Abstract
Exosomes are small membrane vesicles secreted into the extracellular compartment by exocytosis. Tumor exosomes may be involved in the sampling of antigens to antigen presenting cells or as decoys allowing the tumor to escape immune-directed destruction. The proteins present in exosomes secreted by tumor cells have been poorly defined. This study describes the protein composition of mesothelioma cell-derived exosomes in more detail. After electrophoresis of exosome preparations, matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) was used to characterize the protein spots. MHC class I was found to be present together with the heat shock proteins HSC70 and HSP90. In addition, we found annexins and PV-1, proteins involved in membrane transport and function. Cytoskeleton proteins and their associated proteins ezrin, moesin, actinin-4, desmoplakin, and fascin were also detected. Besides the molecular motor kinesin-like protein, many enzymes were detected revealing the cytoplasmic orientation of exosomes. Most interesting was the detection of developmental endothelial locus-1 (DEL-1), which can act as a strong angiogenic factor and can increase the vascular development in the neighborhood of the tumor. In conclusion, mesothelioma cells release exosomes that express a discrete set of proteins involved in antigen presentation, signal transduction, migration, and adhesion. Exosomes may play an important role in the interaction between tumor cells and their environment.
Like most cells of hematopoietic origin, tumor cells secrete exosome-like vesicles. These subcellular membrane vesicles from endosomal origin are secreted on fusion of multi-vesicular bodies with the plasma membrane.1,2 As a consequence, exosomes have a “cellular” membrane orientation with a limited range of proteins derived from the cytosol, endocytic compartment membranes, and plasma membranes.3 They are 60 to 110 nm in diameter, and may be involved in the communication between cells. Exosomes from a murine dendritic cell (DC) line D1 are best characterized for protein composition.4,5 Proteins expressed on these DC-derived exosomes are involved in the regulation of basic processes like signal transduction, adhesion, activation, and migration. In addition, MHC-I and MHC-II, proteins normally involved in antigen presentation, are expressed on DC-derived exosomes.
Although DC-derived exosomes are able to activate cytotoxic T cells and to elicit potent anti-tumor immune responses,4 the function of tumor cell-derived exosomes is unknown. They may serve as decoys by allowing the tumor to escape immune-directed destruction or for sampling antigens to DC. Wolfers et al6 demonstrated that tumor-derived exosomes are capable of transferring tumor antigens to DC, inducing a CD8+ T-cell-dependent cross-immunization of tumor-bearing mice. These exosomes seem to concentrate a set of whole native shared tumor antigens opening the possibility that exosomes could be used as a source of antigen in vaccination protocols.6,7 Proteomics offers the possibility to understand more about human tumor-derived exosomes and these organelles may, like DC-derived exosomes, give new perspectives to improve the diagnosis and therapy of cancer patients.8–10
Malignant mesothelioma (MM) is a tumor of mesodermally derived tissue lining the coelomic cavities with no satisfactory curative treatment.11 This tumor was chosen as a model system to study the characteristics of tumor-derived exosomes because only a small amount of data are available on tumor antigens in this tumor.
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry was used for the proteomic analysis of exosomes derived from well-characterized mesothelioma cell lines. The focus of this article will be on the proteins present in tumor exosomes.
Materials and Methods
Establishment of Human Mesothelioma Cell Lines
Mesothelioma cell lines have been derived from pleural effusions or primary solid tumor biopsy material. After informed consent, patient material was collected under sterile conditions and transported immediately to the laboratory. Solid tissue was minced into small pieces with sterile scissors and gently pressed through a 100-μm mesh cell strainer (Falcon/Becton Dickinson Labware, Franklin Lakes, NJ) with a syringe piston. Dispersed cells and clumps were washed through gauze with HBBS (GIBCO/Invitrogen, Breda, The Netherlands), and the suspension was transferred to a second finer (40-μm mesh) gauze (Falcon/Becton Dickinson Labware). Suspension was centrifuged at 400 × g for 15 minutes at room temperature (RT) and cells placed into culture flasks (Falcon/Becton Dickinson Labware). Pleural effusions were centrifuged 400 × g for 15 minutes and cells were placed into culture flasks. Cells were cultured at 37°C in RPMI 1640 medium containing 25 mmol/L HEPES, Glutamax, 50 μg/ml gentamicin, and 10% (v/v) fetal bovine serum (FBS) (all obtained from GIBCO/Invitrogen) in a humidified atmosphere of 5% CO2, in air. Media were changed once or twice a week and when flasks were confluent, then cells were passaged to a new flask by treatment with 0.05% trypsin and 0.53 mmol/L EDTA in phosphate-buffered saline (PBS, all from GIBCO/Invitrogen). Two cell lines (PMR-MM7 and PMR-MM8) were extensively characterized and kept in long-term cell culture (>50 passages, 6 months of culturing) while using for exosome isolation.
Characterization of Cell Lines
Cellular DNA Content
Cell lines were characterized for cellular DNA content by propidium iodide. In short, cells were trypsinized and washed twice in 0.1% (w/v) glucose (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands) in PBS. A pellet containing 1 × 106 cells was resuspended by slowly adding 1 ml of ice-cold 70% ethanol under vigorous vortexing. The suspension was fixed overnight at 4°C and the next day the pellet was resuspended in 1 ml 0.1% (w/v) glucose in PBS supplemented with 50 μg/ml propidium iodide (Sigma-Aldrich Chemie BV) and 100 Kunitz units RNAseA (Amersham Pharmacia Biotech, Essex, UK) and incubated for 60 minutes at RT. Flow cytometric analysis of nuclear DNA content was performed on a FACSCalibur (BD Immunocytometry Systems, Erembodegem, Belgium).
Immunohistochemical Studies
Cytocentrifuge preparations were stained using the rabbit-anti-mouse (RαM) and APAAP method for the following mouse antibody clones: 5B5 (anti-prolyl 4-hydroxylase (collagen synthesis)), E29 (anti-epithelial membrane antigen), II-7 (anti-carcinoembryonic antigen), HBME-1 (anti-mesothelial cell), Ber-EP4 (epithelial antigen), RCK108 (anti-cytokeratin 19) (all antibodies were obtained from DAKO, Glostrup, Denmark). Appropriate positive controls were used in each case. Specificity of the primary and secondary antibodies was checked by using protein concentration and isotype-matched non-relevant monoclonal antibodies and PBS. Naphtol-AS-MX-phosphate (0.30 mg/ml, Sigma-Aldrich Chemie BV) and new fuchsine (160 mg/ml in 2 mol/L HCl, Chroma-Gesellschaft, Kongen, Germany) were used as substrate for alkaline phosphatase (AP). Levamisol (0.25 mg/ml, Sigma-Aldrich Chemie BV) was added to block endogenous AP activity. Finally, sections were counter-stained with Mayer’s hematoxylin (Merck, Darmstadt, Germany) and mounted in Kaiser’s glycerol-gelatin (Merck).
Tumorigenicity in Vitro and in Immune-Deficient Mice
The tumorigenicity of cell lines was determined by their capacity of forming colonies in semi-solid media.12 An agarose underlay was prepared by adding 1 ml autoclaved 0.8% (w/v) agarose (GIBCO/Invitrogen) in PBS to a 6-well plate per well and allowed to gel for 30 minutes. Cells were collected by trypsinization and adjusted to a concentration of 5 × 104 cells per 3 ml RPMI 1640 medium containing HEPES, Glutamax, gentamicin, and 10% FBS. The cells were diluted in 3 ml StemPro 2.3% methylcellulose (GIBCO/Invitrogen), and the tube was vigorously vortexed until the cells were uniformly suspended. After 10 minutes of allowing the air bubbles to rise, the suspension was added to the agarose underlay and incubated in a humidified incubator at 37°C for 14 days or until colonies were formed. The tumor-forming capacity of the cell lines was also tested in athymic nude mice. Monolayer cells were harvested by trypsinization, and 2 × 106 cells suspended in 0.2 ml of PBS were injected subcutaneously into 4- to 6-week-old BALB/c athymic nu/nu mice (Jackson Laboratory, Bar Harbor, ME). Mice were maintained in sterile-air laminar flow cage racks and examined regularly for tumor development for at least 2 months following the injection.
Virus Contamination, HLA Typing, and Karyotyping
Contamination of the cell lines with HCV, HBV, and HIV viruses was analyzed with (quantitative) polymerase chain reaction at the virology laboratory of the Erasmus MC-Dijkzigt according to World Health Organization references. The Department of Immunohematology and Blood Transfusion of the Leiden University Medical Center performed HLA typing. Karyotyping was carried out in the Department of Clinical Genetics of the Erasmus MC.
Isolation of Mesothelioma-Derived Exosomes
Mesothelioma cell lines at 80% confluency were washed twice with PBS and incubated in RPMI medium (containing HEPES, Glutamax, and gentamicin) and the serum replacer TCH (1X working strength [ICN, Irvine, CA]) for 48 hours in a humidified atmosphere of 5% CO2, 95% air. Cell culture supernatants were subjected to three successive centrifugations to remove cells and debris: 300 × g for 10 minutes, 2000 × g for 20 minutes, and finally at 10,000 × g for 30 minutes, all at 4°C. Exosomes were then pelleted at 64,000 × g for 100 minutes using a SW28 rotor (Beckman Coulter Instruments, Fullerton, CA). Pellets were resuspended and washed in PBS and centrifuged at 100,000 × g for 1 hour (SW60 rotor, Beckman Coulter Instruments). Exosomes were resuspended in PBS, aliquoted, and stored at −80°C. The quantification of exosomal proteins recovered was measured by CBQCA kit according to the manufacturer’s recommendations (Molecular Probes, Leiden, The Netherlands). In the presence of cyanide, the ATTO-TAG CBQCA reagent reacts with the primary amides found on proteins and functions well in the presence of lipids and detergents. The fluorescence emission was measured at ∼550 nm (filter 530 ± 30 nm) with excitation at ∼465 nm (filter 485 ± 20 nm) in a CytoFluor 4000 fluorescence microplate reader (gain 40) (PerSeptive Biosystems, Foster City, CA).
Electron Microscopy
Exosomes obtained after centrifugation of cell-culture supernatants were loaded onto Formvar carbon-coated grids. Adsorbed exosomes were fixed in 2% paraformaldehyde and immunolabeled with CLB-gran1/2, 435 (anti-CD63; CLB, Amsterdam, The Netherlands) and 10 nm protein A gold particles.
Protein Electrophoresis
One-dimensional electrophoresis of mesothelioma-derived exosomes onto 10% SDS-PAGE gels was performed according to manufacturer’s recommendations (PROTEAN II xi Cell, BioRad Laboratories, Hemel Hempstead, UK). Samples were taken-up in 8 mol/L urea (Sigma-Aldrich Chemie BV), 2% CHAPS (Amersham Pharmacia Biotech), 20 mmol/L dithiothreitol (DTT, Sigma-Aldrich Chemie BV), 0.01% bromophenol blue (Sigma-Aldrich Chemie BV), and transferred onto a 1.0-mm thick 10% SDS-PAGE gel. A constant current of 7 mA per gel at 10°C was applied. After 16 hours, gels were stained with Novex Colloidal blue staining kit according to the manufacturer’s instructions (Invitrogen).
Enzymatic Digestion of Protein Spots
Colloidal blue stained protein spots were excised manually with a plastic plunger and transferred onto a 96-well low protein binding microtiter plate (Nunc A/S, Roskide, Denmark). Each excised plug was washed with 100 μl milli-Q for 5 minutes with shaking (650 rpm, Eppendorf Geratebau GmbH, Hamburg, Germany). Gel plugs were de-stained with 0.4% (w/v) ammonium hydrogen carbonate (Sigma-Aldrich Chemie BV), 30% acetonitrile in water by incubating two times for 20 minutes at RT. After a short wash with Milli-Q, gel spots were dried in a rotary evaporator (Savant, Farmingdale, NY) for 30 minutes. Protein digestion was performed with the addition of 4 μl of 100 μg/ml sequencing grade-modified trypsin (Promega, Madison, WI) to each gel piece. The plate was sealed with an adhesive aluminum foil and incubated overnight at RT.
MALDI-TOF Analysis of Peptides
After the specific hydrolysis at the carboxylic sides of lysine and arginine residues by trypsin, 7 μl (1:2) acetonitrile:0.1% trifluoroacetic acid was added to the gel plugs. After mixing, 1 μl of the tryptic digest was taken and co-crystallized with 2.5 μl 2 mg/ml of the photoactive compound α-cyano-4-hydroxy-trans-cinnamic acid (α-HCCA, Bruker Daltonics, Billerica, MA) in acetonitrile. This sample-matrix solution (0.5 μl) was pipetted onto a 400-μm 384-well anchor chip MALDI-TOF plate and air-dried for 5 minutes. Peptide mass spectra were acquired on a Biflex III MALDI-TOF mass spectrometer equipped with a 337-nm nitrogen laser (Bruker Daltonics, Bremen, Germany). The instrument was calibrated with a peptide calibration standard (Bruker Daltonics). Spectra were compared using autolytic fragments from trypsin. A mass list of peptides was obtained from each digest and submitted to Matrix Science Mascot UK software to identify the proteins in the MSDB database of the NCBI. The criteria for identification of proteins were determined as follows: maximum allowed peptide mass error of 200 ppm, at least five matching peptide masses, molecular weight of identified protein should match estimated values by comparing with marker proteins, and top scores given by software higher than 61 (P < 0.05).
Western Blotting
For Western blotting following one-dimensional SDS-PAGE, proteins were electroblotted onto Immobilon P membranes (Millipore Corp, Etten-Leur, The Netherlands) and incubated with specific antibodies, followed by horseradish peroxidase-conjugated secondary antibodies, and detected using SuperSignal West Pico chemiluminescent substrate (Pierce Perbio Science, Etten-Leur, The Netherlands). Antibodies used in this study to confirm the proteins detected by MALDI-TOF were: anti-HSC70 (clone 13D3; Affinity BioReagents, Golden, CO), anti-HSP90 (clone AC88; Stressgen, Victoria, Canada), anti-fascin (clone FCN01, Abcam Ltd, Cambridge, UK), and anti-β-tubulin (clone E7, Developmental Studies Hybridoma Bank, Iowa City, IA).
Results
Establishment and Characterization of Human Mesothelioma Cell Lines
Since 1997, the Department of Pulmonary Medicine Rotterdam (PMR) has established 10 continuously growing cell lines originally initiated from pleural effusions from patients diagnosed as malignant mesothelioma (MM). One cell line was derived from a postmortem pleural biopsy. The 10 patients from whom cell lines were derived were all males ranging in age from 45 to 79 years (mean, 61 years). Two mesothelioma cell lines, PMR-MM7 and PMR-MM8, were characterized as summarized in Table 1.
Table 1.
Characteristics of the Mesothelioma Cell Lines PMR-MM7 and PMR-MM8
| PMR-MM7 | PMR-MM8 | |
|---|---|---|
| Patient | ||
| Gender | Male (Caucasian) | Male (Caucasian) |
| Age | 63 | 57 |
| Cellular DNA content | Aneuploid | Diploid |
| Immunohistochemistry | ||
| 5B5 | Positive | Positive |
| II-7 (CEA) | Negative | Negative |
| HBME-1 | Negative | Negative |
| Ber-EP4 | Negative | Negative |
| RCK108 | Negative | Positive |
| Tumorigenicity | Yes | Yes |
| Virus contamination | HCV, HBV, HIV negative | HCV, HBV, HIV negative |
| Bacterial contamination | No | No |
| Karyotyping | 71∼78<3>,XY,X,+Y,del(1p),add (2p),add(2p),?add(3q),del(5q),+del(5q),+del(5q),der(6)t(6;7),der(6)t(6;7),del(7p),−7,−7,add(8q),add(9p),add(9p),del(9p),del(10p),+11,+11,+der(12),t(2;12),add(13p),−14,−15,add(17q),add(19q),+20,+add(20q),+21?,22?,+mar1,+mar2,+mar3 | 40∼41,add(Xq),−Y,add(1p),−1,add(2q),der(3)t(2;3),−4,add(5q),?5,del(6p),del(6q),inv(7),−8,add(10p),del(10q),del(11q),+add(12q),−13,−13,der(14p)t(14;15),der(15)t(8;15),der(16)t(14;16),−19,22? |
| HLA typing | A*02,A*68,A*28,B*27,B*40,Bw*04,Bw*06,Cw*0304,Cw*03Cw*0704,Cw*07 | A*01,B*39,B*16,Bw*06,Cw*0501,Cw*05 |
Based on these characteristics and by judgment of the Dutch mesothelioma expert panel these cell lines were regarded as true mesothelioma and were differentiated from pleural metastasis of adenocarcinoma. Furthermore, the cells were free from bacterial and viral contaminants, excluding the possibility of viral and bacterial proteins in the exosome preparation.
Isolation and Characterization of Mesothelioma-Derived Exosomes
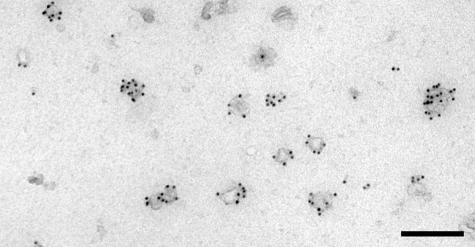
Exosomes from seven mesothelioma cell lines were collected from 80% confluent cultures after culturing for 48 hours in medium supplemented with a serum replacer. Exosomes were purified by successive (ultra)-centrifugation steps. Initial experiments with medium containing fetal bovine serum deprived of cells pelleted also protein components from the serum. Medium containing the serum replacer TCH gave no protein contamination in the exosome preparation (data not shown). Typically, 15 to 50 μg proteins were isolated from 25-ml culture medium after 48 hours of incubation with an 80% confluent layer of mesothelioma cells (175-cm2 flask). Protein content was based on the CBQCA quantitation kit because it functions well in the presence of lipids and can be used directly to determine the amount of proteins in lipid-protein samples. Electron microscopically, the extracellular particles isolated from the culture supernatant after removal of cells by centrifugation consisted of membrane vesicles as shown in Figure 1. Cellular debris was rarely found.
Figure 1.
Electron micrograph of mesothelioma cell line PMR-MM7-derived exosomes, showing cup-shaped membrane vesicles. Exosomes were fixed in 2% formaldehyde and immunolabeled for CD63 as described in the Materials and Methods section (bar, 200 nm). Similar results were obtained with the PMR-MM8-derived exosome preparation (not shown).
Proteomic Analysis
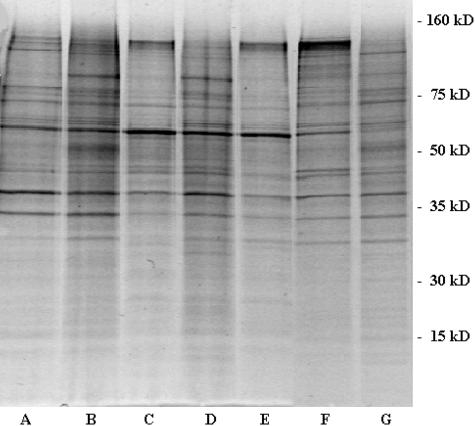
The protein composition of exosomes isolated from seven different mesothelioma cell lines was determined by electrophoretic separation onto a 10% SDS-PAGE gel (Figure 2). Because protein bands showed similar patterns between the different exosome preparations, two cell lines were depicted to characterize all distinct bands by MALDI-TOF mass spectrometry. Therefore, 50 μg of exosomes derived from PMR-MM7 cells and PMR-MM8 cells were loaded onto a 10% SDS-PAGE gel (Figure 3). All distinct bands were subjected to MALDI-TOF analysis. As mentioned in the Materials and Methods section, criteria for positive protein identification were set as follows: maximum allowed peptide mass error of 200 ppm, at least five matching peptide masses, molecular weight of identified protein should match estimated values, and top score given by software. Results are presented in Table 2. The first column corresponds to the numbers on the SDS-PAGE gel followed by a description from which cell lines the exosomes were derived. Protein names, accession numbers, and calculated molecular weights were deduced from the mass fingerprint analysis in the MSDB database of the NCBI. Observed molecular weights were measured by interpolation by image analysis software with the molecular weight curve obtained from the molecular weight marker proteins run as a separate track on the gel. SDS-PAGE allows only an estimation of the mass of a protein. Differences between the calculated molecular weight and observed molecular weight can be caused by excessive post-translational modifications, which were not predicted in the theoretical digestion of the proteins in the database as well as precluding peptides from the fingerprint. The last column corresponds to the score given by the Matrix Science Mascot UK software analysis, which was significant (P < 0.05) when higher than 61.
Figure 2.
Separation of mesothelioma cell-derived exosomal proteins on 10% SDS-PAGE and stained by colloidal blue. Lanes A to G represent the different mesothelioma cell lines, PMR-MM1, PMR-MM3, PMR-MM5, PMR-MM7, PMR-MM8, PMR-MM9, and PMR-MM10, respectively.
Figure 3.
Exosomes derived from the mesothelioma cell lines PMR-MM7 (B) and PMR-MM8 (C) after electrophoresis in a denaturing polyacrylamide gel (A, broad range marker in kilodaltons (kd)). Numbers correspond to the excised protein bands (see Table 2).
Table 2.
Identified Exosomal Proteins Secreted by Mesothelioma Cells (Ordered by Observed Molecular Weight)
| No. | PMR MM | Protein | Accession number | Calculated mol wt | Observed mr | Peptides | Coverage | Top score* |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | desmoplakin I | A38194 | 309.797 | >272 | 36 | 11% | 116 |
| 2 | 8 | fibronectin precursor | CAA26536 | 256.529 | 228 | 31 | 15% | 148 |
| 3 | 8 | myosin | CAB05105 | 226.392 | 205 | 29 | 13% | 97 |
| 4 | 8 | putative P150 | O00378 | 148.786 | 174 | 15 | 10% | 72 |
| 5 | 7 | integrin alpha-3 chain precursor | A40021 | 116.538 | 136 | 15 | 17% | 69 |
| 6 | 8 | hypothetical protein fragment | Q9HAj5 | 71.444 | 115 | 17 | 15% | 87 |
| 7 | 8 | epithelial microtubule-associated protein | I37356 | 84.002 | 115 | 16 | 13% | 68 |
| 8 | 7 | actinin-4 | BAA24447 | 102.204 | 106 | 12 | 11% | 67 |
| 9 | 7 | heat shock protein 90-alpha | HS9A_HUMAN | 84.490 | 95 | 8 | 21% | 112 |
| 10 | 8 | heat shock protein 90 | AAA36026 | 83.212 | 93 | 24 | 27% | 197 |
| 11 | 7 | ezrin | Q96CU8 | 69.370 | 84 | 16 | 25% | 134 |
| 12 | 8 | ezrin | EZRI_HUMAN | 69.225 | 81 | 14 | 20% | 125 |
| 13 | 7 | moesin | MOES_HUMAN | 67.647 | 79 | 9 | 13% | 75 |
| 14 | 7 | albumin | 1A06A | 65.695 | 74 | 15 | 14% | 107 |
| 15 | 7 | annexin VI | ANX6_HUMAN | 75.695 | 74 | 14 | 16% | 75 |
| 16 | 8 | moesin | MOES_HUMAN | 67.647 | 73 | 17 | 24% | 161 |
| 17 | 8 | heat shock cognate protein 70 | A27077 | 70.854 | 70 | 12 | 17% | 85 |
| 18 | 7 | pyruvate kinase | KPY1_HUMAN | 57.710 | 63 | 7 | 9% | 87 |
| 19 | 8 | integrin-binding protein DEL1 precursor | O43854 | 53.730 | 60 | 12 | 20% | 102 |
| 20 | 7 | beta-tubulin | I38369 | 48.848 | 60 | 7 | 13% | 71 |
| 21 | 8 | fascin | FSC1_HUMAN | 54.365 | 60 | 8 | 15% | 72 |
| 22 | 8 | kinesin-like protein 2 | Q9NS87 | 160.061 | 57 | 12 | 7% | 70 |
| 23 | 7 | enolase alpha | ENOA_HUMAN | 47.008 | 52 | 8 | 17% | 65 |
| 24 | 8 | PV1 protein | Q9BX97 | 50.562 | 50 | 8 | 16% | 65 |
| 25 | 8 | protein kinase | A38643 | 53.676 | 50 | 11 | 21% | 62 |
| 26 | 8 | translation initiation factor | FIMS4A | 46.125 | 50 | 12 | 31% | 77 |
| 27 | 7 | actin | AAH08633 | 40.978 | 49 | 12 | 29% | 111 |
| 28 | 8 | actin | AAH08633 | 40.978 | 47 | 12 | 36% | 126 |
| 29 | 8 | 2′3′-cyclic-nucleotide 3′-phosphodiesterase | BAA02435 | 45.070 | 45 | 11 | 28% | 115 |
| 30 | 8 | MHC class I HLA-B | I68774 | 31.669 | 45 | 9 | 39% | 103 |
| 31 | 8 | MHC class I antigen (fragment) | Q9TP25 | 21.011 | 45 | 8 | 44% | 67 |
| 32 | 7 | glyceraldehyde 3-phosphate dehydrogenase | CAA25833 | 36.031 | 41 | 8 | 23% | 89 |
| 33 | 8 | glyceraldehyde 3-phosphate dehydrogenase | G3P2_HUMAN | 35.899 | 39 | 7 | 14% | 75 |
| 34 | 8 | annexin I | 1AIN | 35.018 | 39 | 8 | 24% | 92 |
| 35 | 7 | annexin II | ANX2_HUMAN | 38.449 | 39 | 21 | 52% | 251 |
| 36 | 8 | annexin II | ANX2_HUMAN | 38.449 | 37 | 13 | 31% | 139 |
| 37 | 7 | annexin V | 1HVE | 35.068 | 36 | 9 | 26% | 88 |
| 38 | 8 | annexin V | 1HVE | 35.068 | 35 | 7 | 22% | 64 |
Top scores higher than 61 were significant (p < 0.05).
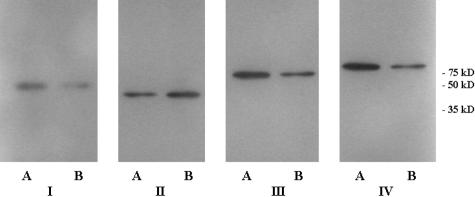
Analysis of the exosomes by Western blot (Figure 4) confirmed the presence of the proteins detected by MALDI-TOF mass spectrometry. Antibodies against fascin, β-tubulin, HSC70, and HSP90 could be visualized by Western blot. As an illustration that some proteins were still present in both cell lines, even when only one gave a statistically significant result using our strict criteria for the MALDI-TOF technique, HSP70, fascin, and β-tubulin proteins were detected in one cell line by MALDI-TOF, whereas they were demonstrated in both cell lines using Western blotting.
Figure 4.
The presence of fascin (I), β-tubulin (II), HSC70 (III), and HSP90 (IV) were confirmed by Western blots of PMR-MM7-derived exosomes (A) and PMR-MM8-derived exosomes (B). The primary antibodies anti-fascin (1:1000), anti-β-tubulin (1:10.000), and anti-HSP90 (1:2000) were followed by horseradish peroxidase-conjugated goat anti-mouse IgG1. Anti-HSC70 (1:1000) was followed by Envision (DAKO). Blots were incubated with SuperSignal West Pico chemiluminescent substrate and exposed to Hyperfilm ECL (Amersham Biosciences, Buckinghamshire, England).
Discussion
Tumor exosomes are poorly defined. In contrast to dendritic cell-derived exosomes, no studies have described an extensive protein characterization of tumor exosomes. Initial exosome isolations from pleural effusion turned out to be troublesome, caused by high amounts of immunoglobulins and complement factors present in the fluid.13 Exosomes in pleural effusions will not only be secreted by mesothelioma cells, but also by mesothelial cells or from cells of hematopoietic origin present in the fluid. Therefore, mesothelioma cell line-derived exosomes were studied for their protein content. The seven mesothelioma cell lines described in this study secrete exosomes into their environment. Exosome-like vesicles were isolated from the culture supernatant after 48 hours of secretion by the cell lines through successive centrifugation steps. Electron microscopy showed 60 to 150 nm diameter vesicles. After one-dimensional electrophoresis of these exosome preparations, protein spots from two mesothelioma cell lines were analyzed by MALDI-TOF mass spectrometry.
Earlier studies showed that exosomes derived from mouse tumors concentrate tumor antigens and contain MHC class I molecules loaded with tumor peptides.6 Similarly, MHC class I was found to be present on human tumor-derived exosomes that may be involved in the presentation of polypeptide fragments of antigens to T cells. Heat shock proteins (HSP) are a group of common proteins that play a role in the cell’s response to elevated temperature, infection, cytokine stimulation, metabolic starvation, and other environmental stresses. Indicated by intense bands on the PAGE-gel, high amounts of HSP90 and heat shock cognate protein (HSC) 70 were present in mesothelioma-derived exosomes. HSP are normally present in small amounts within the cytoplasm of all cells in all life forms but can also be released into the extracellular environment in the absence of cellular necrosis.14 The precise mechanisms by which HSP are actively released by viable cells have not yet been elucidated but we propose that exosomes may play a role in releasing HSP from cells. Inside cells, HSP play a role in protein trafficking, whereby they fold other proteins properly, keep them in correct and functional shape, or transport them from one location to another.15 They act thus as chaperones, bringing along with them small fragments, or peptides, derived from other proteins expressed in that cell, providing a “fingerprint” of the cell’s content.16 Therefore, exosomes carrying high amounts of HSP from a patient’s tumor may be good candidates for a cancer immune therapy without the need to identify what those antigens are. HSP in exosomes can be taken up by dendritic cells and macrophages (perhaps by CD91 receptor-mediated endocytosis17–19), and processed for presentation to the immune system in the lymph nodes. The tumor-specific antigens are released from the HSP inside the cell and presented to cytotoxic T cells (CTL), or “killer cells,” which are then activated. Different studies showed that immune cells stimulated with heat shock proteins can eliminate different kinds of cancers,20–26 and phase III trails are underway in renal cancer and metastatic melanoma. HSC70 has furthermore been described as an important factor in the release of exosomes during reticulocyte maturation27 and HSP73, present in dendritic cell-derived exosomes, induced antitumor immune responses in vivo.4
Annexins comprise a structurally conserved family of proteins capable of binding in a Ca2+-dependent manner to phospholipids.28 Annexins participate in the regulation of membrane organization, membrane traffic, and the regulation of calcium currents across cell membranes or within cells.29 After being exported outside of cells, some annexins have been shown to function as receptors for extracellular proteins and proteases and can interact with glycoconjugates.30 Annexin A2 and annexin A6 participate in disconnecting the clathrin lattice from the spectrin membrane cytoskeleton during the final stages of coated pit budding.31–33
The annexins found in this study (annexin A1 (synonyms: annexin I/lipocortin 1)), annexin A2 (annexin II/calpactin 1), annexin A5 (annexin V), and annexin A6 (annexin VI)) may regulate membrane-cytoskeleton dynamics and besides being involved in membrane-fusion events between intracellular compartments, they play a role in the inward vesiculation process.34
We and others could not detect tetraspanin molecules by mass spectrometry techniques.35,36 However, immunogold electron microscopy using an anti-CD63 (specific tetraspanin marker of late endosomes) antibody showed the presence of this protein at the exosome surface suggesting it was under the threshold of detection of the SDS-PAGE/MALDI-TOF technique.
Plasmalemma vesicle-associated protein (PLVAP) or PV-1 is a caveolae-specific glycoprotein associated with stomatal diaphragms of caveolae, transendothelial channels, and fenestrae and is highly conserved across species.37 While for the diaphragms of fenestrae a “sieving” function of the blood plasma components has been documented,38 there is no data documenting the function of diaphragms of caveolae. PV-1 is anchored in the membrane and could participate in protein-protein interactions via the proline-rich region at the C terminus.38 Results of strong affinity between PV-1 and heparin suggests that PV-1 may interact with heparan sulfate proteoglycans located on cell surfaces or in the extracellular matrix.39 Although data of PV-1 expression on mesothelial cells are lacking, PV-1 is expressed in many types of multiple endocrine and endothelial cell types.39 Our data provide evidence for the presence of PV-1 protein in exosomes from mesothelioma cell lines but the role of this protein both in exosomes and normal mesothelial cells is a matter under current investigation.
Cytoskeleton proteins as actin and tubulin give structure to the exosome together with the associated proteins ezrin, moesin, actinin-4, desmoplakin I, and fascin. Ezrin and moesin belong to the ERM family that attach actin filaments to transmembrane glycoproteins and thereby stabilize cell-surface protrusions.40–42 α-actinin is a microfilament bundling and cross-linking protein that is ubiquitously expressed in numerous actin structures of virtually all cells. Four different isoforms encoding α-actinin have been identified in humans. α-actinin-1 exists as a non-muscle or a smooth muscle isoform, α-actinin-2 and -3 are skeleton muscle isoforms, and α-actinin-4 is a non-muscle isoform.43–46 Although both are non-muscle isoforms, α-actinin-4 exerts contradictory functions from those of α-actinin-1 with respect to their involvement in cell movement. α-actinin-4 is described to be associated with enhanced cell motility and cancer invasion, especially in patients with a cytoplasmic localization of this protein.46 After disassembly of the actin skeleton, α-actinin-4 may reorganize the cytoskeleton by cross-linking actin filaments, a process supposedly requisite for exosome formation. Recently, mutations in the ACTN4 gene, which codes for α-actinin-4, were reported to cause familial focal segmental glomerulosclerosis.47 Although rarely detected in lung tumors, a point-mutation (adenine → thymine) in the ACTN4 gene of a non-small cell lung cancer (NSCLC) cell line resulting in an asparagine instead of lysine in actinin-4, leading to the expression of a tumor-specific antigen recognized by autologous cytotoxic T lymphocytes (CTL).48–50 Preliminary data suggest that the mesothelioma cell line (PMR-MM7, HLA-A02, A68) is not recognized by the mutated actinin-4-specific CTL clone (HLA-A02, A68, under current investigation (F. Mami-Chouaib, Institut Gustave Roussy, Villejuif, France)).
Like actinin-4, fascin organizes actin filaments into bundles and is predominantly present in dendrites, microspikes, microvilli, filopodia, and pseudopodia (or called lamellipodia depending on the morphology) at the cell periphery and in stress fibers in some cells. The expression of fascin is described in cells that have the morphological characteristic of membrane protrusions in common, like glial and neuronal cells, microcapillary endothelial cells, and antigen-presenting dendritic cells. Therefore, it is suggested that fascin plays a role in extending the membrane either for cell motility or for interactions with other cell types. Fascin expression is dramatically increased during maturation of DC.51 On maturation of DC and their travel to lymph nodes to present the antigen to T cells, numerous fascin-containing membrane extensions appear. At this stage, the secretion of exosomes by DC is increased.9 High levels of fascin is also observed in many cancer cells and appears to be correlated with aggressive cell behavior.52,53 We suggest that fascin may also be involved in the inward budding of multi-vesicular bodies, which creates internal vesicles that after fusion with the plasma membrane leads to release of exosomes into the extracellular milieu.
Desmoplakin is required for assembly of functional desmosomes (a type of junction that attaches one cell to its neighbor) during epithelial sheet formation, maintaining cytoskeletal architecture, and reinforcing membrane attachments essential for stable intercellular adhesion.54 This intracellular anchor protein is responsible for connecting the cytoskeleton to transmembrane adhesion proteins.
Molecular motor proteins mediate the intracellular transport of membrane-enclosed organelles. Kinesin-like protein, a microtubule-based motor protein, was found.
The membrane orientation of exosomes is identical to that of cells, during their formation cytoplasm is included into the vesicle. The metabolic enzymes, glyceraldehyde 3-phosphate dehydrogenase, enolase-1, and pyruvate kinase, are involved in the glycolysis. This process in which glucose is converted into pyruvate with the concomitant production of ATP occurs in the cytosol. Regulation of the production of cyclic AMP is done by 2′3′-cyclic-nucleotide 3′ phosphodiesterase. The phenomenon of increased expression of glucose transporters and glycolytic enzymes in tumor cells was described as the Warburg effect55 and is one of the most universal characteristics of solid tumors.56–58 The genes of these products are also found to be up-regulated in expression mapping in mesothelioma oncogenesis (Singhal S, personal communication). High amounts of glycolytic enzymes in the cytoplasm of cells will thus be reflected by the presence of these enzymes in exosomes. There are no data published on the distribution and function of the putative P150 protein (code O00378).
Some of the proteins present in mesothelioma-derived exosomes like MHC class I, HSC70, HSP90α, annexins (A1, A2, A5, A6), actin, and tubulin were also described to be present in B cell-derived exosomes35 and dendritic cell-derived exosomes.5 Furthermore, proteomic analysis of B cell-derived exosomes revealed the presence of moesin, glyceraldehyde 3-phosphate dehydrogenase, pyruvate kinase, and enolase in common with our results on mesothelioma-derived exosomes.35 Common proteins with intestinal epithelial cell exosomes are MHC class I, actin, tubulin, and enolase-1 and with other tumor-derived exosomes are MHC class I and HSC70.59
Most interesting was the detection of a protein, not described previously on exosomes, a precursor of the developmental endothelial locus-1 (DEL-1) protein. DEL-1 has structural homology to lactadherin and has a regulatory function in vascular remodeling during embryo genesis.60 It is described as an extracellular matrix protein that promotes adhesion of endothelial cells via the αvβ3 integrin receptor present on endothelial cells. The αvβ3 integrin receptor is also present on dendritic cells and mediates the uptake of apoptotic vesicles, important for cross priming of tumor antigens. It is speculative that DEL-1 on tumor-exosomes is important for targeting exosomes to DC for cross-presentation. Recently, Aoka et al61,62 suggested that DEL-1 acts as an angiogenic factor in the context of solid tumor formation and that the increase in vascular development accelerates tumor growth through decreased apoptosis. The role of DEL-1 in tumor exosomes can be bilateral first, attachment to dendritic cells and secondly, to increase the vascular development in the neighborhood of the tumor. Exosomes may also bind to extracellular matrix components by fibronectin, a ligand for integrins.
Proteomic analysis using MALDI-TOF mass spectrometry revealed several new proteins not previously described on (tumor) exosomes or for mesothelioma cell lines. In conclusion, mesothelioma cell line-derived exosomes express a discrete set of proteins involved in antigen presentation, signal transduction, migration, and adhesion and thereby may be an important pathway in the communication between cells.
Acknowledgments
We thank Hans Dalebout, Lies-Anne Severijnen, and Janice Griffith for their assistance in the MALDI-TOF mass spectrometry studies and electron microscopy studies, respectively. We also thank the Department of Clinical Genetics of the Erasmus MC for karyotyping the mesothelioma cell lines.
Footnotes
Address reprint requests to Joost P.J.J. Hegmans, Erasmus MC, Department of Pulmonary Medicine, P.O. Box 1738; 3000 DR, Rotterdam, The Netherlands. E-mail: j.hegmans@erasmusmc.nl.
Supported by “Stichting Asbestkanker Rotterdam.”
References
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multi-vesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis, and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes: selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- Amigorena S. Anti-tumour immunotherapy using dendritic cell-derived exosomes. Res Immunol. 1998;149:661–662. doi: 10.1016/s0923-2494(99)80035-2. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Fernandez N, Lozier A, Wolfers J, Regnault A, Raposo G, Amigorena S. Dendritic cells or their exosomes are effective biotherapies of cancer. Eur J Cancer. 1999;35:S36–S38. doi: 10.1016/s0959-8049(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Hoogsteden HC, Langerak AW, van der Kwast TH, Versnel MA, van Gelder T. Malignant pleural mesothelioma. Crit Rev Oncol Hematol. 1997;25:97–126. doi: 10.1016/s1040-8428(96)00231-4. [DOI] [PubMed] [Google Scholar]
- Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, Van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN: Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol, 2004, Feb 19 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins in health and disease: therapeutic targets or therapeutic agents? Exp Rev Mol Med. 2001. Sept;21:1–21. doi: 10.1017/S1462399401003556. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Young D, Roman E, Moreno C, O’Brien R, Born W. Molecular chaperones and the immune response. Philose Trans R Soc Lond B Biol Sci. 1993;339:363–367. doi: 10.1098/rstb.1993.0035. 367–368. [DOI] [PubMed] [Google Scholar]
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, Schild H. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–5403. [PubMed] [Google Scholar]
- Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med. 1999;189:797–802. doi: 10.1084/jem.189.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166:490–497. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- Robert J, Menoret A, Basu S, Cohen N, Srivastava PR. Phylogenetic conservation of the molecular and immunological properties of the chaperones gp96 and hsp70. Eur J Immunol. 2001;31:186–195. doi: 10.1002/1521-4141(200101)31:1<186::AID-IMMU186>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- Goldman B. Cancer vaccines: finding the best way to train the immune system. J Natl Cancer Inst. 2002;94:1523–1526. doi: 10.1093/jnci/94.20.1523. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. Purification of heat shock protein-peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods. 1997;12:165–171. doi: 10.1006/meth.1997.0464. [DOI] [PubMed] [Google Scholar]
- Geminard C, Nault F, Johnstone RM, Vidal M. Characteristics of the interaction between Hsc70 and the transferrin receptor in exosomes released during reticulocyte maturation. J Biol Chem. 2001;276:9910–9916. doi: 10.1074/jbc.M009641200. [DOI] [PubMed] [Google Scholar]
- Creutz CE. The annexins and exocytosis. Science. 1992;258:924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Gerke V, Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders: calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984;3:227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Ying Y, Anderson RG. Annexin VI-mediated loss of spectrin during coated pit budding is coupled to delivery of LDL to lysosomes. J Cell Biol. 1998;142:937–947. doi: 10.1083/jcb.142.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin E, Russo-Marie F, Dubois T, de Paillerets C, Alfsen A, Bomsel M. In adrenocortical tissue, annexins II and VI are attached to clathrin-coated vesicles in a calcium-independent manner. Biochim Biophys Acta. 1998;1402:115–130. doi: 10.1016/s0167-4889(97)00151-1. [DOI] [PubMed] [Google Scholar]
- Futter CE, Felder S, Schlessinger J, Ullrich A, Hopkins CR. Annexin I is phosphorylated in the multi-vesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993;120:77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts RW, Leckie RS, Veenhuizen PT, Schwartzmann G, Moebius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes: potential implications for their function and multi-vesicular body formation. J Biol Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multi-vesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Stan RV, Arden KC, Palade GE. cDNA and protein sequence, genomic organization, and analysis of cis regulatory elements of mouse and human PLVAP genes. Genomics. 2001;72:304–313. doi: 10.1006/geno.2000.6489. [DOI] [PubMed] [Google Scholar]
- Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci USA. 1999;96:13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko R, McFarland M, Ben-Jonathan N. Distribution and characterization of plasmalemma vesicle protein-1 in rat endocrine glands. J Endocrinol. 2002;175:649–661. doi: 10.1677/joe.0.1750649. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S. ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci. 1997;22:53–58. doi: 10.1016/s0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Henry M, Solomon F, Jacks T. RhoA-dependent phosphorylation and relocalization of ERM proteins into apical membrane/actin protrusions in fibroblasts. Mol Biol Cell. 1998;9:403–419. doi: 10.1091/mbc.9.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM. Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267:9281–9288. [PubMed] [Google Scholar]
- Millake DB, Blanchard AD, Patel B, Critchley DR. The cDNA sequence of a human placental α-actinin. Nucleic Acids Res. 1989;17:6725. doi: 10.1093/nar/17.16.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H, McAfee M, Kwiatkowski DJ. Cloning and chromosomal localization of the human cytoskeletal α-actinin gene reveals linkage to the β-spectrin gene. Am J Hum Genet. 1990;47:62–71. [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, Yamada Y, Chiba H, Hirohashi S. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998;140:1383–1393. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- Echchakir H, Mami-Chouaib F, Vergnon I, Baurain JF, Karanikas V, Chouaib S, Coulie PG. A point mutation in the α-actinin-4 gene generates an antigenic peptide recognized by autologous cytolytic T lymphocytes on a human lung carcinoma. Cancer Res. 2001;61:4078–4083. [PubMed] [Google Scholar]
- Echchakir H, Dorothee G, Vergnon I, Menez J, Chouaib S, Mami-Chouaib F. Cytotoxic T lymphocytes directed against a tumor-specific mutated antigen display similar HLA tetramer binding but distinct functional avidity and tissue distribution. Proc Natl Acad Sci USA. 2002;99:9358–9363. doi: 10.1073/pnas.142308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mami-Chouaib F, Echchakir H, Dorothee G, Vergnon I, Chouaib S. Antitumor cytotoxic T-lymphocyte response in human lung carcinoma: identification of a tumor-associated antigen. Immunol Rev. 2002;188:114–121. doi: 10.1034/j.1600-065x.2002.18810.x. [DOI] [PubMed] [Google Scholar]
- Pinkus GS, Pinkus JL, Langhoff E, Matsumura F, Yamashiro S, Mosialos G, Said JW. Fascin, a sensitive new marker for Reed-Sternberg cells of Hodgkin’s disease: evidence for a dendritic or B cell derivation? Am J Pathol. 1997;150:543–562. [PMC free article] [PubMed] [Google Scholar]
- Grothey A, Hashizume R, Sahin AA, McCrea PD. Fascin, an actin-bundling protein associated with cell motility, is up-regulated in hormone receptor-negative breast cancer. Br J Cancer. 2000;83:870–873. doi: 10.1054/bjoc.2000.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothey A, Hashizume R, Ji H, Tubb BE, Patrick CW, Jr, Yu D, Mooney EE, McCrea PD. C-erbB-2/ HER-2 up-regulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene. 2000;19:4864–4875. doi: 10.1038/sj.onc.1203838. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3:1076–1085. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Galarraga J, Loreck DJ, Graham JF, DeLaPaz RL, Smith BH, Hallgren D, Cummins CJ. Glucose metabolism in human gliomas: correspondence of in situ and in vitro metabolic rates and altered energy metabolism. Metab Brain Dis. 1986;1:279–291. doi: 10.1007/BF00999357. [DOI] [PubMed] [Google Scholar]
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Artemov D, Bedi A, Bhujwalla Z, Chiles K, Feldser D, Laughner E, Ravi R, Simons J, Taghavi P, Zhong H. “The metabolism of tumours”: 70 years later. Novartis Found Symp. 2001;240:251–260. 260–254. [PubMed] [Google Scholar]
- van Niel G, Heyman M. The epithelial cell cytoskeleton and intracellular trafficking. II. Intestinal epithelial cell exosomes: perspectives on their structure and function. Am J Physiol. 2002;283:G251–G255. doi: 10.1152/ajpgi.00102.2002. [DOI] [PubMed] [Google Scholar]
- Hidai C, Zupancic T, Penta K, Mikhail A, Kawana M, Quertermous EE, Aoka Y, Fukagawa M, Matsui Y, Platika D, Auerbach R, Hogan BL, Snodgrass R, Quertermous T. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the αvβ3 integrin receptor. Genes Dev. 1998;12:21–33. doi: 10.1101/gad.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoka Y, Johnson FL, Penta K, Hirata Ki K, Hidai C, Schatzman R, Varner JA, Quertermous T. The embryonic angiogenic factor Del1 accelerates tumor growth by enhancing vascular formation. Microvasc Res. 2002;64:148–161. doi: 10.1006/mvre.2002.2414. [DOI] [PubMed] [Google Scholar]
- Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, Coleman M, Boudreau N, Varner JA. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest. 2003;112:30–41. doi: 10.1172/JCI17034. [DOI] [PMC free article] [PubMed] [Google Scholar]