Abstract
Transcription factor Ets-1 has been reported to regulate angiogenesis in vascular endothelial cells. Here, we investigated a mechanism that may regulate the expression of Ets-1 in vascular endothelial growth factor (VEGF)- and hypoxia-induced retinal neovascularization and that may have potential to inhibit ocular neovascular diseases. VEGF and hypoxia increased Ets-1 expression in cultured bovine retinal endothelial cells. The VEGF-induced mRNA increase of Ets-1 was suppressed by a tyrosine kinase inhibitor (genistein), by inhibitors of MEK (mitogen-activated protein and extracellular signal-regulated kinase kinase) (PD98059 and UO126), and by inhibitors of protein kinase C (GF109203X, staurosporine, and Gö6976). Dominant-negative Ets-1 inhibited VEGF-induced cell proliferation, tube formation, and the expression of neuropilin-1 and angiopoietin-2. In a mouse model of proliferative retinopathy, Ets-1 mRNA was up-regulated. Intravitreal injection of dominant-negative Ets-1 suppressed retinal angiogenesis in a mouse model of proliferative retinopathy. In conclusion, VEGF induces Ets-1 expression in bovine retinal endothelial cells and its expression is protein kinase C/ERK pathway-dependent. Ets-1 up-regulation is involved in the development of retinal neovascularization, and inhibition of Ets-1 may be beneficial in the treatment of ischemic ocular diseases.
Pathological growth of new blood vessels is the common final pathway in ocular neovascular diseases such as diabetic retinopathy, retinopathy of prematurity, and age-related macular degeneration, and often leads to catastrophic loss of vision. Vascular endothelial growth factor (VEGF) has been proven to be a predominant angiogenic factor that mediates such ocular neovascularization. VEGF is increased by hypoxia,1 which is one of the primary stimuli for ocular neovascularization. VEGF inhibition by soluble VEGF receptor 1 protein or adenovirus vector-encoding soluble VEGF receptor 1 have been reported to reduce retinal neovascularization effectively.2,3
The Ets gene family conserves an 85-amino acid DNA-binding ETS domain that binds the consensus sequence 5′-GGA(A/T)-3′ in the promoter region of the target genes,4 and have various biological functions, including cellular growth, differentiation, and organ development.5 Ets-1, first identified among the Ets gene family, has been shown to also be associated with pathological angiogenesis. Increased Ets-1 expression is observed in cultured endothelial cells and in endothelial cells of new vessels during tumor angiogenesis in the adult.6,7 A number of angiogenesis-related molecules, including matrix metalloproteinase (MMP)-1, MMP-3, MMP-9, urokinase-type plasminogen activator, integrin β3, vascular endothelial-cadherin (VE-cadherin), and neuropilin-1 (NRP1) are reported to be targets of Ets-1 in endothelial cells.7–10 Receptor tyrosine kinases such as VEGF receptor 1, VEGF receptor 2, and TIE1/2 (tyrosine kinase that contains immunoglobulin-like loops and epidermal growth factor-similar domains), have been reported to have ETS-binding motif in their promoter regions.11–13 Despite reports of the role of Ets-1 in angiogenesis of various tissues, its role in ocular angiogenesis has not been investigated. In this study, we investigated Ets-1 regulation and its function in VEGF- and ischemia-induced retinal neovascularization.
Materials and Methods
Reagents
Human recombinant VEGF was obtained from Genzyme (Cambridge, MA). Goat anti-human VEGF neutralizing antibody was purchased from R&D Systems (Minneapolis, MN). Rabbit polyclonal anti-Ets-1 antibody and rabbit polyclonal anti-extracellular signal-regulated kinase 1 (ERK1) antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-phospho-p44/p42 antibody was purchased from New England Biolabs (Beverly, MA). PD98059, wortmannin, genistein, GF109203X, staurosporine, rottlerin, and Gö6976 were obtained from Calbiochem (La Jolla, CA). UO126 was obtained from Cell Signaling Technology (Beverly, MA). All other materials were obtained from Sigma (St. Louis, MO).
Cell Culture
Bovine retinal endothelial cells (BRECs) were grown under previously described condition.14 BRECs were cultured in Dulbecco’s modified Eagle’s medium with 5.5 mmol/L glucose, 10% plasma-derived horse serum (Wheaton, Pipersville, PA), 50 mg/ml heparin, and 50 U/ml endothelial cell growth factor (Roche Diagnostics, Indianapolis, IN). Cells were characterized for their homogeneity by immunoreactivity with anti-factor VIII antibody, and remained morphologically unchanged under these conditions, as confirmed by light microscopy. BRECs were exposed to human recombinant VEGF or exposed to hypoxic conditions as described.14 For hypoxic study, cells were exposed to 1 ± 0.5% oxygen using a water-jacketed mini-CO2/multigas incubator with reduced oxygen control (model BL-40M; Jujikagaku, Tokyo, Japan). All cells were maintained at 37°C in a constant carbon dioxide atmosphere with oxygen deficit induced by nitrogen replacement. To determine the signaling pathways involved in VEGF-induced Ets-1 mRNA expression, BRECs were treated for 4 hours with VEGF (25 ng/ml) after pretreatment for 30 minutes with genistein, a tyrosine kinase inhibitor (200 μmol/L); GF109203X, a general protein kinase C (PKC) inhibitor (5 μmol/L); staurosporine, a general PKC inhibitor (100 nmol/L); rottlerin, an inhibitor of PKCδ (5 μmol/L); Gö6976, an inhibitor of classical PKC (5 μmol/L); PD98059, an inhibitor of MEK (mitogen-activated protein and ERK kinase) (50 μmol/L); UO126, an inhibitor of MEK (10 μmol/L); or wortmannin, a phosphatidylinositol 3-kinase (PI3-kinase) inhibitor (100 nmol/L), respectively. To investigate the effects of VEGF on ERK phosphorylation, BRECs were treated for 10 minutes with VEGF (10 ng/ml) after pretreatment with GF109203X (5 μmol/L) or PD98059 (50 μmol/L) for 30 minutes.
Recombinant Adenovirus-Mediated Gene Transfer
The sequence encoding the Ets domain and lacking the transactivation domain of murine Ets-1 was amplified from murine c-ets-1 cDNA by the polymerase chain reaction with oligonucleotides CCG CTC GAG CCA CCA TGG CTC CTG CTG CTG CCC T and GGC CTC GAG CTA AGC ATA ATC TGG AAC ATC ATA TGG ATA GTC AGC ATC CGG CT having XhoI restriction sites and HA epitope. The amplified product was digested with XhoI and subcloned into pGEM11zf(+), as described previously.15 Adenovirus vector encoding dominant-negative Ets-1 was constructed by homologous recombination in 293 cells between the transfer cassette bearing the expression unit of dominant-negative Ets-1 and almost the entire adenovirus genome and restriction enzyme-digested adenovirus genome tagged with terminal protein.15 The adenovirus was applied at a concentration of 1 × 108 plaque-forming units/ml, and adenovirus with the genome carrying an enhanced green fluorescent protein gene (GFP) (Clontech, Palo Alto, CA) or lacZ were used as controls as described.16 Infection efficiency was monitored by fluorescence, which showed expression in >80% of cells. Expression of recombinant protein was confirmed by Western blot analysis.
Mouse Model of Proliferative Retinopathy
The study adhered to the Association for Research in Vision and Ophthalmology (ARVO) Standards for the Use of Animals in Ophthalmic and Vision Research. The well-established mouse model of proliferative retinopathy was created as described.17 Briefly, litters of 7-day-old (postnatal day 7, P7) C57BL/6J mice were exposed to 75% oxygen for 5 days and then were returned to room air at P12 to produce retinal neovascularization. Mice of the same age, maintained in room air, served as controls.
Northern Blot Analysis
Total RNA was isolated from cells and retinas of mice using guanidinium thiocyanate, and Northern blot analysis was performed as described.18 Total RNA (20 μg) was electrophoresed through 1% formaldehyde-agarose gels and then transferred to a nylon membrane (Pall BioSupport, East Hills, NY). After ultraviolet cross-linking, blots were prehybridized, and hybridized with 32P-labeled cDNA. All signals were analyzed using a densitometer (BAS-2000 II; Fuji Photo Film, Tokyo, Japan). The signal for each sample was normalized by reprobing the same blot using 36B4 cDNA control probe. cDNA template of murine Ets-1, human NRP1, and human angiopoietin-2 (Ang-2) were prepared by reverse transcriptase-polymerase chain reaction using the following primer pairs: 5′-CCC TGG GTA AAG AAT GCT TTC TCG-3′ (Ets-1 sense), 5′-GGA CTG ACA AGA CTT ATC AGT GAG-3′ (Ets-1 anti-sense), 5′-CAC ATT GGG CGT TAC TGT GGA C-3′ (NRP1 sense), 5′-CCT TTG TGG TTG GGG TGT CTA C-3′ (NRP1 anti-sense), 5′-AGC TGT GAT CTT GTC TTG GC-3′ (Ang-2 sense), and 5′-GTT CAA GTC TCG TGG TCT GA-3′ (Ang-2 anti-sense). For the animal studies, total RNA was isolated from retinas of mice at different time points (10 retinas from five mice at each time point: P12 immediately after return to room air, P13, P15, P17, P19, P21, P23, and P26).
Western Blot Analysis
Total protein from cells was assessed by Western blot analysis as described.19 BRECs were washed with ice-cold phosphate-buffered saline (PBS) and lysed in 1× Laemmli buffer (50 mmol/L Tris, pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol) containing protease inhibitors (10 mmol/L sodium pyrophosphate, 100 mmol/L NaF, 1 mmol/L Na3VO4, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 2mmol/L phenylmethyl sulfonyl fluoride). Total protein (30 μg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and was transferred to nitrocellulose membrane (Bio-Rad Laboratories, Richmond, CA). The blots were incubated overnight at 4°C with primary antibodies followed by incubation for 2 hours with horseradish peroxidase-conjugated secondary antibody (1:2000 dilution) (Amersham International, Buckinghamshire, UK). Primary antibodies specific for Ets-1, phospho-p44/p42, and ERK1 were used at 1:500, 1:2000, and 1:2000 dilutions, respectively. Visualization was performed by enhanced chemiluminescence detection system (Amersham International).
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear cell extracts were prepared by the freezing-thawing method as described.20 Synthetic oligonucleotide was as follows: Ets-1 consensus, 5′-GTC AGT TAA GCA GGA AGT GAC TAA C-3′.21 The sequences of the double-strand oligonucleotide probes labeled with T4 kinase and γ-32P ATP were generated by use of labeling kits (Promega, Madison, WI). Reactions of nuclear cell extract and 32P-labeled consensus oligonucleotides were electrophoresed through a 4% nondenaturing polyacrylamide gel, and the signal was analyzed using a densitometer (BAS-2000 II; Fuji Photo Film).
Cell Growth Assay and Tube Formation Assay
Cell growth assay was studied by DNA concentration measured by a fluorometer as described.16 BRECs were plated in 12-well plates at a density of 1 × 104 cells/well in Dulbecco’s modified Eagle’s medium containing 5% calf serum. After 24 hours of incubation, cells were infected with adenovirus, and the medium was replaced with Dulbecco’s modified Eagle’s medium containing 5% calf serum with or without VEGF (10 ng/ml). Four days later, the cells were lysed in 0.1% sodium dodecyl sulfate, and DNA concentrations in each well were measured using Hoechst 33258 dye (Calbiochem) and a fluorometer (model DyNA Quant 200; Hoefer, San Francisco, CA). In vitro tube formation assays were performed as described.18 Vitrogen 50 (Cohesion, Palo Alto, CA), 0.2 N NaOH, and 200 mmol/L HEPES (8:1:1, v/v/v) and 10× RPMI medium (Gibco BRL-Invitrogen, Carlsbad, CA) were made to 400 μl and added to 24-well plates. After polymerization of the gels, 1 × 105 BRECs were seeded and incubated with Dulbecco’s modified Eagle’s medium containing 5% plasma-derived horse serum for 24 hours. After infection with adenovirus, additional collagen gel was added, and then BRECs were incubated with medium containing 3% plasma-derived horse serum with or without VEGF (25 ng/ml). Five days later, five different fields (×4 objective) were chosen, and total tube-like structures were measured using public domain NIH image software (downloaded from http://rsb.info.nih.gov/nih-image/Default.html).
Intraocular Injection
A solution (1 μl) containing adenovirus vector encoding dominant-negative Ets-1 (1 × 1010 plaque-forming units/ml) was injected into the vitreous of one eye of anesthetized C57BL/6J mice with a 33-gauge needle (Ito, Hamamatsu, Japan) on P12, as previously described.2,3,22 As a control, an equivalent amount of control adenovirus was injected into the contralateral eye. At P19, the mice were killed by cardiac perfusion of 1 ml 4% paraformaldehyde in PBS, and the eyes were enucleated and fixed in 4% paraformaldehyde overnight at 4°C before paraffin embedding. Serial 6-μm paraffin-embedded axial sections were obtained from the optic nerve and stained with hematoxylin and periodic acid-Schiff, according to a standard protocol. All retinal vascular nuclei anterior to the internal limiting membrane were counted in each section by a fully masked protocol. For each eye, 10 intact sections of equal length, each 30 μm apart, were evaluated. The mean number of neovascular nuclei per section per eye was then determined.
Statistical Analysis
Determinations were performed in triplicate, and experiments were repeated at least three times. Results are expressed as the mean ± SEM. One-way analysis of variance followed by the Fisher t-test was used to evaluate significant differences, and P < 0.05 was selected as the statistically significant value. For evaluation of in vivo retinal angiogenesis, the chi-square test for categorical data and the paired Student’s t-test or the Mann-Whitney rank sum test for quantitative data with unequal variance are used.
Results
Ets-1 mRNA Expression Is Induced by VEGF
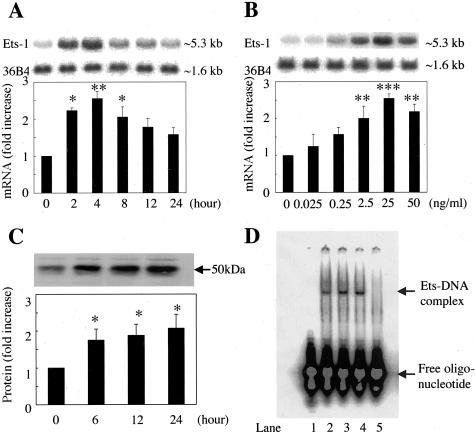
The effects of VEGF on the expression of Ets-1 mRNA were studied by Northern blot analysis in BRECs. VEGF (25 ng/ml) increased Ets-1 mRNA levels in a time-dependent manner, reaching a maximum after 4 hours (2.5 ± 0.2-fold, P < 0.01) (Figure 1A). The dose response to VEGF-induced Ets-1 mRNA expression was studied after 4 hours of VEGF stimulation. The expression of Ets-1 mRNA was up-regulated in a dose-dependent manner, with an EC50 of ∼0.25 ng/ml; maximal increase was observed at a VEGF concentration of 25 ng/ml (2.5 ± 0.1-fold, P < 0.001) (Figure 1B).
Figure 1.
Stimulation of Ets-1 expression by VEGF. A: Time-dependent induction of Ets-1 mRNA after stimulation by VEGF. Total RNA was isolated at the indicated time points after the cells were stimulated with VEGF (25 ng/ml), and Northern blot analysis was performed. B: Dose-dependent induction of Ets-1 mRNA 4 hours after stimulation by VEGF. BRECs were treated with the indicated concentrations of VEGF for 4 hours. Representative Northern blots and control 36B4 (top) and quantitation after normalization to the control signals are shown (bottom). C: Effect of VEGF on Ets-1 protein expression. BRECs were studied after 6, 12, and 24 hours of incubation with VEGF (25 ng/ml). Total protein (30 μg) was assessed by Western blot analysis using anti-Ets-1 antibody. The size of Ets-1 protein corresponded to ∼55 kd. Representative Western blots (top) and quantitation (bottom). Results are shown as fold increases of control. Mean ± SEM of three separate experiments (each in triplicate). *, P < 0.05; **, P < 0.01; ***, P < 0.001. D: Stimulation of DNA-binding activity by VEGF. DNA-binding activity of nuclear protein was analyzed by EMSA. BRECs were incubated for 12 or 24 hours with VEGF (25 ng/ml), and then nuclear protein was obtained and applied to EMSA. Lane 1, negative control (free probe alone); lane 2, BRECs basal level (control); lane 3, VEGF stimulation for 12 hours; lane 4, VEGF stimulation for 24 hours; lane 5, VEGF stimulation for 12 hours with unlabeled competitor.
VEGF Increases Ets-1 Protein Production
Ets-1 protein expression was studied by Western blot analysis in BRECs using anti-Ets-1 antibody. VEGF increased significantly Ets-1 protein expression after 6 hours (1.7 ± 0.3-fold, P < 0.05), 12 hours (1.8 ± 0.3-fold, P < 0.05), and 24 hours (2.0 ± 0.4-fold, P < 0.05) (Figure 1C).
DNA-Binding Activity Is Stimulated by VEGF
The DNA-binding activity of nuclear proteins was studied by EMSA. After 12 hours and 24 hours of stimulation of BRECs with VEGF, significant increases of DNA-Ets-1 complex were observed. The DNA-Ets-1 complex disappeared by adding unlabeled competitor (Figure 1D).
Ets-1 Induction by Hypoxia Is Mediated by VEGF
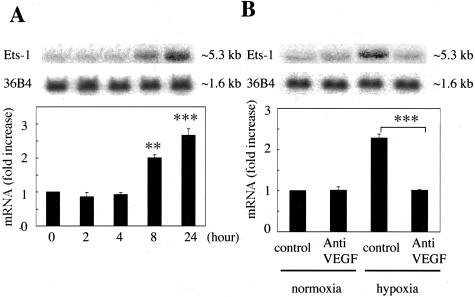
The effects of hypoxia on the expression of Ets-1 mRNA were studied by Northern blot analysis in BRECs. Ets-1 mRNA levels were increased significantly after 8 hours (2.0 ± 0.1-fold, P < 0.01) and 24 hours of hypoxia stimulation (2.6 ± 0.2-fold, P < 0.001) (Figure 2A). Because VEGF expression is up-regulated by hypoxia, we studied whether or not VEGF mediation is involved in the observed hypoxia regulation of Ets-1 by using anti-VEGF neutralizing antibody. The increased Ets-1 mRNA levels induced by hypoxia for 24 hours were almost completely reversed by anti-VEGF neutralizing antibody (Figure 2B).
Figure 2.
Stimulation of Ets-1 mRNA expression by hypoxia. A: Time-dependent induction of Ets-1 mRNA by hypoxia. BRECs were incubated under hypoxic conditions (1% O2). Total RNA was isolated at the indicated time points after hypoxic stimulation, and Northern blot analysis was performed. B: Effects of anti-VEGF neutralizing antibody on hypoxia induced Ets-1 mRNA expression. Total RNA was isolated from BRECs 24 hours after stimulation by hypoxic (1% O2) or normoxic conditions with or without anti-VEGF neutralizing antibody (10 μg/ml), and Northern blot analysis was performed. Representative Northern blots and control 36B4 (top) and quantitation (bottom). Results are shown as fold increases of control. Mean ± SEM of three separate experiments (each in triplicate). **, P < 0.01; ***, P < 0.001.
Ets-1 mRNA Expression Is Increased During Retinal Neovascularization
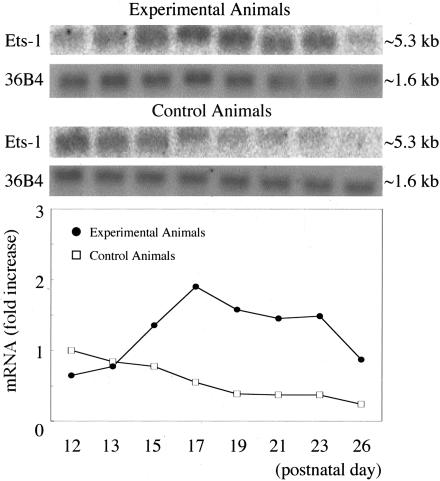
Using Northern blot analysis, we further studied regulation of the Ets-1 gene in an in vivo model of proliferative retinopathy. Total RNA was isolated from retinas of mice at different time points (10 retinas from five mice at each time point: P12 immediately after return to room air, P13, P15, P17, P19, P21, P23, and P26). In the animals in which retinal ischemia was induced, Ets-1 mRNA levels were lower compared with those in the control animals at P12, but a remarkable increase of the mRNA level was observed from P15 to P23. In contrast, in age-matched control animals, Ets-1 mRNA levels decreased gradually from P12 to P26 (Figure 3). A maximal 4.0-fold increase was detected at P19 compared with that in the normal age-matched controls.
Figure 3.
Ets-1 mRNA expression during retinal neovascularization. Results of the Northern blot analysis of total RNA isolated from retinas of animals of proliferative retinopathy and from retinas of normal age-matched controls. Northern blots and control 36B4 (top) and quantification after normalization to 36B4 signal (bottom). After normalization to the 36B4 signal, the increases in Ets-1 mRNA at each time point compared with age-matched control at P12 are shown. Black circle, experimental animals; white square, control animals.
Effects of Tyrosine Kinase, PKC, MEK, and PI3-Kinase Inhibition on VEGF-Dependent Ets-1 Induction
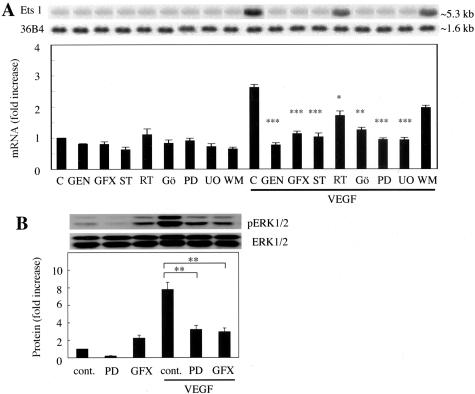
The effects of tyrosine kinase, PKC, MEK, and PI3-kinase inhibitors were determined by Northern blot analysis. Wortmannin, a PI3-kinase inhibitor caused no significant change in the effect of VEGF on Ets-1 mRNA expression in BRECs, but genistein, a tyrosine kinase inhibitor did inhibit the effects of VEGF by 71 ± 4.1% (P < 0.001). MEK inhibitors, PD98059 and UO126 inhibited the effects of VEGF by 65 ± 2.0% (P < 0.001) and 65 ± 2.8% (P < 0.001), respectively. General PKC inhibitors, GF109203X and staurosporine inhibited the effects of VEGF by 58 ± 4.5% (P < 0.001) and 61 ± 6.7% (P < 0.001), respectively. PKCδ inhibitor, rottlerin, and classical PKC inhibitor, Gö6976 inhibited the effects of VEGF by 35 ± 7.7% (P < 0.05) and 53 ± 3.9% (P < 0.01), respectively (Figure 4A).
Figure 4.
Role of tyrosine kinase, PKC, ERK, and PI3-kinase in VEGF-induced Ets-1 expression. A: BRECs were pretreated with genistein (GS, 200 μmol/L), GF109203X (GFX, 5 μmol/L), staurosporine (ST, 100 nmol/L), rottlerin (RT, 5 μmol/L), Gö6976 (Gö, 5 μmol/L), PD98059 (PD, 50 μmol/L), UO126 (UO, 10 μmol/L), or wortmannin (WM, 100 nmol/L) followed by stimulation with 25 ng/ml of VEGF for 4 hours. Total RNA was assessed by Northern blot analysis. Representative Northern blots and control 36B4 (top) and quantitation (bottom). *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus control with VEGF. B: VEGF-induced ERK phosphorylation is PKC-dependent. BRECs were stimulated with VEGF (10 ng/ml) for 10 minutes after pretreatment with GF109203X (GFX, 5 μmol/L) or PD98059 (PD, 50 μmol/L) for 30 minutes. Total proteins were assessed by Western blot analysis using anti-phospho-ERK1(p44)/ERK2(p42) antibody and with anti-ERK1 (total) antibody. Signals of phospho-ERK1/2 (pERK1/2) protein were quantified by those of total ERK1/2 protein. Representative Western blots (top) and quantitation (bottom). Results are shown as fold increases of control. Mean ± SEM of three separate experiments (each in triplicate). **, P < 0.01.
VEGF-Induced ERK Phosphorylation Is PKC-Dependent
We investigated the involvement of PKC in ERK pathway by Western blot analysis. We investigated the effects of a PKC inhibitor, GF109203X, or a MEK inhibitor, PD98059, on ERK1/2 phosphorylation in BRECs. Both GF109203X and PD98059 decreased ERK1/2 phosphorylation by 40 ± 6.7% (P < 0.01), and 36 ± 6.8% (P < 0.01), respectively (Figure 4B).
Effects of Dominant-Negative Ets-1 on NRP1 and Ang-2 Expression
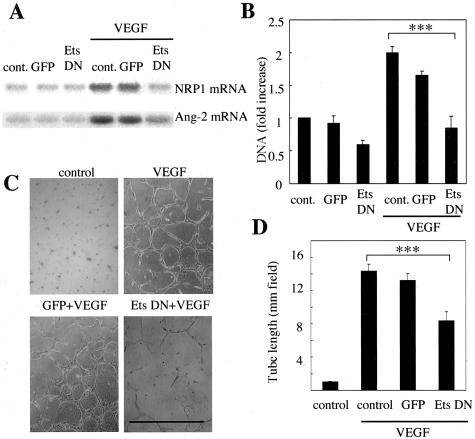
To further investigate the role of Ets-1 in retinal neovascularization, we determined effects of Ets-1 dominant-negative on expression of NRP1 and Ang-2 in BRECs stimulated by VEGF. Both NRP1 and Ang-2 mRNA expression were decreased by dominant-negative Ets-1 (Figure 5A).
Figure 5.
Effects of Ets-1 blockade on angiogenic activity in BRECs. A: Effects of Ets-1 blockade on neuropilin-1 (NRP1) and angiopoietin-2 (Ang-2) expression. BRECs were infected with adenovirus vector encoding dominant-negative Ets-1 (Ets DN) or adenovirus with the genome carrying an enhanced GFP followed by stimulation with VEGF (25 ng/ml) for 4 hours. Northern blot analysis was performed. Representative Northern blots were shown; top, neuropilin-1 mRNA expression; bottom, angiopoietin-2 mRNA expression. Similar data were obtained from another Northern blot analysis (data not shown). B: The cell growth was analyzed by DNA concentrations measured by a fluorometer. BRECs were incubated with vehicle (PBS), or infected with adenovirus encoding dominant-negative Ets-1 (Ets DN) and GFP, followed by incubation in the medium with or without VEGF (10 ng/ml). After 4 days of incubation, the cells were lysed in 0.1% sodium dodecyl sulfate and DNA concentrations were measured. Results are shown as fold increases of control. ***, P < 0.001. C: Effects of Ets-1 blockade on in vitro tube formation. BRECs were seeded in a three-dimensional collagen gel and incubated with VEGF (25 ng/ml) for 5 days. Total lengths of tube formation of each well were measured and tube lengths (mm/field) were compared. Representative phase-contrast micrographs of tube formation in three experiments; top left, control; top right, VEGF stimulation; bottom left, adenovirus encoding GFP transfectants with VEGF; bottom right, adenovirus encoding dominant-negative Ets-1 transfectants (Ets DN) with VEGF. D: Results are shown as tube length. Mean ± SEM of three separate experiments (each in triplicate). ***, P < 0.001. Scale bar, 1 mm (C).
Effects of Ets-1 Blockade on Angiogenic Activity in BRECs
To investigate the role of Ets-1 in VEGF-dependent angiogenesis in vitro, we examined whether or not dominant-negative Ets-1 would affect cell proliferation and tube formation of BRECs. VEGF (10 ng/ml) increased DNA synthesis (2.0 ± 0.1-fold, P < 0.01). DNA synthesis of adenovirus encoding dominant-negative Ets-1 transfectants was significantly reduced in comparison with that of VEGF stimulation alone (42 ± 11%, P < 0.001) or GFP transfectants (Figure 5B). VEGF induced tube formation at 14.3 ± 0.3 mm/field, whereas treatment with dominant-negative Ets-1 transfectants inhibited the VEGF-induced tube formation by 8.3 ± 0.4 mm/field compared to VEGF stimulation alone (P < 0.001) (Figure 5, C and D).
Effects of Dominant-Negative Ets-1 on Retinal Angiogenesis in the Mouse Model
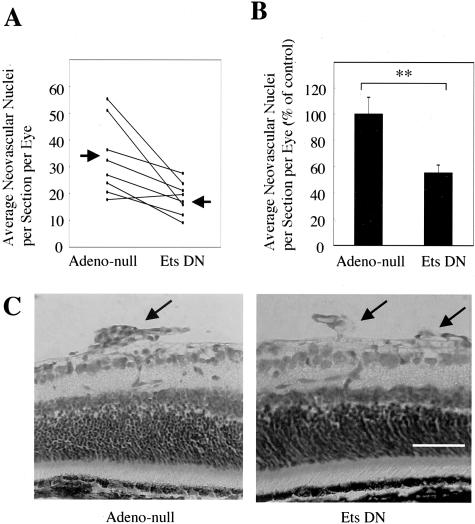
To further determine the role of Ets-1 in pathological angiogenesis in vivo, we examined the effect of Ets-1 inhibition in the mouse model of proliferative retinopathy. The mice exposed to 75% oxygen from P7 to P12 exhibited extensive retinal capillary obliteration. When the mice were returned to room air, the inner retina became hypoxic, expression of VEGF was up-regulated, and retinal neovascularization occurred above the internal limiting membrane into the vitreous. These neovascular tufts were most prominent at P17 to P19. Adenovirus vector encoding dominant-negative Ets-1 treatment resulted in a significant reduction in retinal neovascularization compared with injections of control null adenovirus vector (45% ± 6%, P < 0.01) (Figure 6, A and B). Suppression of the neovascular response was evident in histological examination of paraffin-embedded ocular cross sections (Figure 6C, arrows). Expression of lacZ was prominent on the retinal surface and in the neovascular tufts (data not shown) as described previously.22 No retinal detachment or other damage related to the needle puncture was observed. We also found no retinal toxicity by microscopy morphologically.
Figure 6.
Inhibitory effects of Ets-1 with intravitreal injection of adenovirus encoding dominant-negative Ets-1 in the mouse model of proliferative retinopathy. A: Mice were injected with a solution (1 μl) containing adenovirus vector encoding dominant-negative Ets-1 (Ets DN) (1 × 1010 plaque-forming units/ml) in one eye. Equivalent dose of control null adenovirus (Adeno-null) was injected into contralateral eye. The average number of neovascular cell nuclei per 6-μm histological section per eye was then determined. Solid lines connect eyes from the same animal. Arrows: mean of the neovascular cell nuclei. B: Retinal neovascularization determined from neovascular nuclei counts is expressed as a percentage of the control eye. Mean ± SEM of all animals. **, P < 0.01. C: Typical histological findings in the corresponding retinal locations in eyes injected with control null adenovirus (left) or adenovirus encoding dominant-negative Ets-1 (right). Arrows: an area of retinal neovascularization with vascular cells internal to the inner limiting membrane. Scale bar, 1 mm.
Discussion
VEGF inhibition effectively suppresses ischemia-induced retinal neovascularization.2,3 However, because VEGF is crucial for the physiological functions of the retina such as normal development of the vasculature through its effects on survival of vascular endothelial cells and retinal neurons,23–26 complete inhibition of VEGF itself could reduce basal level and cause undesirable effects on homeostasis of endothelial cell or neuron. Ets-1 is a transcription factor that is induced by angiogenic stimuli such as VEGF, basic fibroblast growth factor, and hypoxia27 in endothelial cells, which regulates angiogenic activity through up-regulation of angiogenic molecules such as MMP-1, MMP-3, MMP-9, integrin β3, and VE-cadherin.7–9 Ets-1 is expressed not only during normal angiogenesis but also during pathological angiogenesis such as wound healing, tumor angiogenesis, and re-endothelialization after denuding injury.6,28,29 Ets-1 anti-sense oligonucleotides reduce new vessel formation in CAM assay30 and hepatocyte growth factor-induced angiogenesis in an ischemic hind limb model.31 Moreover, the elimination of the activity Ets-1 by a dominant-negative molecule inhibit basic fibroblast growth factor-induced normal angiogenesis and tumor angiogenesis in vivo.15,32 The inhibition of Ets-1 probably suppresses VEGF-induced angiogenic activities. Thus far, we focused on this transcription factor as a candidate target against retinal neovascular diseases. In the present study, we demonstrated that VEGF and hypoxia up-regulate Ets-1, and Ets-1 mediates VEGF-dependent angiogenic activity in cultured BRECs and ischemia-dependent retinal neovascularization in a mouse model. Our data for the first time demonstrate that Ets-1 inhibition effectively suppresses retinal neovascularization in the ischemic retina. Ets-1 is a novel target for suppressing ischemia-induced retinal neovascularization, and Ets-1 inhibition is thought to be beneficial for the treatment of ocular neovascular diseases.
VEGF increases the level of Ets-1 mRNA in BRECs in a time- and dose-dependent manner. The dose-response study revealed an EC50 of ∼0.25 ng/ml, which is well below the range of the previously reported VEGF concentrations in vitreous fluid from patients with active neovascular ocular disease.33 Using Western blot analysis, we demonstrated that the Ets-1 protein expression level increased by VEGF stimulation. Furthermore, the DNA-binding activity of Ets-1 increased by VEGF stimulation using EMSA. The magnitudes of the VEGF-induced increases in Ets-1 mRNA, protein synthesis, and DNA-binding activity correlated well each other. These data clearly indicates that VEGF activates Ets-1 expression in retinal vascular endothelial cells.
Hypoxia is the major stimulus that leads to ischemia-induced angiogenesis. In this study, Ets-1 mRNA expression increased by hypoxia. The hypothesis that Ets-1 is probably mediated by induction of VEGF is further confirmed by the experiment showing that anti-VEGF neutralizing antibody has a significant suppressive effect on hypoxic Ets-1 mRNA expression.
To confirm Ets-1 induction in vivo under angiogenic condition, we investigated Ets-1 mRNA expression in a mouse model proliferative retinopathy. We found a marked increase in Ets-1 mRNA levels from P15 to P23. In this model, the VEGF mRNA level peaked at P12,34 which is earlier than the Ets-1 increase seen in the present study. Although further analysis is necessary, hypoxia-induced VEGF probably contributed to the observed increase of Ets-1 mRNA in the ischemic retina.
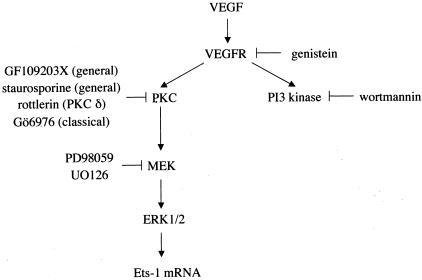
Recent reports have shown that VEGF promotes activation of several signaling molecules, including phospholipase Cγ, phospholipase D, PKC, PI3-kinase, and mitogen-activated protein kinase (MAPK).35–37 Previous studies revealed the involvement of ERK and p38 MAPK in basic fibroblast growth factor-induced Ets-1 expression.38 Accordingly, we investigated the signaling pathway that mediates the VEGF-induced Ets-1 mRNA expression. Genistein, a tyrosine kinase inhibitor; PD98059 and UO126, MEK inhibitors; GF109203X and staurosporine, general PKC inhibitors; and Gö6976, a classical PKC inhibitor abrogated the increase in Ets-1 mRNA expression induced by VEGF. The inhibitory effect by rottlerin, a PKCδ inhibitor was partial. PD 98059 and UO126 inhibit phosphorylation of MEK, which then inhibit phosphorylation of ERK. These data suggest that ERK and both of classical and novel PKC isoforms are involved in the signaling pathways for VEGF-induced Ets-1 mRNA expression. However, wortmannin, a PI3-kinase inhibitor had no effect on VEGF-induced Ets-1 mRNA induction, which suggests that PI3-kinase is not involved in the signaling pathway of VEGF-induced Ets-1 mRNA expression (Figure 7). The observed inhibitory effects of GF109203X and staurosporine were less than that of genistein, PD98059, or UO126. This suggests a contribution of the PKC-independent pathway to MAPK activation by VEGF stimulation, such as the Ras-dependent pathway and the nitric oxide-dependent pathway.39,40 This is the first demonstration that ERK and PKC are involved in the signaling pathway for VEGF-induced Ets-1 mRNA expression.
Figure 7.
Mechanism of VEGF-induced Ets-1 mRNA expression in BRECs. Schematic representation of potential mechanism for VEGF-induced Ets-1 mRNA expression as supported by the data presented. VEGF-induced Ets-1 mRNA expression is primarily mediated by PKC-mediated ERK1/2 pathway.
Numerous angiogenesis-related genes have been demonstrated to be the target of Ets-1.7–12 To further elucidate a role of Ets-1 in retinal neovascularization, we studied whether or not NRP1 or Ang-2, which have been suggested to be important regulators of angiogenesis,41–43 would be the target of Ets-1. NRP1, previously identified as a neuronal receptor, has been shown recently to function also in endothelial cells as an isoform-specific receptor for VEGF165 and as a co-receptor in vitro of VEGF receptor 2 (VEGFR2). Recent studies reported a major role of the angiopoietin/TIE2 systems in postnatal pathological angiogenesis as well as in vascular development in embryogenesis through the regulation of vascular integrity and to promote angiogenesis when potent angiogenic factor such as VEGF exists.42,43 We previously reported the up-regulation of NRP1 and Ang-2 in retinal ischemia and its potential contribution to the development of ischemia-induced retinal neovascularization.14,44 In this study, our observation that dominant-negative Ets-1 suppresses the increase of VEGF-induced NRP1 mRNA and Ang-2 mRNA expression suggests Ets-1 involvement in VEGF-induced gene regulation of these molecules.
To investigate the role of Ets-1 in angiogenic activities in vitro and in vivo, we determined the effect of Ets-1 blockade, by using adenovirus vector encoding dominant-negative Ets-1. We demonstrated that dominant-negative Ets-1 abrogated 58% of the DNA synthesis and 43% of the tube-forming activity induced by VEGF in BRECs, which suggests that VEGF-induced Ets-1 is involved in the angiogenic activities in BRECs. Intravitreal injections of adenovirus vector provide good transduction in proliferative retinopathy.3,22 In vivo experiments, intravitreal injection of adenoviral vector encoding dominant-negative Ets-1 significantly reduced retinal neovascularization. We also found prominent expression of lacZ on the retinal surfaces and in the neovascular tufts in a mouse model of proliferative retinopathy. The magnitude of inhibition was 45%, which is comparable to previous reports of VEGF inhibitors such as direct injection of soluble VEGFR1 (47% inhibition) or adenoviral injection of soluble VEGFR1 (50% inhibition).2,3 The inhibition efficacy for in vitro tube formation and in vivo neovascularization observed in this study were partial. It might be because of incomplete inhibition of Ets-1 activity caused by transfection efficacy of adenovirus, or uninhibited activity of another angiogenic pathway except VEGF/Ets-1.
Taken together, our data suggest that Ets-1 in retinal vascular endothelial cells are potentially up-regulated and contributed to the formation of robust angiogenesis in ischemic retina. Accordingly, inhibition of Ets-1 activity may prove to be beneficial for the treatment of ocular angioproliferative diseases.
Footnotes
Address reprint requests to Hitoshi Takagi, Department of Ophthalmology and Visual Sciences, Kyoto University Graduate School of Medicine, 54 Shogoinkawara-cho, Sakyo-ku, Kyoto 606-8507, Japan. E-mail: hitoshi@kuhp.kyoto-u.ac.jp.
Supported by grants-in-aid for scientific research from the Ministry of Education, Science, and Culture and the Ministry of Health and Welfare of the Japanese government (grant no. 70283596).
Present address of D.W.: Department of Ophthalmology and Visual Sciences, Kyoto University Graduate School of Medicine, 54 Shogoinkawara-cho, Sakyo-ku, Kyoto 606-8507, Japan.
References
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Mistry A, De Alwis M, Paleolog E, Baker A, Thrasher AJ, Ali RR. Inhibition of retinal neovascularization by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther. 2002;9:320–326. doi: 10.1038/sj.gt.3301680. [DOI] [PubMed] [Google Scholar]
- Wasylyk B, Hahn SL, Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- Wernert N, Raes MB, Lassalle P, Dehouck MP, Gosselin B, Vandenbunder B, Stehelin D. c-ets1 proto-oncogene is a transcription factor expressed in endothelial cells during tumor vascularization and other forms of angiogenesis in humans. Am J Pathol. 1992;140:119–127. [PMC free article] [PubMed] [Google Scholar]
- Iwasaka C, Tanaka K, Abe M, Sato Y. Ets-1 regulates angiogenesis by inducing the expression of urokinase-type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J Cell Physiol. 1996;169:522–531. doi: 10.1002/(SICI)1097-4652(199612)169:3<522::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Lelievre E, Mattot V, Huber P, Vandenbunder B, Soncin F. ETS1 lowers capillary endothelial cell density at confluence and induces the expression of VE-cadherin. Oncogene. 2000;19:2438–2446. doi: 10.1038/sj.onc.1203563. [DOI] [PubMed] [Google Scholar]
- Oda N, Abe M, Sato Y. ETS-1 converts endothelial cells to the angiogenic phenotype by inducing the expression of matrix metalloproteinases and integrin beta3. J Cell Physiol. 1999;178:121–132. doi: 10.1002/(SICI)1097-4652(199902)178:2<121::AID-JCP1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Teruyama K, Abe M, Nakano T, Takahashi S, Yamada S, Sato Y. Neuropilin-1 is a downstream target of transcription factor Ets-1 in human umbilical vein endothelial cells. FEBS Lett. 2001;504:1–4. doi: 10.1016/s0014-5793(01)02724-7. [DOI] [PubMed] [Google Scholar]
- Wakiya K, Begue A, Stehelin D, Shibuya M. A cAMP response element and an Ets motif are involved in the transcriptional regulation of flt-1 tyrosine kinase (vascular endothelial growth factor receptor 1) gene. J Biol Chem. 1996;271:30823–30828. doi: 10.1074/jbc.271.48.30823. [DOI] [PubMed] [Google Scholar]
- Kappel A, Schlaeger TM, Flamme I, Orkin SH, Risau W, Breier G. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood. 2000;96:3078–3085. [PubMed] [Google Scholar]
- Dube A, Akbarali Y, Sato TN, Libermann TA, Oettgen P. Role of the Ets transcription factors in the regulation of the vascular-specific Tie2 gene. Circ Res. 1999;84:1177–1185. doi: 10.1161/01.res.84.10.1177. [DOI] [PubMed] [Google Scholar]
- Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274:15732–15739. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- Nakano T, Abe M, Tanaka K, Shineha R, Satomi S, Sato Y. Angiogenesis inhibition by transdominant mutant Ets-1. J Cell Physiol. 2000;184:255–262. doi: 10.1002/1097-4652(200008)184:2<255::AID-JCP14>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Suzuma K, Takahara N, Suzuma I, Ishiki K, Ueki K, Leitegs M, Aiello LP, King GL. Characterization of protein kinase c β isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci USA. 2002;22:721–726. doi: 10.1073/pnas.022644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res. 1998;82:619–628. doi: 10.1161/01.res.82.5.619. [DOI] [PubMed] [Google Scholar]
- Suzuma I, Suzuma K, Ueki K, Hata Y, Feener EP, King GL, Aiello LP. Stretch-induced retinal vascular endothelial growth factor expression is mediated by phosphatidylinositol 3-kinase and protein kinase C (PKC)-ζ but not by stretch-induced ERK1/2, Akt, Ras, or classical/novel PKC pathways. J Biol Chem. 2002;277:1047–1057. doi: 10.1074/jbc.M105336200. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts,’ prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgardh-Nilsson A, Cercek B, Wang JW, Naito S, Lovdahl C, Sharifi B, Forrester JS, Fagin JA. Regulated expression of the ets-1 transcription factor in vascular smooth muscle cells in vivo and in vitro. Circ Res. 1996;78:589–595. doi: 10.1161/01.res.78.4.589. [DOI] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Ando A, Wahlin K, Gunther V, McVey D, Wei L, Campochiaro PA. Intraocular adenoviral vector-mediated gene transfer in proliferative retinopathies. Invest Ophthalmol Vis Sci. 2002;43:1610–1615. [PubMed] [Google Scholar]
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Cepko CL. Flk-1, a receptor for vascular endothelial growth factor (VEGF), is expressed by retinal progenitor cells. J Neurosci. 1996;16:6089–6099. doi: 10.1523/JNEUROSCI.16-19-06089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourey PA, Gohari S, Su JL, Alderson RF. Vascular endothelial cell growth factors promote the in vitro development of rat photoreceptor cells. J Neurosci. 2000;20:6781–6788. doi: 10.1523/JNEUROSCI.20-18-06781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa M, Abe M, Kurosawa H, Hida W, Shirato K, Sato Y. Hypoxia induces transcription factor ETS-1 via the activity of hypoxia-inducible factor-1. Biochem Biophys Res Commun. 2001;289:39–43. doi: 10.1006/bbrc.2001.5927. [DOI] [PubMed] [Google Scholar]
- Bolon I, Gouyer V, Devouassoux M, Vandenbunder B, Wernert N, Moro D, Brambilla C, Brambilla E. Expression of c-ets-1, collagenase 1, and urokinase-type plasminogen activator genes in lung carcinomas. Am J Pathol. 1995;147:1298–1310. [PMC free article] [PubMed] [Google Scholar]
- Wernert N, Gilles F, Fafeur V, Bouali F, Raes MB, Pyke C, Dupressoir T, Seitz G, Vandenbunder B, Stehelin D. Stromal expression of c-Ets-1 transcription factor correlates with tumor invasion. Cancer Res. 1994;54:5683–5688. [PubMed] [Google Scholar]
- Wernert N, Stanjek A, Kiriakidis S, Hugel A, Jha HC, Mazitschek R, Giannis A. Inhibition of angiogenesis in vivo by ets-1 antisense oligonucleotides-inhibition of Ets-1 transcription factor expression by the antibiotic fumagillin. Angew Chem Int Ed. 1999;38:3228–3230. doi: 10.1002/(sici)1521-3773(19991102)38:21<3228::aid-anie3228>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Tomita N, Morishita R, Taniyama Y, Koike H, Aoki M, Shimizu H, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Angiogenic property of hepatocyte growth factor is dependent on upregulation of essential transcription factor for angiogenesis, ets-1. Circulation. 2003;107:1411–1417. doi: 10.1161/01.cir.0000055331.41937.aa. [DOI] [PubMed] [Google Scholar]
- Pourtier-Manzanedo A, Vercamer C, Van Belle E, Mattot V, Mouquet F, Vandenbunder B. Expression of an Ets-1 dominant-negative mutant perturbs normal and tumor angiogenesis in a mouse ear model. Oncogene. 2003;22:1795–1806. doi: 10.1038/sj.onc.1206215. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Jia Q, Song HY, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J Biol Chem. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- Xia P, Aiello LP, Ishii H, Jiang ZY, Park DJ, Robinson GS, Takagi H, Newsome WP, Jirousek MR, King GL. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest. 1996;98:2018–2026. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Abe M, Sato Y. Roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the signal transduction of basic fibroblast growth factor in endothelial cells during angiogenesis. Jpn J Cancer Res. 1999;90:647–654. doi: 10.1111/j.1349-7006.1999.tb00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering BM, Bos JL. Regulation of Ras-mediated signaling: more than one way to skin a cat. Trends Biochem Sci. 1995;20:18–22. doi: 10.1016/s0968-0004(00)88944-6. [DOI] [PubMed] [Google Scholar]
- Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Oh H, Takagi H, Otani A, Koyama S, Kemmochi S, Uemura A, Honda Y. Selective induction of neuropilin-1 by vascular endothelial growth factor (VEGF): a mechanism contributing to VEGF-induced angiogenesis. Proc Natl Acad Sci USA. 2002;99:383–388. doi: 10.1073/pnas.012074399. [DOI] [PMC free article] [PubMed] [Google Scholar]