Abstract
Obstructive diseases of blood vessels and the lung are characterized by degradation and synthesis of new extracellular matrix (ECM) components. Regulated remodeling of the ECM in diseases such as atherosclerosis and lymphangioleiomyomatosis (LAM), both characterized by excessive accumulation of smooth muscle cells (SMCs), is thought to be controlled in part by cell surface receptors for specific ECM components. Discoidin domain receptors (DDR) 1 and 2 represent a family of tyrosine kinase collagen receptors that are activated by fibrillar collagens. To test the hypothesis that DDR may be involved in ECM remodeling by SMCs in vivo, we analyzed DDR expression by reverse transcriptase-polymerase chain reaction and immunohistochemistry and demonstrate that both DDR1 and DDR2 are up-regulated in nodules of LAM as compared to normal controls, and are expressed in lesions of atherosclerosis. In vitro, retroviral overexpression of DDR1 or DDR2 in human SMCs cultured on polymerized collagen gels leads to a reduction of collagen expression and induces matrix metalloproteinase (MMP) 1 at both mRNA and protein levels, but only DDR2 enhances MMP2 activation. Moreover, DDR2 overexpression increases SMC-mediated collagen and elastin degradation in vitro. Using laser microdissection, we extend our studies to the analysis of SMCs from LAM nodules where we observe higher MMP1 expression and MMP2 activation. Taken together, these data provide evidence for the potential roles of DDR1 and DDR2 in the regulation of collagen turnover mediated by SMCs in obstructive diseases of blood vessels and the lung.
The extracellular matrix (ECM) is a dynamic structure that not only provides a scaffold for mechanical support and organization of tissues, but also regulates critical events in normal development and pathological conditions. Therefore, synthesis and degradation of the ECM must be tightly regulated, and the net balance between neosynthesis and breakdown will determine the final composition of ECM components in tissues.1 Although the action of several growth factors and cytokines can directly regulate collagen biosynthesis,2 and three classes of enzymes—matrix metalloproteinase (MMPs), serine proteases, and cysteine proteases—have been implicated in ECM breakdown,1,3,4 the molecular mechanisms regulating the remodeling of major ECM components, such as collagen and elastin, are still poorly understood.
During the formation of the lesions of atherosclerosis, deposition of new ECM components, in particular collagens type I and III, and increased expression of MMP are observed.5–10 Dynamic changes in ECM composition within the vessel wall have been proposed to play an important role in excessive smooth muscle cell (SMC) accumulation, a key event in atherosclerosis.3,5,8,11 Moreover, a critical factor in the clinical sequelae associated with lesions of atherosclerosis, such as myocardial infarction, is the integrity of interstitial type I collagen present in the fibrous cap that covers the atherosclerotic lesions—the higher the content of SMCs and intact type I collagen, the less prone the fibrous cap is to rupture.5,12 Excessive accumulation of SMCs around bronchioles, pulmonary arteries, and venules, in association with MMP-dependent degradation of fibrillar collagen and elastic fibers is also observed during lymphangioleiomyomatosis (LAM).13–16 LAM is a rare disorder that occurs either sporadically or in association with tuberous sclerosis affecting primarily women of reproductive age.13,15 SMC accumulation and degeneration of collagen and elastin fibers leads to respiratory failure in LAM patients, who normally die within 5 to 10 years after the onset of symptoms.13,15
Regulated remodeling of the ECM in disease processes such as atherosclerosis and LAM is poorly understood but is thought to be regulated in part by cell surface receptors for specific ECM. Until recently, cell-ECM interactions have been ascribed primarily to integrins.17,18 The identification of discoidin domain receptors 1 and 2 (DDR1 and DDR2) as collagen receptors with tyrosine kinase activity constitutes an alternative mechanism for mediating cell-matrix adhesion and transmitting ECM signals to cells.19,20 Evidence from the generation of DDR1- and DDR2-null mice, and in vitro studies, suggest that DDR can regulate cell proliferation and ECM remodeling mediated by MMP activities during normal development and/or pathological conditions.21–26
In the present study, we investigate the role of DDRs in SMC-mediated collagen remodeling, and characterize their expression at different stages of atherosclerotic lesion development and in LAM disease. Our data demonstrate that signaling from DDR1 and DDR2 down-regulates collagen type I production and induces collagen degradation through the induction of collagenase I (MMP1) and activation of gelatinase A (MMP2). Using laser microdissection technology, we extend our observations in cultured human SMCs to LAM nodules where we observe higher expression of MMP1 and activation of MMP2 as compared to normal lung. Thus, our study provides evidence that collagen-mediated activation of both DDR1 and DDR2 in SMCs may contribute to the turnover and remodeling of collagen in lesions of atherosclerosis and LAM.
Materials and Methods
Cell Culture and Collagen Matrix Preparation
Human newborn arterial SMCs were isolated from the thoracic aorta as previously described.27 SMCs between passages 5 and 9 were cultured in 1% plasma-derived serum/Dulbecco’s modified Eagle’s medium (DMEM) on the surface of the following collagen type I preparations: three-dimensional polymerized collagen gels (1.0 mg/ml final concentration) prepared by neutralization of the collagen solution (Vitrogen 100; Cohesion Technologies, Palo Alto, CA) with 1/6 vol of 7× concentrated DMEM, dilution to a final 1× DMEM solution, and incubation at 37°C for 24 hours.28 Monomer collagen-coated dishes were prepared by incubating 0.1 mg/ml of collagen solution in 0.1 mol/L of acetic acid at 37°C for 24 hours and washing twice with DMEM before cell seeding.
Reagents and Antibodies
The broad-based metalloproteinase inhibitor GM6001 was purchased from Elastin Products Co., and Coomassie Blue R250 from Sigma Chemical Co. (St. Louis, MO). The following antibodies were used for immunostaining: rabbit polyclonal anti-DDR1 (C20, 3 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), goat polyclonal anti-DDR2 (N20, 9 μg/ml; Santa Cruz Biotechnology), normal rabbit IgG (Vector Laboratories, Burlingame, CA), normal goat IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), anti-α-actin (1 μg/ml; DAKO, Carpinteria, CA), and anti-HAM-56.29 For competition experiments, DDR1-blocking peptide (SC-532 P, Santa Cruz Biotechnology) and DDR2-blocking peptide (sc-7555 P, Santa Cruz Biotechnology) were used. For Western blot analysis, the following antibodies were used: rabbit polyclonal anti-HA (Zymed Laboratories, South San Francisco, CA); mouse monoclonal to phosphotyrosine residues (clone 4G10; Upstate Technology, Lake Placid, NY); mouse monoclonal anti-MMP1 (Calbiochem, La Jolla, CA); rabbit polyclonal anti-DDR1 (C20, Santa Cruz Biotechnology); goat polyclonal anti-DDR2 (H-108, Santa Cruz Biotechnology). Protein G-Plus agarose was purchased from Santa Cruz Biotechnology.
Protein Analysis and Immunoprecipitation
Cells were washed twice with phosphate-buffered saline (PBS) and lysed (50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 0.5% Nonidet P-40, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L NaVO4, 1 μg/ml aprotinin, 1μg/ml leupeptin, and 1 μg/ml pepstatin) for 30 minutes on ice. Cell lysates were cleared by centrifugation at 15,000 × g for 20 minutes, and protein concentrations were determined using the BCA protein assay (Pierce, Rockford, IL). For protein analysis of control and LAM tissues, five 20-μm-thick frozen sections were placed into a microfuge tube, washed twice with PBS, and lysed as described above. Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions, transferred to Immobilon polyvinylidene difluoride (Millipore, Bedford, MA), and subsequently immunoblotted with specific antibodies, before visualization by enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL). For immunoprecipitations, 200 μg of protein extracts from human SMCs were incubated with 5 μg of anti-HA antibody at 4°C. After 2 hours, protein G-Plus Agarose was added and the incubation was continued overnight. Samples were washed three times with lysis buffer and eluted with 1× sodium dodecyl sulfate electrophoresis-loading buffer.
Gelatin Zymography
Cell lysates were prepared as described above, and conditioned media were collected from SMC cultures. Samples were prepared in nonreducing loading buffer and separated on 10% sodium dodecyl sulfate-polyacrylamide gel containing 1 mg/ml of gelatin. After electrophoresis, gels were washed three times in washing solution (150 mmol/L NaCl and 2.5% Triton X-100) for 30 minutes, rinsed in water, and incubated for 12 to 16 hours in collagenase buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 10 mmol/L CaCl2). Gels were subsequently fixed and stained in Coomassie Blue fixative solution (25% methanol, 7% acetic acid, and 0.25% Coomassie Blue R250) for 2 hours at room temperature and destained with washing solution (25% methanol and 7% acetic acid in water) for 4 to 5 hours.
RNA Preparation and Northern Blot
Total RNA was prepared from SMCs cultured on polymerized collagen gels with StrataPrep Total RNA Miniprep kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. Ten μg of total RNA were subjected to a Northern blot procedure using a modification of the methods pioneered by Alwine and colleagues.30 Briefly, total RNA samples were separated on 1.5% agarose gels containing formaldehyde, transferred by capillary action to a nylon membrane (Hybond, Amersham Pharmacia Biotech), and UV cross-linked to the membrane. Probes were radiolabeled with α-32P dCTP by using the Rediprime II kit (Amersham Pharmacia Biotech) according to manufacturer’s instructions. Radiolabeled probes were added to express-hyb solution (Clontech, Palo Alto, CA) and allowed to hybridize for 1 hour at 68°C after a prehybridization for 30 minutes at 68°C. After hybridization, membranes were washed and the gel exposed to Kodak BioMax film (Eastman-Kodak, Rochester, NY).
Probes for specific hybridization to DDR1 a, b, and c isoforms (1543 to 1873 bp), DDR1 b and c isoforms (1657 to 1770 bp), DDR2 (1708 to 2093 bp), MMP1 (284 to 845 bp), MMP2 (361 to 899 bp), Col1α(I) (1212 to 1690 bp), and GAPDH (360 to 695 bp) were generated by polymerase chain reaction (PCR) amplification of cDNA derived from human SMCs.
Reverse Transcriptase (RT)-PCR
For RT-PCR total RNA was extracted from 50 to 100 mg of frozen tissues using the StrataPrep Total RNA Miniprep kit (Stratagene) according to the manufacturer’s instructions. First-strand cDNA synthesis was performed with 1 μg of total RNA and oligo-dT primer, using SuperScript Preamplification System for First Strand cDNA Synthesis (Invitrogen, Carlsbad, CA). PCR reactions were performed using specific human DDR1 isoform primers previously described (5′-ATCCTGCTCCTGCTGCTCATCA-3′, 5′-ATCTTGAGGGCTGTCGACC TCA-3′),31 DDR2 primers spanning the juxtamembrane and transmembrane domains (5′-ATCCTGATTGGCTGCTTGGTGGCC-3′, 5′-CTCAAAGGACCCCTTG-3′), and GAPDH primers 5′TGAAGGTCGGAGTCAACGGATTTGGT-3′, 5′CATGTGGGCCATGAGGTC CACCAC-3′).
Generation of Expression Constructs and Retroviral Infection
Standard molecular biology techniques were used for all constructs. For subcloning into retroviral vectors, full-length human DDR1 isoform b was generated by PCR using primers containing 5′ BamHI and 3′BamHI sites and DDR2 used primers with 5′ XhoI 3′ NotI sites. Both constructs have a hemagglutinin (HA) epitope tag on the 3′ end. The sequences of all PCR-generated constructs were confirmed by sequencing. Primer sequences used for cloning are available on request. All retroviral expression plasmids were constructed using the pBM-IRES-PURO,32 expressing the puromycin resistance gene (PAC) as a selectable second cis-tron gene, generated from the original pBM-IRES-EGFP, generously provided by GP Nolan (Stanford University, Stanford, CA).
Immunocytochemistry and Laser Microdissection
Segments of the aortae of nonhuman primates fed a hypercholesterolemic diet were fixed in methyl Carnoy’s fixative and embedded in paraffin before sectioning. Tissue from control lungs was provided by Lynn Schnapp, David Thorning, Charles Frevert (University of Washington, Seattle, WA), and Marilyn Glassberg (University of Miami, Miami, FL). Specimens from LAM lungs were supplied by Joel Moss (National Institutes of Health, Bethesda, MD) and the LAM Registry. Hematoxylin and eosin staining was performed to evaluate the preservation of frozen and fixed tissues before further analyses. Fixed tissues (methyl Carnoy’s and paraformaldehyde fixatives) were used for immunocytochemical analysis. Serial sections were blocked in PBS and 0.1% Triton X-100 and PBS containing 3% H2O2 and 0.1% azide to eliminate endogenous peroxidase activity and with avidin/biotin blocking kit (Vector), with 5% normal sera (same species as secondary antibody). Primary antibody incubations were 4 hours followed by horseradish peroxidase-conjugated secondary biotinylated antibodies (Jackson ImmunoResearch Laboratories, Inc.) for 1 hour, and then developed with avidin-peroxidase (Vectastain ABC kit elite, Vector), all at room temperature. Antibody binding was visualized with diaminobenzidine and sections were counterstained with methyl green.
For microdissection 10-μm-thick frozen sections from normal and LAM tissue were placed on glass slides containing a 1.35-μm thick PEN membrane (Stem Cell Technology), acetone-fixed for 10 minutes at 4°C, blocked with 5% normal goat serum, and incubated with anti-α-actin antibody (1 μg/ml) overnight at 4°C. After washing, the slides were incubated with biotinylated anti-mouse IgG (Vector), then horseradish peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc.), and visualized with diaminobenzidine. Slides were kept at −20°C until use. Only α-actin-positive cells were collected using the PALM Laser Microbeam Microdissection plus Laser Pressure Catapulting system (P.A.L.M. Mikrolaser Tachnologie, Munich, Germany) that allows the precise outline and collection of positive cells without the inclusion of unstained cells in the same area. To collect a similar number of cells from each specimen, images of α-actin-stained slides were captured using Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI) and area occupied by SMCs was quantified using color threshold with Image Pro Plus (Media Cybernetics, Silver Spring, MD) software. Comparable total areas were then microdissected, and collected cells were lysed as described under protein analysis and immunoprecipitation.
Collagen Degradation Assay
For the collagen degradation assay, SMCs were cultured on fluorescein isothiocyanate (FITC)-conjugated polymerized collagen gels (1.0 mg/ml final concentration; Elastin Products Co.), and at subsequent time points conditioned media were collected and FITC-conjugated collagen fragments measured using a Fusion microplate analyzer (Packard BioScience, Inc., Meriden, CT).
Results
mRNA Levels for DDR1 and DDR2 Are Up-Regulated in the Lungs of LAM Patients, but Do Not Change Significantly between Normal and Atherosclerotic Arteries
A major component of obstructive diseases of blood vessels and the lung is degradation and abnormal repair and deposition of new ECM, typified by the deposition of fibrillar type I collagen.33,34 Regulated remodeling of the ECM in disease processes such as atherosclerosis and LAM, two diseases characterized by excessive accumulation of large numbers of SMCs, is poorly understood but is thought to be regulated in part by cell surface receptors for specific ECM molecules. A family of collagen receptors with protein tyrosine kinase activity, DDR1 and DDR2, have been identified in injury-induced remodeling of blood vessels22 and the liver.25 To begin to examine the potential role of DDR in collagen remodeling in atherosclerosis and LAM, we evaluated their expression.
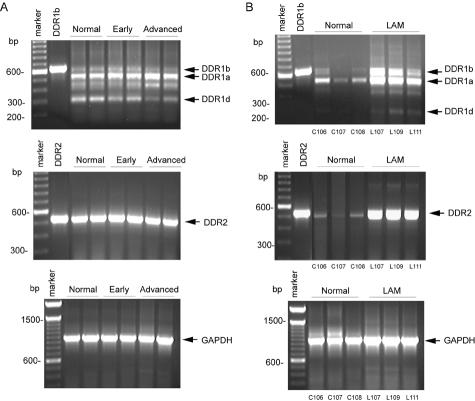
Five different isoforms of human DDR1, all generated by alternative splicing, have been previously described,19,31,35 whereas only one DDR2 transcript has been identified. We used semiquantitative RT-PCR and primers designed from the human sequence to compare levels of DDR1 and DDR2 in diseased and normal tissue with GAPDH to control for the relative quality and quantity of total RNA. In normal and diseased arteries from nonhuman primates on a high cholesterol diet, we detect three DDR1 isoforms (a, b, and d; Figure 1A). Although the overall expression of DDR1 does not change dramatically, there is an apparent decrease in DDR1d mRNA levels and an increase in expression of DDR1a isoform in advanced atherosclerotic lesions (Figure 1A). In contrast, DDR2 mRNA levels are not modulated between normal aortae and aortae with early or advanced lesions (Figure 1A). Human and nonhuman primate lesions at early and late stages of development are characterized by different cellular compositions within lesions—early lesions contain primarily macrophage-rich foam cells and late lesions have well-developed fibrous caps with SMCs covering the foam cells and necrotic core (Figure 2). Although 95% of the chimpanzee and human genome can be directly aligned with an average sequence divergence of 1.2%,36,37 we cannot eliminate the possibility that DDR1 isoforms may vary between nonhuman primates analyzed in this study and humans. However, fragments of comparable size were observed in nonhuman primate and human tissue (Figure 1, A and B).
Figure 1.
DDR1 and DDR2 mRNA are differentially expressed in lesions of atherosclerosis and LAM nodules. A: Total RNA was extracted from frozen aortae of control nonhuman primates (n = 2, normal) or from animals fed a hypercholesterolemic diet for varying periods of time resulting in different stages of lesion development (n = 2, early and advanced). B: Normal human lung (n = 3) was compared with LAM lung (n = 3). First-strand cDNA synthesis was generated from isolated RNA and the PCR reaction was performed as described in Material and Methods. Plasmid DNA containing the human sequence for DDR1b and DDR2 was used as a positive control. PCR products for DDR1 isoforms a, b, and d, DDR2, and GAPDH are indicated with arrowheads.
Figure 2.
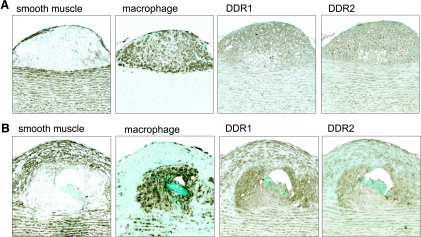
DDR1 and DDR2 are present in SMCs in lesions of atherosclerosis. Immunohistochemical analysis was performed on serial sections of aortae of nonhuman primates fed a hypercholesterolemic diet with early (A) and advanced (B) atherosclerotic lesions. Adjacent sections were stained with antibodies recognizing a marker for SMCs (α-actin) or macrophages (HAM-56), and DDR1 (C-20, rabbit polyclonal; Santa Cruz Biotechnology) or DDR2 (N-20, goat polyclonal; Santa Cruz Biotechnology). A: Recruited macrophages and medial SMCs express DDR1 and DDR2. B: SMCs present in the fibrous cap and macrophages in the core of advanced atherosclerotic lesions express DDR1 and DDR2.
Comparison of normal lung and LAM tissue using the same set of primers demonstrated an increase in mRNA levels of DDR1a and DDR1b isoforms in lung affected by LAM disease as compared to normal controls (Figure 1B). DDR1d was variably detected in LAM specimens, and always represented a very small percentage of the total DDR1 (Figure 1B and data not shown). In contrast to arteries, DDR2 mRNA was increased in LAM specimens compared with normal lung (Figure 1B). Although DDR1 and DDR2 mRNA levels are not significantly altered during the development of lesions of atherosclerosis, levels of both DDR1 and DDR2 mRNA seem to be up-regulated in LAM nodules.
Multiple Cell Types Express DDR1 and DDR2 in Lesions of Atherosclerosis and the Lungs of LAM Patients
Immunohistochemistry was used to evaluate cell type and relative expression of DDR1 and DDR2 in lesions of atherosclerosis and in lungs of patients with LAM. As shown in Figure 2, both DDR1 and DDR2 are expressed in early and advanced lesions of atherosclerosis, as well as in the underlying medial SMCs. In early lesions, we detect expression of both DDR1 and DDR2 in macrophages that are the major component of the developing lesions (Figure 2A). DDR1 and DDR2 are also abundant in advanced lesions within SMCs localized in the fibrous cap and macrophages in the necrotic core (Figure 2B). This distribution is typical of lesions in eight different nonhuman primates examined.
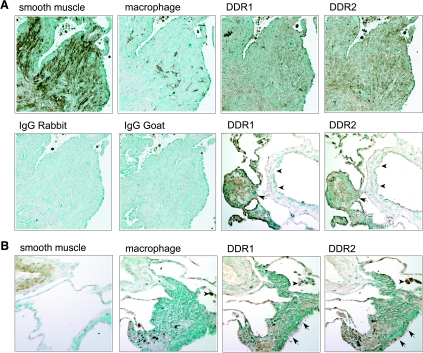
Seven segments of lung tissue from LAM patients were evaluated, and positive staining for α-smooth muscle actin is observed in the SMCs that comprise the nodules characteristic of LAM lesions (Figure 3A). In LAM nodules, many of the SMCs express DDR1 and DDR2 (Figure 3A), and SMCs surrounding blood vessels in LAM lungs also express both receptors, but at lower levels than LAM nodules in the same section (Figure 3A and data not shown). DDR1 and DDR2 are also found in macrophages in and around LAM nodules (Figure 3A and data not shown). In normal lung, DDR1 and DDR2 staining is prominent in ciliated epithelial cells and alveolar macrophages, with lighter staining of vascular and interstitial SMCs (Figure 3B and data not shown). The specificity of the antibodies recognizing DDR1 and DDR2 was confirmed by the complete abrogation of immunoreactivity by co-incubation with the peptides used to generate the antibodies (data not shown).
Figure 3.
DDR1 and DDR2 are induced in SMCs in LAM nodules. Immunohistochemical analysis was performed on serial sections of specimens from LAM patients (A) and normal controls (B). Adjacent sections were stained with antibodies recognizing a marker for SMCs (α-actin) or macrophages (HAM-56), and DDR1 (C-20, rabbit polyclonal; Santa Cruz Biotechnology) or DDR2 (N-20, goat polyclonal; Santa Cruz Biotechnology), and control isotype-matched antibodies. A: SMC-rich nodules, characteristic of the lungs of LAM patients, express both DDR1 and DDR2, but no significant staining is seen with isotype-matched normal IgG. SMCs in vessels (arrowheads) express lower levels of DDR1 and DDR2 than adjacent LAM nodules (arrow). B: DDR1 and DDR2 are also expressed at low levels in interstitial and arterial SMCs present in normal lungs but are expressed at higher levels in epithelial cells, including ciliated epithelium (arrows), and alveolar macrophages (arrowheads).
DDR1 and DDR2 Are Activated by Polymerized Collagen Gels in Human SMCs
Cultured SMCs maintain some of the characteristics of SMCs in vivo, but some features, such as the expression of specific integrin receptors for ECM can change dramatically.38 To begin, to examine the potential role of DDR1 and DDR2 in SMC remodeling of the ECM, we investigated their expression and regulation in normal human arterial SMCs cultured on polymerized collagen gels—a model used to evaluate ECM remodeling.
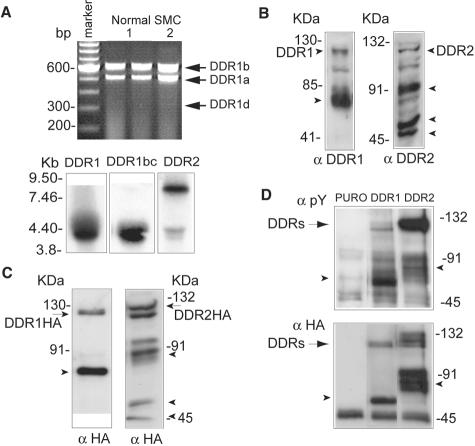
By RT-PCR, we detect the expression of DDR1a and DDR1b message in cultured SMCs with variable expression of DDR1d (Figure 4A, top). To determine the relative expression of DDR1 isoforms by Northern analysis, we designed two different probes—the first recognizing all five DDR1 isoforms and the second specific for isoforms b and c that contain additional cytoplasmic sequences not present in other DDR1 isoforms. As shown in Figure 4A, bottom, both probes detect the predicted DDR1 message of ∼4.2 kb35 in SMCs cultured on polymerized collagen gels. The probe for DDR2 detects two separate mRNA species of 9.5 kb and 4.5 kb (Figure 4A, bottom). Thus, both receptors are present in human SMCs cultured on polymerized collagen gels and they appear to express two of the three DDR1 isoforms observed in tissues from atherosclerotic and LAM lesions.
Figure 4.
DDR1 and DDR2 are expressed in cultured human SMCs and activated by polymerized collagen gels. A: RT-PCR (top) and Northern blot analysis (bottom) were performed with total RNA isolated from human SMCs cultured on polymerized collagen gels for 24 hours. RT-PCR for DDR1 was performed as described in Figure 1 using two different SMC isolates. For Northern blot analysis, the membrane was hybridized with probes recognizing all DDR1 isoforms, DDR1 isoforms b and c, and DDR2. B: Expression of DDR1 and DDR2 was evaluated by Western blot analysis of total cell lysates prepared from SMCs cultured on polymerized collagen gels for 24 hours. Arrowheads indicate smaller molecular species of DDR1 (62 kd) and DDR2 (90, 50, and 45 kd). C: Expression of HA epitope-tagged DDR1 and DDR2 in retrovirally transduced human SMCs cultured on polymerized type 1 collagen for 24 hours was evaluated by Western blot analysis with antibody recognizing the HA epitope tag. D: Activation state of DDR1 and DDR2 in human SMCs cultured on polymerized type 1 collagen for 4 hours was evaluated by immunoprecipitation with the anti-HA epitope antibody and Western blotting with anti-phosphotyrosine antibody. For all data shown in this figure, results are representative of three independent experiments.
Western blot analysis of detergent cell extracts using antibodies recognizing either DDR1 or DDR2 reveal numerous species including 125- and 130-kd forms, that have been previously shown to represent mature DDR1 and DDR2, respectively (Figure 4B). To determine the specificity of these antibodies and subsequently study the function of DDR1 and DDR2, we retrovirally overexpressed HA epitope-tagged forms of DDR1 and DDR2 in SMCs. After retroviral transduction, we observe ≈10- to 20-fold increase in expression levels of DDR1 and DDR2 by Western blot analysis (data not shown). DDR2 expression levels were at least twofold higher than those in cells overexpressing DDR1. Antibodies to the HA epitope tag recognize a 125- and 130-kd species of DDR1 and DDR2 (Figure 4C), similar to the mature forms of endogenous DDR1 and DDR2 (Figure 4B). Western blot performed after depletion of cell surface proteins by biotinylation of the cell surface and incubation with agarose-streptavidin confirmed that these 125- and 130-kd forms were the only DDR1 and DDR2 species present at the cell surface (data not shown).
Additional molecular species of DDR1 (≈62-kd species) and DDR2 (≈90-, 50-, and 45-kd species) are observed with antibodies to DDR (Figure 4B) and to HA in cell lysates from SMCs overexpressing HA epitope-tagged DDR1 and DDR2 (Figure 4C). The 62-kd product of DDR1 corresponds in size to a previously described C-terminal cleavage product of the full-length DDR1 generated by shedding.39 However, the multiple DDR2 species have not been previously characterized, and incubation with inhibitors for MMP, cysteine proteases, proteasome, lysosome, γ-secretase did not change the relative expression of the lower molecular weight species of DDR2 in SMCs cultured on polymerized collagen gels (data not shown).
Both soluble and immobilized type I collagen has been previously shown to activate DDR1 and DDR2.19,20 To test the activation of DDR1 and DDR2 in response to polymerized collagen gels, we used SMCs overexpressing DDR1 and DDR2. Tyrosine phosphorylation of both DDR1 and DDR2 are detected in SMCs on polymerized collagen gels (Figure 4D). Similar activation is observed after 4 or 24 hours of culture (data not shown). The lower molecular weight protein products of DDR1 (62 kd) and DDR2 (90 kd) are also phosphorylated on tyrosine residues (Figure 4D). Taken together, our data show that cultured human vascular SMCs express both DDR1 and DDR2, and polymerized collagen gels specifically induce their tyrosine phosphorylation.
Overexpression of DDR1 or DDR2 in SMCs Leads to Down-Regulation of Collagen Production and Increased Matrix Metalloproteinase Activity
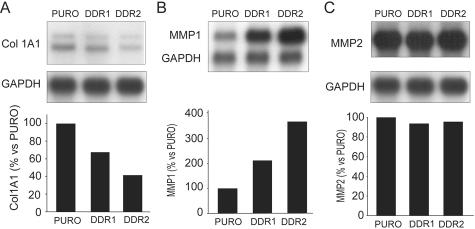
To characterize the role of DDR1 and DDR2 in the modulation of SMC ECM remodeling, we compared the mRNA expression levels of Col1α(I), MMP1, and MMP2—the major MMP secreted by SMCs—after 72 hours of culture on polymerized collagen gels. Overexpression of DDR1 and DDR2 results in a reduction of 46.0 ± 11.7% and 58.8 ± 17.3% of Col1α(I) mRNA levels, respectively (Figure 5A), whereas overexpression increases MMP1 mRNA levels by 1.87 ± 0.23-fold and by 2.62 ± 1.0-fold, respectively (Figure 5B). In contrast, under the same experimental conditions we did not observe any significant change in the levels of MMP2 mRNA (Figure 5C). At the protein level, evaluation of collagen synthesis by measuring [3H]-proline incorporation into secreted collagen demonstrates that overexpression of DDR2, and to a lesser extent DDR1, leads to a reduction of collagen production by 30.3 ± 5.5% and 19.5 ± 2.1%, respectively (n = 3). Consistent with the observed induction of mRNA, MMP1 levels increase by 3.23 ± 0.21-fold and 4.57 ± 0.56-fold for DDR1 and DDR2 (n = 3), respectively (Figure 6A). Gelatin zymography also reveals that DDR2, but not DDR1, enhanced the MMP2/pro-MMP2 ratio in human SMCs cultured on polymerized collagen gels (Figure 6A).40
Figure 5.
Collagen 1α(I) and MMP1 mRNA are regulated in human SMCs overexpressing DDR1 or DDR2. A to C: Northern blot analysis was performed with total RNA isolated from human SMCs retrovirally transduced with pBM-IRES-PURO retrovirus encoding control vector (PURO), DDR1, or DDR2 and cultured on polymerized collagen gels for 72 hours. Nylon membranes were hybridized with probes recognizing Col1α(I), MMP1, MMP2, and GAPDH. Expression levels of Col1α(I) (A), MMP1 (B), and MMP2 (C) mRNA were normalized to the GAPDH signal, all quantified with phosphoimager analysis and Imagequant software (bottom). All results are representative of three independent experiments.
Figure 6.
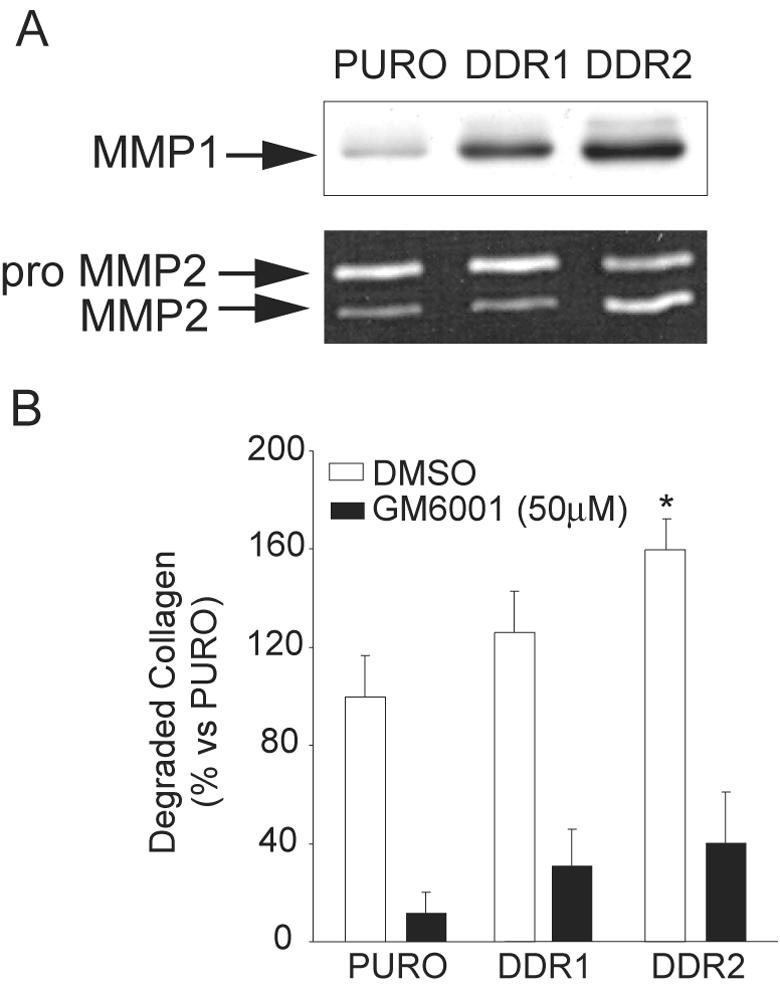
MMP-mediated collagen degradation is increased in SMCs overexpressing DDR1 or DDR2. Human SMCs were transduced with pBM-IRES-PURO retrovirus encoding control vector (PURO), DDR1, or DDR2. All of the data are representative of three independent experiments. A: MMP1 expression was analyzed in the conditioned media by Western blot analysis, and MMP2 expression and processing were analyzed in conditioned media by gelatin zymography after 72 hours of culture on polymerized collagen gels. B: Retrovirally transduced human SMCs were cultured on polymerized FITC-conjugated collagen in the presence or absence of the MMP inhibitor GM6001 (50 μmol/L final concentration). Conditioned media were collected after 72 hours. Release of FITC-conjugated collagen was measured with a 96-well plate reader. *, P < 0.05 by Student’s t-test.
Evaluation of the impact of DDR expression on ECM remodeling demonstrates that induction of DDR1 or DDR2 is sufficient to increase the ability of SMCs to degrade polymerized collagen in vitro (Figure 6B). Analysis of collagenolytic activity, measured as a release of collagen fragments into the media of SMCs cultured on FITC-conjugated polymerized collagen gels, shows a significant increase in collagen degradation in SMCs overexpressing DDR2 (151.6 ± 16.1%, n = 3) (Figure 6B). Although a trend toward increased degradation is observed in SMCs overexpressing DDR1, the difference is not statistically significant (111.9 ± 12.4%, n = 3) and may reflect the lower level of overexpression of DDR1 relative to DDR2. The basal and stimulated degradation of collagen is dependent on MMPs as demonstrated by almost complete abrogation by the metalloproteinase inhibitor, GM-6001 (Figure 6B). Thus, overexpression of DDR2, and to some extent DDR1, increases collagenolytic activity of human SMCs in vitro, through the induction of MMP1 and/or activation of MMP2.
Characterization of MMP Activity in Tissues Derived from LAM Patients
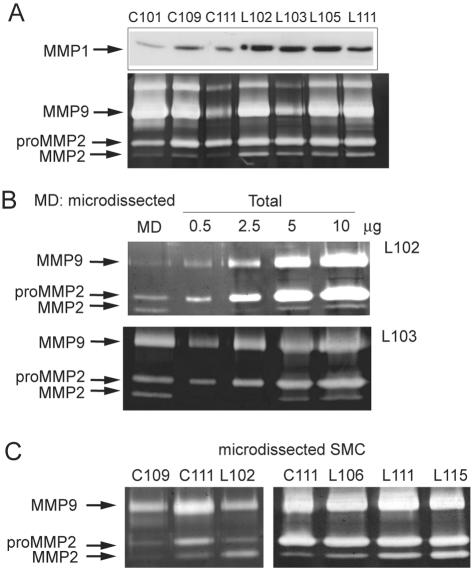
Our studies in cultured SMCs demonstrate that DDR2, and to some extent DDR1, play a significant role in collagen turnover through the simultaneous increase of MMP-dependent collagen breakdown and reduction of collagen production. Evaluation of changes in the expression levels of DDR1 and DDR2 in normal vessels versus diseased arteries and normal lung compared with LAM lesions demonstrate an apparent increase in expression levels only in LAM lesions, particularly the induction of DDR2 (Figure 1). Thus, collagen and elastin degradation mediated by MMP in the remodeling of the tissue architecture during LAM14,16 may be related to the observed changes in DDR expression. To examine the MMPs involved in LAM, we first compared the protein levels of MMP1 and the gelatinolytic activities in protein extracts from LAM tissues with normal control lungs (Figure 7A). We detect a significant increase in MMP1 expression in all LAM tissues (n = 7) as compared to normal lung (n = 4) (3.00 ± 1.27-fold) (Figure 7A and data not shown). Two prominent enzymes are observed by zymography: MMP2 (72 kd/68 kd) and MMP9 (92 kd), based on their molecular weights and ability to degrade gelatin (Figure 7A). Although we observe variable MMP9 levels between samples, increased processing of pro-MMP2 to active MMP2 is a consistent finding in all LAM tissues as compared to normal lung tissue (Figure 7A).
Figure 7.
SMCs present in LAM lesions have higher levels of MMP1 and activated MMP2 than those from normal lung. A: Total protein extracts were prepared from five 20-μm-thick frozen sections of normal (C101, C109, C111) and LAM (L102, L103, L105, L111) lungs. Protein concentrations were determined and MMP1 expression was analyzed by Western blot and gelatinase activity by gelatin zymography. B: α-Actin-positive cells were dissected with a laser-dissecting microscope (MD) from 10-μm-thick frozen sections from two different LAM patients for comparison by zymography with total protein extracts of the same specimens. C: α-Actin-positive cells were dissected from vessels present in normal lung (C109, C111) and from LAM nodules (L102, L106, L111, L115) for analysis by gelatin zymography.
To investigate the cellular source for MMP2, we used laser microdissection technology to selectively analyze the gelatinolytic activities of protein extracts from α-smooth muscle actin-positive cells within LAM nodules. SMCs dissected from two different LAM specimens contain ∼40% of the total MMP2 in the active form (Figure 7B), whereas the percentage of active MMP2 is significantly lower in total tissue protein extracts (10 to 15% of the total) (Figure 7B). A direct comparison of the gelatinolytic activity of protein extracts isolated from normal perivascular SMCs and LAM SMCs reveals a shift to an increased proportion of active MMP2 in SMCs derived from LAM tissues (Figure 7C). Taken together, these results suggest that SMCs are the major source of active MMP2 in LAM disease, and provide evidence that SMCs in LAM nodules are present in a phenotypic state characterized by higher MMP2 activation than normal lung vascular SMCs.
Discussion
DDR1- and DDR2-Mediated Signaling Regulates Collagen Turnover in Human SMCs
A number of different factors can regulate the expression of MMP1 and activation of MMP2, including MMP substrates. Hence ECM components, such as polymerized collagen gels, increase fibroblast expression of MMP1,41–43 and activate MMP2 in SMCs.40 These studies show that cell-ECM interactions, in particular those mediated through both α1β1 and α2β1 integrin collagen receptors, regulate MMP1 expression and MMP2 activation.40,41 However, other collagen receptors have been identified, and in the present study we tested the hypothesis that DDR1- and DDR2-mediated signals can regulate collagen remodeling in SMCs. Our studies demonstrate that signaling from both DDR1 and DDR2 negatively regulates collagen biosynthesis and induces MMP1 expression. In addition, we observe a specific activation of MMP2 in SMCs overexpressing DDR2, but not DDR1.
Studies of the role of DDR in cellular function have primarily focused on the analysis of mouse cells because of the availability of mice with targeted deletion of DDR1 and DDR2.21,24 However, mice do not possess a homologous gene to human MMP1, and consequently very little is known about DDR-mediated regulation of MMP1 expression. Only DDR2 has been associated with altered MMP1 activity using the human fibrosarcoma cell line HT 1080 transduced with DDR2.19 Therefore, our study is the first to establish a role for DDR1 and DDR2 in MMP1 expression in primary human cells. We also show that increased levels of MMP1 and activation of MMP2 correlate well with the ability of cultured SMCs to degrade fibrillar collagen.
Our in vitro study linking DDR with collagen remodeling is supported by the in vivo finding that ECM deposition is substantially increased in the mammary gland in DDR1-null mice.21 In addition, a defect in blastocyst implantation in DDR1-null mice has been ascribed to a reduction of MMP activity.21 In contrast, the expression pattern of MMP2, MMP9, MMP13, and MT1-MMP are normal in DDR2-null mice,24 although it is possible that DDR1 functions compensate for the loss of DDR2 in these mice. Interestingly, the absence of the major collagen integrin receptor expressed in SMCs in vivo, such as α1β1 integrin, causes loss of feedback regulation of collagen synthesis, revealing a common, receptor-mediated regulatory mechanism of collagen deposition.44 However, in α1-null mice a simultaneous increase in collagenase 3 (MMP13) overcomes the loss of negative feedback by increasing collagen breakdown, whereas DDR1-null mice and DDR2-null fibroblasts, have decreased MMP2 expression.22,26,41
The role of DDR2 in MMP2 expression has been previously shown in mouse fibroblast and hepatic stellate cells.25,26 In particular, analysis of 293T cells transiently transfected with DDR2 and the comparison between DDR2-null and heterozygous skin fibroblasts, show that DDR2 signaling induces MMP2 promoter activity.26 In contrast, in the present study, human SMCs overexpressing DDR2 do not show any difference in MMP2 mRNA expression, but a significant increase in MMP2 levels and activity, suggesting a different level of regulation between cell types. It is important to note that our observation is consistent with several examples showing that MMP2 expression levels are usually regulated at the translational level.1
Potential Role of DDR1 and DDR2 in Collagen Remodeling Observed in Atherosclerosis and LAM Disease
At late stages of atherosclerosis, stability of the fibrous cap can be compromised by enzyme-mediated degradation of type I collagen and elastin that provide most of the cap’s biomechanical strength. Focal degradation of the fibrous cap often leads to its rupture that is followed by thrombosis and can result in complete vessel occlusion associated with heart attacks and strokes. In cultured human SMCs, we have established a role for DDR in reduced collagen biosynthesis and increased MMP-dependent collagen degradation. Moreover, an elastinolytic activity assay performed using human SMCs, has shown that DDR2 overexpression leads to 3.58 ± 1.4-fold increase in elastin degradation, which was completely blocked by the MMP inhibitor GM-6001 (data not shown). These data strongly suggest a potential role for DDR activation in influencing plaque stability. Consistent with this possibility, it has been shown that expression of DDR1 is increased after acute balloon injury of a normal rat carotid artery and DDR1-null mice develop smaller neointima 14 days after balloon injury.22 In vitro, DDR1-null SMCs appear to have a decreased proliferative and migratory response and reduced MMP2 and MMP9 expression.22
However, the role of DDR-mediated signaling in collagen remodeling during atherosclerosis is still uncertain, although our analyses of atherosclerotic lesion development demonstrate that both DDR1 and DDR2 are expressed in SMCs localized to the fibrous cap and macrophages in early lesions and within the necrotic core. Nevertheless, we failed to observe a significant modulation of DDR1 or DDR2 mRNA or protein expression levels at different stages of atherosclerosis as compared with normal vessels from nonhuman primates. Until the complete sequence of the nonhuman primate is available, our studies cannot exclude the possibility of differences between nonhuman primate and human DDR isoforms. However, our data demonstrate comparable fragments from human and nonhuman primate tissue consistent with their extremely low sequence divergence.36,37
Very little is known about the regulation of DDR signaling in vivo. At least three different mechanisms have been proposed to regulate DDR signaling: alternative splicing,35 ectodomain shedding,39 and the extracellular concentration of collagen.25 The latter mechanism is supported by evidence that collagen content, which increases after liver injury, probably constitutes the limiting factor for DDR2 activation.25 In fact, DDR2 phosphorylation is detected only after injury in which collagen deposition is increased, despite its expression in both normal and diseased liver.25 Moreover, our in vitro data on DDR-mediated reduction of collagen deposition and MMP-dependent collagen breakdown may be indicative of a negative feedback loop whereby DDR signaling reduces the ligand availability turning off DDR tyrosine phosphorylation.
Our data in LAM specimens demonstrating apparent sustained up-regulation of DDR1 and DDR2 implicate the DDR in ECM remodeling in LAM disease. Although SMC accumulation and matrix remodeling are features shared in common by atherosclerosis and LAM, the pathogenesis of LAM is not understood and animal models are not available to analyze cellular and molecular changes at early time points. The differences observed in DDR levels between normal and LAM lungs may be dependent on either specific up-regulation or changes in cellular composition in LAM specimens. Very little is known about MMP expression in LAM. Immunohistochemical studies conducted on LAM biopsy specimens have provided evidence for the presence of MMP2 in areas of ECM remodeling.14,16 We demonstrate by Western blot analysis that MMP1 is consistently up-regulated in LAM lung specimens as compared with control lung, and provide evidence for the presence of active MMP2 in LAM tissue. Using laser microdissection technology, we establish that the major source for MMP2 in LAM nodules is SMCs, and that these cells express higher levels of active MMP2 than SMCs isolated from normal lung arteries. Although several factors may contribute to MMP expression during LAM, increased DDR1 and DDR2 expression levels in SMCs contained in LAM nodules are indicative of their involvement in ECM remodeling in LAM disease.
In conclusion, we show that DDR1 and DDR2 are expressed in SMCs in lesions of atherosclerosis and LAM. Phosphorylation of DDR1 and DDR2 in cultured human SMCs in response to collagen leads to decreased collagen biosynthesis and increased collagen and elastin breakdown mediated by MMP1 and MMP2 that may contribute to the ECM remodeling observed during these pathological conditions.
Acknowledgments
We thank Garry Nolan (Stanford University, Stanford, CA) for generously providing the pBM series retroviral vectors and Phoenix-A retroviral packaging cells; Lynn Schnapp, David Thorning, Charles Frevert (University of Washington, Seattle, WA), and Marilyn Glassberg (University of Miami, Miami, FL) for provision of normal lung; Joel Moss (National Institutes of Health, Bethesda, MD) and Marty Wallace (LAM Registry) for LAM specimens; Carole Balach for her editorial assistance; and Roderick Browne, Li-Chuan Huang, and Julie Philalay for their technical assistance.
Footnotes
Address reprint requests to Elaine W. Raines, Department of Pathology, Harborview Medical Center, 325 9th Ave., Box 359675, Seattle, WA 98104-2499. E-mail: ewraines@u.washington.edu.
Supported by the National Institutes of Health (grant no. HL18645 to E.W.R.), a LAM Foundation Award (to N.F.), and National Center for Research Resources grant RR-00166 to the Northwest Primate Center.
Current address of N.F.: Department of Pharmacologic Sciences, University of Milan, Milan 20133 Italy.
Current address of N.O.C.: Beatson Institute for Cancer Research, Cancer Research UK, Glasgow G61 1BD, Scotland UK.
References
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 2002;227:301–314. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation. 2000;102:2861–2866. doi: 10.1161/01.cir.102.23.2861. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- Barnes MJ. Collagens in atherosclerosis. Coll Relat Res. 1985;5:65–97. doi: 10.1016/s0174-173x(85)80048-0. [DOI] [PubMed] [Google Scholar]
- Schroeder AP, Falk E. Pathophysiology and inflammatory aspects of plaque rupture. Cardiol Clin. 1996;14:211–220. doi: 10.1016/s0733-8651(05)70274-5. [DOI] [PubMed] [Google Scholar]
- Southgate KM, Fisher M, Banning AP, Thurston VJ, Baker AH, Fabunmi RP, Groves PH, Davies M, Newby AC. Upregulation of basement membrane-degrading metalloproteinase secretion after balloon injury of pig carotid arteries. Circ Res. 1996;79:1177–1187. doi: 10.1161/01.res.79.6.1177. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Zempo N, Kenagy RD, Au YP, Bendeck M, Clowes MM, Reidy MA, Clowes AW. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg. 1994;20:209–217. doi: 10.1016/0741-5214(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans VJ, Yu ZX, Nelson WK, Valencia JC, Tatsuguchi A, Avila NA, Riemenschn W, Matsui K, Travis WD, Moss J. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch. 2000;67:311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Kawamoto M, Yamamoto A, Ishizaki M, Basset F, Masugi Y. Role of elastic fiber degradation in emphysema-like lesions of pulmonary lymphangiomyomatosis. Hum Pathol. 1990;21:1252–1261. doi: 10.1016/s0046-8177(06)80039-0. [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri RA, Jr, Krymskaya VP. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fleming MV, Stetler-Stevenson WG, Liotta LA, Moss J, Ferrans VJ, Travis WD. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM). Hum Pathol. 1997;28:1071–1078. doi: 10.1016/s0046-8177(97)90061-7. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107:727–735. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90:1147–1149. doi: 10.1161/01.res.0000022166.74073.f8. [DOI] [PubMed] [Google Scholar]
- Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, Bruckner K, Goergen JL, Lemke G, Yancopoulos G, Angel P, Martinez C, Klein R. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2:446–452. doi: 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–1378. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaso E, Labrador JP, Wang L, Ikeda K, Eng FJ, Klein R, Lovett DH, Lin HC, Friedman SL. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem. 2002;277:3606–3613. doi: 10.1074/jbc.M107571200. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, Raines EW, Nakano T, Graves LM, Krebs EG, Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994;93:1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- Tsukada T, Rosenfeld M, Ross R, Gown AM. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986;6:601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- Alwine JC, Kemp DJ, Stark GR. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci USA. 1977;74:5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves F, Saupe S, Ledwon M, Schaub F, Hiddemann W, Vogel WF. Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines. EMBO J. 2001;15:1321–1323. doi: 10.1096/fj.00-0626fje. [DOI] [PubMed] [Google Scholar]
- Garton KJ, Ferri N, Raines EW. Efficient expression of exogenous genes in primary vascular cells using IRES-based retroviral vectors. Biotechniques. 2002;32:830–834. doi: 10.2144/02324rr01. [DOI] [PubMed] [Google Scholar]
- Goldstein RH. Control of type I collagen formation in the lung. Am J Physiol. 1991;261:L29–L40. doi: 10.1152/ajplung.1991.261.2.L29. [DOI] [PubMed] [Google Scholar]
- McCullagh KG, Duance VC, Bishop KA. The distribution of collagen types I, III and V (AB) in normal and atherosclerotic human aorta. J Pathol. 1980;130:45–55. doi: 10.1002/path.1711300107. [DOI] [PubMed] [Google Scholar]
- Vogel W. Discoidin domain receptors: structural relations and functional implications. EMBO J. 1999;13:S77–S82. doi: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- Britten RJ. Divergence between samples of chimpanzee and human DNA sequences is 5%, counting indels. Proc Natl Acad Sci USA. 2002;99:13633–13635. doi: 10.1073/pnas.172510699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama A, Watanabe H, Toyoda A, Taylor TD, Itoh T, Tsai SF, Park HS, Yaspo ML, Lehrach H, Chen Z, Fu G, Saitou N, Osoegawa K, de Jong PJ, Suto Y, Hattori M, Sakaki Y. Construction and analysis of a human-chimpanzee comparative clone map. Science. 2002;295:131–134. doi: 10.1126/science.1065199. [DOI] [PubMed] [Google Scholar]
- Skinner MP, Raines EW, Ross R. Dynamic expression of alpha 1 beta 1 and alpha 2 beta 1 integrin receptors by human vascular smooth muscle cells. Alpha 2 beta 1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- Vogel WF. Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett. 2002;514:175–180. doi: 10.1016/s0014-5793(02)02360-8. [DOI] [PubMed] [Google Scholar]
- Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol. 1999;147:619–630. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha 1 beta 1 and alpha 2 beta 1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zutter MM, Santoro SA, Clark RA. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J Cell Biol. 1998;140:709–719. doi: 10.1083/jcb.140.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Clark RA, Parks WC. p38 mitogen-activated kinase is a bidirectional regulator of human fibroblast collagenase-1 induction by three-dimensional collagen lattices. Biochem J. 2001;355:437–447. doi: 10.1042/0264-6021:3550437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112:263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]