendrogliomas. Tumors of central neuroepithelial origin. Binger DD, McLendon RE, Bruner JM, editors. London: Arnold; Russell and Rubinstein’s Pathology of Tumors of the Nervous System. (ed 4) 1998:pp 370–387. [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Wegner M. Expression of transcription factors during oligodendroglial development. Microsc Res Tech. 2001;15:746–752. doi: 10.1002/jemt.1059. [DOI] [PubMed] [Google Scholar]
- Marie Y, Sanson M, Mokhtari K, Leuraud P, Kujas M, Delattre JY, Poirier J, Zalc B, Hoang-Xuan K. OLIG2 as a specific marker of oligodendroglial tumour cells. Lancet. 2001;358:298–300. doi: 10.1016/S0140-6736(01)05499-X. [DOI] [PubMed] [Google Scholar]
- Lu QR, Park JK, Noll E, Chan JA, Alberta J, Yuk D, Alzamora MG, Louis DN, Stiles CD, Rowitch DH, Black PM. Oligodendrocyte lineage genes (OLIG) as molecular markers for human glial brain tumors. Proc Natl Acad Sci USA. 2001;98:10851–10856. doi: 10.1073/pnas.181340798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Sugimori M, Takebayashi H, Kosako H, Nagao M, Yoshida S, Nabeshima Y, Shimamura K, Nakafuku M. Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron. 2001;31:757–771. doi: 10.1016/s0896-6273(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Nakazato Y, Ishizeki J, Takahashi K, Yamaguchi H, Kamei T, Mori T. Localization of S-100 protein and glial fibrillary acidic protein-related antigen in pleomorphic adenoma of the salivary glands. Lab Invest. 1982;46:621–626. [PubMed] [Google Scholar]
- Yokoo H, Isoda K, Sakura M, Sasaki A, Hirato J, Nakazato Y. A novel monoclonal antibody that recognizes human perivascular cells of the central nervous system under a specific immune reaction. Neuropathology. 2000;20:216–220. doi: 10.1046/j.1440-1789.2000.00345.x. [DOI] [PubMed] [Google Scholar]
- Nakazato Y, Sasaki A, Hirato J, Ishida Y. Immunohistochemical localization of neurofilament protein in neuronal degenerations. Acta Neuropathol. 1984;64:30–36. doi: 10.1007/BF00695603. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Perentes E, Rubinstein LJ. Immunohistochemical characterization of oligodendrogliomas: an analysis of multiple markers. Acta Neuropathol. 1986;72:15–22. doi: 10.1007/BF00687942. [DOI] [PubMed] [Google Scholar]
- Sung CC, Collins R, Li J, Pearl DK, Coons SW, Scheithauer BW, Johnson PC, Yates AJ. Glycolipids and myelin proteins in human oligodendrogliomas. Glycoconj J. 1996;13:433–443. doi: 10.1007/BF00731476. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Watkins BA, Thomas DG, Noble MD. Antigenic expression by cells derived from human gliomas does not correlate with morphological classification. Neuropathol Appl Neurobiol. 1987;13:327–347. doi: 10.1111/j.1365-2990.1987.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Perentes E, Rubinstein LJ. Non-specificity of anti-carbonic anhydrase C antibody as a marker in human neurooncology. J Neuropathol Exp Neurol. 1987;46:451–460. doi: 10.1097/00005072-198707000-00004. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Smith HK, Sun T, Pringle NP, Hall A, Woodruff R. Oligodendrocyte lineage and the motor neuron connection. Glia. 2000;29:136–142. doi: 10.1002/(sici)1098-1136(20000115)29:2<136::aid-glia6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev. 2000;99:143–148. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Bouvier C, Bartoli C, Aguirre-Cruz L, Virard I, Colin C, Fernandez C, Gouvernet J, Figarella-Branger D. Shared oligodendrocyte lineage gene expression in gliomas and oligodendrocyte progenitor cells. J Neurosurg. 2003;99:344–350. doi: 10.3171/jns.2003.99.2.0344. [DOI] [PubMed] [Google Scholar]
- Ohnishi A, Sawa H, Tsuda M, Sawamura Y, Itoh T, Iwasaki Y, Nagashima K. Expression of the oligodendroglial lineage-associated markers Olig1 and Olig2 in different types of human gliomas. J Neuropathol Exp Neurol. 2003;62:1052–1059. doi: 10.1093/jnen/62.10.1052. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kondo T, Takebayashi H, Taga T. Negative regulatory effect of an oligodendrocytic bHLH factor OLIG2 on the astrocytic differentiation pathway. Cell Death Differ. 2004;11:196–202. doi: 10.1038/sj.cdd.4401332. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figarella-Branger D, Söylemezoglu, Kleihues P, Hassoun J. Central neurocytoma. Pathology and genetics. Kleihues P, Cavenee WK, editors. Lyon: IARC Press; Tumours of the Nervous System. 2000:pp 107–109. [Google Scholar]
- Figarella-Branger D, Daniel L, André P, Guia S, Renaud W, Monti G, Vivier E, Rougon G. The PEN5 epitope identifies an oligodendrocyte precursor cell population and pilocytic astrocytomas. Am J Pathol. 1999;155:1261–1269. doi: 10.1016/S0002-9440(10)65228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan Y, Nishiyama A, Chang A, Mork S, Barnett GH, Cowell JK, Trapp BD, Staugaitis SM. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci USA. 1999;96:10361–10366. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Mokhtari K, Sanson M, Marie Y, Kujas M, Huguet S, Leuraud P, Capelle L, Delattre JY, Poirier J, Hoang-Xuan K. Glioblastomas with an oligodendroglial component: a pathological and molecular study. J Neuropathol Exp Neurol. 2001;60:863–871. doi: 10.1093/jnen/60.9.863. [DOI] [PubMed] [Google Scholar]
- Kraus JA, Lamszus K, Glesmann N, Beck M, Wolter M, Sabel M, Krex D, Klockgether T, Reifenberger G, Schlegel U. Molecular genetic alterations in glioblastomas with oligodendroglial component. Acta Neuropathol. 2001;101:311–320. doi: 10.1007/s004010000258. [DOI] [PubMed] [Google Scholar]
- Hoang-Xuan K, Aguirre-Cruz L, Mokhtari K, Marie Y, Sanson M. OLIG-1 and 2 gene expression and oligodendroglial tumours. Neuropathol Appl Neurobiol. 2002;28:89–94. doi: 10.1046/j.1365-2990.2002.00395.x. [DOI] [PubMed] [Google Scholar]
- Smith JS, Wang XY, Qian J, Hosek SM, Scheithauer BW, Jenkins RB, James CD. Amplification of the platelet-derived growth factor receptor-A (PDGFRA) gene occurs in oligodendrogliomas with grade IV anaplastic features. J Neuropathol Exp Neurol. 2000;59:495–503. doi: 10.1093/jnen/59.6.495. [DOI] [PubMed] [Google Scholar]