Abstract
The interaction of L-selectin on lymphocytes with sulfated ligands on high endothelial venules (HEVs) of lymph nodes results in lymphocyte rolling and is essential for lymphocyte homing. The MECA-79 monoclonal antibody reports HEV-expressed ligands for L-selectin by recognizing a critical sulfation-dependent determinant on these ligands. HEC-GlcNAc6ST, a HEV-localized sulfotransferase, is essential for the elaboration of functional ligands within lymph nodes, as well as the generation of the MECA-79 epitope. Here, we use an antibody against murine HEC-GlcNAc6ST to study its expression in relationship to the MECA-79 epitope. In lymph nodes, the enzyme is expressed in the Golgi apparatus of high endothelial cells, in close correspondence with luminal staining by MECA-79. In lymph node HEVs of HEC-GlcNAc6ST-null mice, luminal staining by MECA-79 is almost abolished, whereas abluminal staining persists although reduced in intensity. HEV-like vessels in several examples of inflammation-associated lymphoid neogenesis, including nonobese diabetic mice, also exhibit concomitant expression of the sulfotransferase and luminal MECA-79 reactivity. The correlation extends to ectopic lymphoid aggregates within the pancreas of RIP-BLC mice, in which CXCL13 is expressed in islets. Analysis of the progeny of RIP-BLC by HEC-GlcNAc6ST-null mice establishes that the enzyme is responsible for the MECA-79 defined luminal ligands.
Effective immune surveillance and the development of an immune response depend on the ability of lymphocytes to enter secondary lymphoid organs where foreign antigens are presented.1 Lymphocytes also perform surveillance and effector functions in extralymphoid tissues. At these sites, excessive recruitment of lymphocytes can result in the development of chronic inflammatory lesions with pathological consequences to the surrounding tissues. In some cases, the inflammatory infiltrates resemble lymphoid organs, and the process is referred to as lymphoid organ neogenesis.2,3 This ectopic formation of lymphoid structures can occur in several common autoimmune diseases, in response to certain infectious agents and in a variety of lymphomas. Priming of lymphocytes may occur in these organized lymphoid aggregates, which could perpetuate disease in autoimmune settings.
During normal lymphocyte recirculation into lymph nodes, lymphocyte trafficking from the blood occurs across specialized postcapillary venules called high endothelial venules (HEVs), which are distinguished by their plump endothelial cells.1 Egress is initiated by transient interactions (tethering) between the lymphocytes and high endothelial cells (HECs) lining the blood vessel wall, leading to rolling of the lymphocytes along the vessel.4 For lymph nodes, the primary adhesion molecule mediating this interaction is L-selectin on lymphocytes. As a C-type lectin, L-selectin interacts with specific carbohydrate-based HEV ligands.5 In mucosal lymphoid organs such as Peyer’s patches (PPs), the integrin α4β7 initiates rolling of effector or memory lymphocytes, while L-selectin predominates in this function for naïve lymphocytes.1 The recruitment cascade is completed by the firm adherence and subsequent transmigration of the lymphocytes across the endothelial cell layer into the parenchyma of the organs.1,4
The HEV-expressed ligands for L-selectin thus far identified consist of a set of heavily O-glycosylated glycoproteins, which include GlyCAM-1 and CD34 in the mouse and podocalyxin and CD34 in the human.5 Recognition of these ligands by L-selectin requires sialylation, fucosylation, and sulfation of their mucin-like domains.6 The minimal recognition epitope appears to be comprised of a capping group known as 6-sulfo sialyl Lewis x (6-sulfo sLex) in which the C-6 position of GlcNAc within sialyl Lewis x (sLex) is esterified with sulfate. The key evidence implicating this structure is based on structural analysis of O-glycans of L-selectin ligands,7–9 testing of chemically synthesized sulfated oligosaccharides as inhibitors of L-selectin binding10 and tissue staining with carbohydrate-directed monoclonal antibodies.11 The most complete structural analysis to date has demonstrated that 6-sulfo sLex can cap both a core 2 branch and an extended core 1 branch of biantennary O-glycans within GlyCAM-1.8,12
A parallel approach to defining L-selectin ligands has taken advantage of a monoclonal antibody, MECA-79.13 This antibody stains HEVs in various secondary lymphoid organs of many species, including mouse and human.14,15 MECA-79 recognizes a set of sialomucins, including the aforementioned L-selectin ligands, together with another recently identified sialomucin called endomucin.16 The complex is referred to as peripheral node addressin (PNAd).13 MECA-79 blocks the attachment of lymphocytes to peripheral lymph node (PN) HEVs in vitro and inhibits lymphocyte homing to PNs in vivo (mouse).13 MECA-79 does not, however, inhibit in vitro lymphocyte attachment to PP HEVs or in vivo homing to this organ. Blocking studies indicate that MECA-79 competes with L-selectin for recognition of the sialomucin ligands.17,18
The utility of MECA-79 extends beyond the analysis of normal secondary lymphoid organs. Thus, the organized lymphoid aggregates that form in chronic inflammatory settings generally contain HEV-like vessels that express PNAd and/or MAdCAM-1.3,19 MECA-79+ vessels, of a high-walled or flat phenotype, are also found in other inflammatory lesions where organized lymphoid structures are not present.14,20 In several examples of chronic inflammation, the L-selectin/PNAd system has been confirmed to be functionally significant in lymphocyte recruitment.21–24
The structural basis of the MECA-79 epitope and its relationship to the L-selectin recognition epitopes has been an issue of considerable interest. The reactivity of MECA-79 with multiple components first suggested that it recognizes a posttranslational modification that is shared by L-selectin ligands. Early studies with the metabolic inhibitor chlorate established that the binding of L-selectin and MECA-79 share a requirement for sulfation.25 Recently, the minimal MECA-79 epitope was shown to consist of an extended core 1 structure modified with GlcNAc-6-sulfate8 Although MECA-79 reactivity is retained in the sialylated and/or fucosylated forms,8 these modifications are not required for the MECA-79 epitope.26 Thus, the MECA-79 epitope overlaps with a subregion of the 6-sulfo sLex determinant, thus explaining the function-blocking activity of the antibody.
Recent work from several groups has identified candidate enzymes for the biosynthesis of L-selectin ligands and the MECA-79 epitope in HEVs. Core 2 β1,6-N-acetylglucosaminyl-transferase-I (Core2GlcNAcT-I) and core 1 β1,3-N-acetylglucosaminyltransferase (Core1-β3GlcNAcT),8,12,27 are involved in the formation of the core structures of O-glycans within these ligands. Core1-β3GlcNAcT is required for the generation of the MECA-79 epitope.8,12 The α1,3-fucosyltransferases FucT-VII and to a lesser extent Fuc-TIV are responsible for fucosylation of ligands within lymph node HEVs.26,28
With respect to sulfation, one member of the GlcNAc-6-O-sulfotransferase (GlcNAc6ST) family, known variously as HEC-GlcNAc6ST, L-selectin ligand sulfotransferase (LSST), GST-3, or GlcNAc6ST-2 (gene name, CHST4) has received the most attention.29–31 Transcripts corresponding to HEC-GlcNAc6ST are abundantly and preferentially expressed in HECs.29,30 The cDNA encoding HEC-GlcNAc6ST, when co-transfected into CHO cells together with cDNAs encoding Core2GlcNAcT-I, FucT-VII, and CD34 confers L-selectin ligand activity onto these cells as measured in both equilibrium binding and flow chamber assays.29,30 Moreover, the phenotype of mice genetically deficient in HEC-GlcNAc6ST has clearly established a role for this enzyme in the generation of L-selectin ligands in vivo.32,33 In particular, lymphocyte homing to PNs is reduced by 50% in the HEC-GlcNAc6ST−/− mice compared with wild-type mice. This is reflected in a smaller size of the PN in the −/− mice, and a 60% decrease in the number of total lymphocytes within this organ. The residual homing to PNs in the −/− mice remains L-selectin-dependent, ruling out the induction of alternative, L-selectin-independent homing mechanisms in this lymphoid organ. The deficiency in homing to PNs in the −/− mice is reflected in a dramatic reduction of luminally disposed, MECA-79-reactive ligands for L-selectin.32 Correspondingly, MECA-79 has no effect on homing to PNs in these mice, and does not perturb rolling on their HEVs.33 However, MECA-79- and L-selectin-reactive ligands remain on the abluminal aspect of the PN HEVs in these mice. These ligands are also present in the HEVs of MN and PP in wild-type mice and correspond to those described by Streeter and colleagues13 in PPs in the original characterization of the MECA-79 monoclonal antibody. The presence of HEC-GlcNAc6ST-independent, MECA-79-reactive L-selectin ligands in the lymph nodes and PPs of the HEC-GlcNAc6ST-null mice strongly implicates another GlcNAc-6-O-sulfotransferase in the synthesis of these ligands (see Discussion).
To further characterize the role of HEC-GlcNAc6ST, we have developed an antibody directed against a peptide predicted from its amino acid sequence. A preliminary description of this antibody has been presented by one of us in an analysis of LTβ-deficient and RIP-LTαβ transgenic mice.19 In the current study, we exploit this antibody as a staining reagent to gain information on the relationship between expression of the HEC-GlcNAc6ST protein and the expression pattern of MECA-79-reactive ligands. We investigate HEVs in both secondary lymphoid organs and HEV-like vessels in several examples of ectopic lymphoid aggregates.
Materials and Methods
Mice
Mice genetically deficient in HEC-GlcNAc6ST have been described.32 Null mice and wild-type controls used for immunofluorescence were 12- to 16-week-old males. Mice expressing B-lymphocyte chemokine (BLC) under the rat insulin promoter (RIP-BLC) have been described.34 To generate RIP-BLC mice deficient in HEC-GlcNAc6ST, we bred HEC-GlcNAc6ST−/− mice to RIP-BLC+/− mice. The genotype of the resulting offspring was determined by polymerase chain reaction using the following primer sets: HEC-GlcNAc6ST; forward primer-1 5′-ttttacattgctccttggatgggaatc-3′, forward primer-2 5′-gacatagcgttggctacccgtgatatt-3′, reverse primer 5′-aggctgtgctgctggtgaaagtcat-3′; RIP-BLC; forward primer 5′-caacccctgactatcttccag-3′, reverse primer 5′-gagatgatagtggcttcaggcag-3′. The resulting HEC-GlcNAc6ST+/−/RIP-BLC+/− mice were bred to HEC-GlcNAc6ST−/− mice to generate HEC-GlcNAc6ST−/−/RIP-BLC+/−, which were subjected to the phenotypic analysis described in the present study. Sections of thymus from AKR/J mice, 19 weeks old (female) and from salivary glands (female) and lacrimal glands (male) of nonobese diabetic (NOD) mice, 30 weeks old (prediabetic) were provided by Sara Michie, Department of Pathology, Stanford University. Jeffrey Bluestone of University of California at San Francisco provided the pancreas from a diabetic 12-week-old NOD mouse (female) that had been treated with cyclophosphamide at 8 weeks. A 10-week-old Sprague-Dawley rat (female) was used for the Golgi localization experiment.
Generation of a HEC-GlcNAc6ST Antibody
Polyclonal rabbit anti-serum directed against murine HEC-GlcNAc6ST was prepared as described.19 Briefly, rabbits were immunized with KLH-conjugates of three different peptides derived from the primary amino acid sequence of murine HEC-GlcNAc6ST (peptide 1, CHMSVHRHLSQREESRR; peptide 2, KIICKSQVDIVKAIQTLPE; and peptide 3, RGKGMGQHAFHTNC). The resulting sera were tested for HEC-GlcNAc6ST reactivity by immunofluorescence staining of cryostat-cut sections of mouse PN, prepared as described below. The sera were diluted to 0.5 μg/ml in blocking solution [phosphate-buffered saline (PBS) containing 3% bovine serum albumin and 5% mouse serum] and applied to sections in the presence or absence of 10 μg/ml of each peptide. The desired anti-peptide antibody was purified by affinity chromatography on a column to which peptide 3 was coupled. The C-terminus of this peptide contains a cysteine residue that was bound to iodoacetyl-substituted agarose using the SulfoLink kit (Pierce Chemical Co., Rockford, IL). The purified antibody was used in subsequent immunofluorescence studies. Comparison of the sequence of peptide 3 within HEC-GlcNAc6ST with corresponding regions of GlcNAc6ST-1, GlcNAc6ST-3, and GlcNAc6ST-4 revealed 23%, 40%, and 23% sequence identity, respectively.
Immunofluorescence
Tissues were dissected and immediately frozen in OCT (Tissue Tek). Ten-μm sections were prepared on poly-l-lysine-coated glass slides and allowed to dry for 1 hour. They were fixed in ice-cold acetone for 5 minutes followed by air-drying for 30 minutes. Sections were blocked 30 minutes in blocking solution: PBS containing 3% bovine serum albumin and 5% serum (mouse serum for mouse sections, rat serum for rat sections). All staining steps were performed in blocking solution. Primary antibodies were incubated on slides for 1 hour at room temperature and used at the following concentrations: purified rabbit anti-murine HEC-GlcNAc6ST (0.25 μg/ml), MECA-79 (1.0 μg/ml), MECA-367 (2.0 μg/ml, a gift from Dr. Eugene Butcher, Department of Pathology, Stanford University), and GM-130 (1.25 μg/ml; BD Biosciences). Anti-HEC-GlcNAc6ST was detected by biotinylated goat anti-rabbit IgG (1.3 μg/ml; Jackson Immuno-Research, West Grove, PA) followed by Cy2-conjugated streptavidin (1.8 μg/ml; Jackson Immuno-Research, Bar Harbor, ME). MECA-79 was detected by Cy3-conjugated goat anti-rat IgM (1.5 μg/ml, Jackson Immuno-Research). MECA-367 was detected by aminomethylcoumarin-conjugated goat anti-rat IgG (15 μg/ml, Jackson Immuno-Research). GM-130 was detected by Cy3-conjugated goat anti-mouse IgG (1.3 μg/ml, Jackson Immuno-Research). Sections were counterstained with Harris hematoxylin (Sigma, St. Louis, MO) and mounted with FluorSave Reagent (Calbiochem, La Jolla, CA).
In Vivo/ex Vivo Immunofluorescence
Unlabeled MECA-79 (50 μg per mouse in 100 μl of PBS) was injected into the tail vein of mice and allowed to circulate for 30 minutes. Mice were killed and frozen sections were prepared as described above. Sections were stained as above with Cy3-conjugated goat anti-rat IgM (1.5 μg/ml, Jackson Immuno-Research) followed by biotinylated MECA-79 (1.0 μg/ml) and Cy2-conjugated streptavidin (1.8 μg/ml, Jackson Immuno-Research). Finally, the sections were counterstained and mounted as above.
Enzyme-Linked Immunosorbent Assay
Peptide 3 was coated onto wells of a 96-well Immulon 2HB plate (Thermo Labsystems, Inc.), at 2 μg/ml in 15 mmol/L Na2CO3, 35 mmol/L NaHCO3, pH 9.6, and incubated overnight at 4°C. The next day, wells were blocked with 3% bovine serum albumin in PBS/0.1% Tween 20 (blocking solution) for 2 hours at room temperature. Purified rabbit anti-murine HEC-GlcNAc6ST antibody was reacted in a twofold-dilution series starting at 5 μg/ml in blocking solution for 2 hours at room temperature. The bound antibody was detected with biotinylated goat anti-rabbit IgG (1.3 μg/ml in blocking solution, Jackson Immuno-Research) followed by streptavidin-alkaline phosphatase (0.5 μg/ml in PBS; Caltag Laboratories, Burlingame, CA) and p-nitrophenyl phosphate substrate (5 mg, Pierce Biotechnology, Inc.) in 5 ml of 10% diethanolamine and 0.5 mmol/L MgCl2, pH 9.8.
Western Blotting
cDNAs encoding murine HEC-GlcNAc6ST in pCDNA3.1/HISMyc (Invitrogen, Carlsbad, CA) or the vector control were transfected into COS cells by lipofectamine (Invitrogen)-mediated transfection. Three days after transfection, lysates were prepared from the transfected cells by lysis in PBS/2% Triton X-100. Aliquots (∼10% of the total) of the lysates were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%) and transferred to Problott (Applied BioSystems, Foster City, CA). The membrane was blocked for 2 hours in blotto (5% dry milk in PBS/0.1% Tween 20). The membrane was incubated in blotto containing 1 μg/ml of purified HEC-GlcNAc6ST antibody for 1 hour at room temperature, followed by biotinylated goat anti-rabbit IgG (1 μg/ml, Jackson Immuno-Research) and streptavidin-horseradish peroxidase (Caltag Laboratories). The blot was developed with an enhance chemiluminescence (ECL) horseradish peroxidase substrate kit (Amersham, Arlington Heights, IL) followed by exposure to X-ray film.
Morphometric Analysis
HEC-GlcNAc6ST−/−/RIP-BLC+/− and HEC-GlcNAc6ST +/+/RIP-BLC+/− mice (four mice in each group, 20 to 25 weeks of age) were killed and cryostat sections of pancreata were prepared as described above. Every 15th 10-μm section was counterstained with Harris hematoxylin (Sigma) and analyzed. The numbers of noninfiltrated and infiltrated islets per section were counted to yield the percentage of infiltrated islets. Infiltrate sizes were also measured using ImageJ 1.25t software (National Institutes of Health, Bethesda, MD) and the mean size of the lymphocytic aggregates on each section was determined. This procedure was applied to 24 to 60 sections per pancreas. Means and standard deviations were calculated. Student’s t-test was applied to determine the statistical significance of the differences between the two types of mice.
Results
Expression of HEC-GlcNAc6ST in Secondary Lymphoid Organs
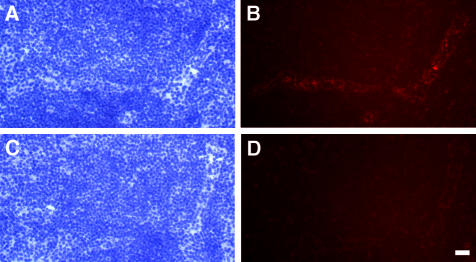
To obtain an antibody against HEC-GlcNAc6ST, we immunized a rabbit with a mixture of three peptide-KLH conjugates. The peptides consisted of residues derived from the predicted sequence of mouse HEC-GlcNAc6ST and were chosen in part because of conservation with the human orthologue of this sulfotransferase. The resulting serum stained HEVs in cryostat sections of mouse lymph node (Figure 1). As shown in Figure 1D, one of the three peptides (but not the other two, data not shown) substantially inhibited staining of HEVs in mouse PN sections. This peptide was therefore chosen as an immunoaffinity reagent for use with the serum. The purified antibody reacted specifically with the peptide by enzyme-linked immunosorbent assay and with recombinant HEC-GlcNAc6ST by Western blot (not shown).
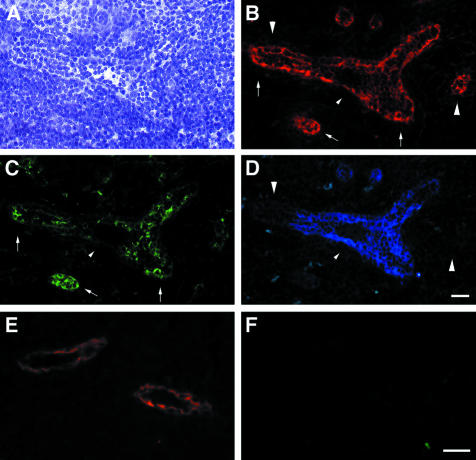
Figure 1.
Staining of lymph nodes with an anti-HEC-GlcNAc6ST serum. An anti-HEC-GlcNAc6ST antiserum was produced by immunization of an individual rabbit with three peptides derived from the HEC-GlcNAc6ST sequence, as described in Materials and Methods. The serum was used to stain cryostat sections of mouse PN in the absence (B) or presence (D) of peptide 3 (RGKGMGQHAFHTNC). A and C show the bright-field images (hematoxylin stained) corresponding to B and D. Scale bar, 25 μm.
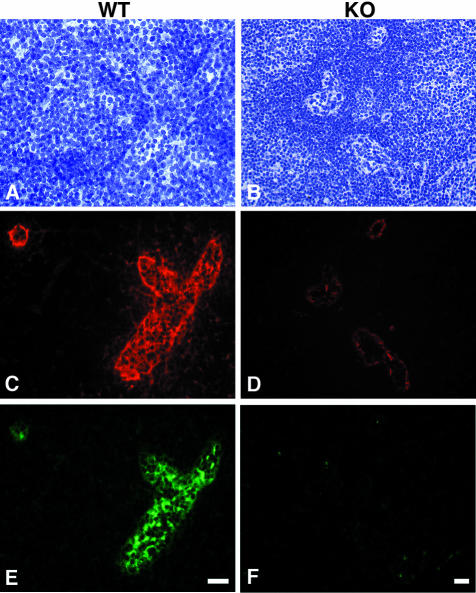
Using this antibody and a two-color immunofluorescence protocol, we examined the relationship between expression of HEC-GlcNAc6ST protein and the presence of the MECA-79 epitope (Figure 2). As shown previously in our characterization of HEC-GlcNAc6ST-null mice,32 the MECA-79 epitope was greatly diminished on the luminal surface of the HEVs in these mice (Cy3, red), whereas MECA-79 staining persisted in an abluminal pattern (Figure 2D). HEVs within PN of wild-type animals expressed HEC-GlcNAc6ST as detected by the peptide-specific antibody (Figure 2E; Cy2, green), whereas the HEVs of null mice were completely negative for the sulfotransferase (Figure 2F). The absence of staining in the null mice confirmed that the gene targeting had eliminated the expression of the encoded protein. Importantly, the expression of HEC-GlcNAc6ST was highly restricted to HEVs within lymph nodes, consistent with previous in situ hybridization findings.29,30 A number of other tissues including kidney, pancreas, and heart in normal mice were devoid of staining (not shown), as predicted from mRNA expression.30
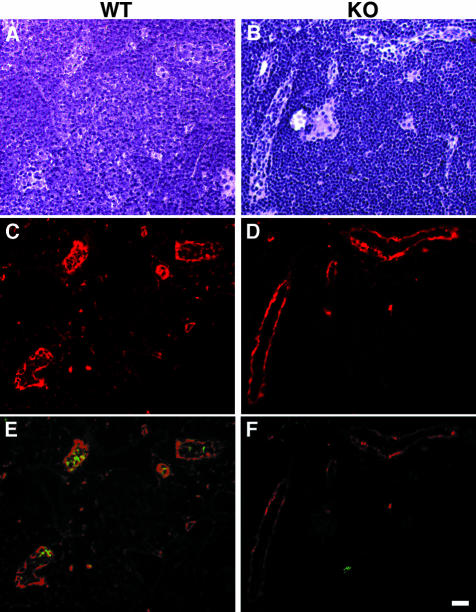
Figure 2.
Expression of HEC-GlcNAc6ST and the MECA-79 epitope in mouse lymph node. Serial cryostat sections were prepared from PNs of wild-type (A, C, E) or −/− (B, D, F) mice. Sections were stained by immunofluorescence with MECA-79 (C, D) or the anti-HEC-GlcNAc6ST antibody (E, F). The bright-field images (A, B) establish the presence of HEVs in both wild-type and −/− lymph nodes. Scale bar, 25 μm.
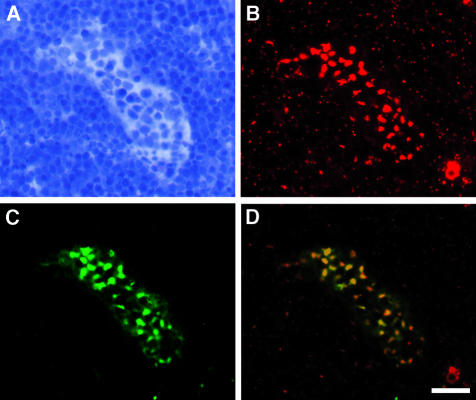
The staining pattern for the sulfotransferase in HEVs was in circular-, crescent-, or domed-shaped spots that were usually in the apical regions of the HEC (Figure 2E). Sulfation of carbohydrates occurs in the lumen of the Golgi apparatus.35,36 To determine whether these spots co-localized with the Golgi apparatus, we performed two-color immunofluorescence with the HEC-GlcNAc6ST antibody and an antibody directed against a Golgi marker, GM-130 (Figure 3). We used sections of rat lymph node to permit the use of a mouse antibody as the primary staining reagent. The expression of HEC-GlcNAc6ST in rat LN HEVs occurred in the same characteristic pattern as observed in mouse (Figure 3C). Dual immunofluorescence showed that positive staining for HEC-GlcNAc6ST was spatially contiguous with the Golgi marker, indicating a Golgi localization for the sulfotransferase within HECs (Figure 3, B and D).
Figure 3.
Golgi localization of HEC-GlcNAC6ST. A cryostat section from a rat lymph node was dual stained with an antibody for a Golgi marker (B; GM-130, Cy3, red) and the anti-HEC-GlcNAc6ST antibody (C; Cy2, green). D: Overlay of the two fluorescent images; yellow indicates overlap of the two stains. A is the bright-field image of the same field showing a single HEV. Scale bar, 25 μm.
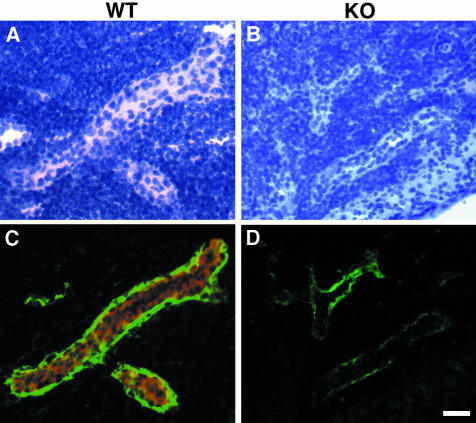
To establish unambiguously that there was a reduction of luminal HEV MECA-79 epitope and to determine whether there was also an effect on abluminal staining in the HEC-GlcNAc6ST−/− mice, we used a dual in vivo/ex vivo staining approach. Unlabeled MECA-79 was injected into the tail vein of mice and allowed to circulate for 30 minutes. Cryostat sections of PNs were prepared and stained with Cy3-conjugated secondary reagents to reveal the injected MECA-79 that had bound to the blood-exposed (ie, luminal) regions of the HEVs. The sections were then treated sequentially with biotin-conjugated MECA-79 and streptavidin-Cy2 to detect the remaining epitopes that were not accessible from the blood. As shown in Figure 4, the HEVs of wild-type mice expressed abundant levels of luminal MECA-79 epitope, whereas luminal staining was barely detectable in the HEVs of −/− mice. Furthermore, although the ex vivo staining indicated the presence of abluminal MECA-79 epitopes in both wild-type and null mice, the staining intensity was consistently reduced in the HEVs of null mice. This result establishes that HEC-GlcNAc6ST also contributes to abluminal expression of the MECA-79 epitope.
Figure 4.
Contribution of HEC-GlcNAC6ST to the generation of luminal and abluminal MECA-79 epitopes. Cryostat sections of PN were prepared from wild-type (A, C) and −/− (B, D) mice that had received intravenous injections of unlabeled MECA-79. The sections were stained first with Cy3-conjugated secondary reagents to detect injected luminally bound MECA-79 and then with biotinylated MECA-79 followed by streptavidin-Cy2 to detect abluminal MECA-79 epitopes. C and D: Overlay of the two fluorescent images. A and B show the corresponding bright-field images. Scale bar, 25 μm.
HEVs of mesenteric lymph nodes (MNs) show a mosaic expression pattern for PNAd and MAdCAM-1 (a ligand for α4β7).13 We performed triple staining on MNs for PNAd (MECA-79), HEC-GlcNAc6ST, and MAdCAM-1 (MECA-367) (Figure 5). We found many HEVs that were mosaic, particularly the large vessels. In most MN HEVs, MECA-79 stained the luminal (and abluminal) aspects, irrespective of the presence of MAdCAM-1. The presence of HEC-GlcNAc6ST in the characteristic Golgi pattern correlated with luminal MECA-79 epitope. Conversely, wherever MECA-79 was confined to the abluminal aspects of the HEVs, HEC-GlcNAc6ST was absent. In some of these segments, MAdCAM-1 was clearly evident.
Figure 5.
Expression of HEC-GlcNAc6ST in MNs and PPs. Cryostat sections from MNs of wild-type mice were stained simultaneously for MECA-79 (B; Cy3, red), HEC-GlcNAc6ST (C; Cy2, green), and MAdCAM-1 (D; aminomethylcoumarin, blue). Large arrowheads in B and D indicate vessels or segments of vessels that are MAdCAM-1-negative but contain luminal MECA-79 epitopes. Arrows in B and C indicate the correspondence between expression of HEC-GlcNAc6ST and luminal MECA-79 epitopes. Small arrowheads in B, C, and D indicate a segment of a vessel that is positive for MAdCAM-1, negative for HEC-GlcNAc6ST, and in which MECA-79 is confined to the luminal aspect. In E and F, a section of PP was dual stained for MECA-79 (E; Cy3, red) and HEC-GlcNAc6ST (F; Cy2, green). Scale bar, 25 μm.
Because we had previously shown that in the PP, the MECA-79 staining pattern was identical between wild-type and HEC-GlcNAc6ST−/− mice and was confined to the abluminal aspect of HEVs,32 we wanted to determine whether HEC-GlcNAc6ST was expressed in PP HEVs. Our previous in situ hybridization analysis had failed to detect transcripts for HEC-GlcNAc6ST in wild-type PP (K Tangemann and SD Rosen, unpublished data). As shown in Figure 5F, the PPs of wild-type mice did not express HEC-GlcNAc6ST at the protein level. Consistent with previous observations,13,32 only abluminal MECA-79 staining was present (Figure 5E). However, as expected, staining with MECA-367 demonstrated strong expression of MAdCAM-1 on HEVs in a pericellular pattern (not shown).
Expression of HEC-GlcNAc6ST in HEV-Like Vessels
As noted above, the MECA-79 epitope is observed on the activated vascular endothelium in many inflammatory sites.14,15,20 We wanted to know whether HEC-GlcNAc6ST expression was induced on such activated endothelium. We first examined AKR mice, which progressively develop hyperplasia of the thymic medulla with age.22 Infiltrated T and B cells become segregated as in lymph nodes, with HEV-like vessels confined to non-B-cell regions. Hiraoka and colleagues30 demonstrated the presence of HEC-GlcNAc6ST mRNA in MECA-79+, HEV-like vessels in this model. As shown in Figure 6, such vessels also expressed HEC-GlcNAc6ST protein in a pattern similar to that seen in lymph nodes.
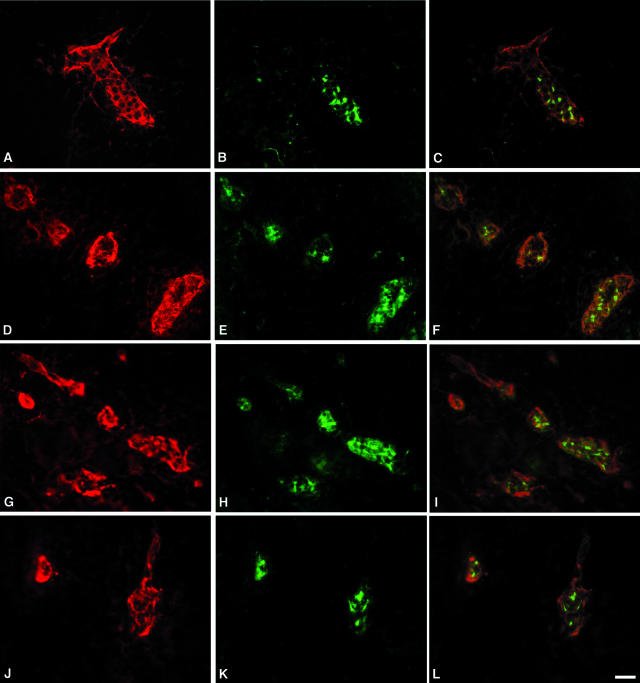
Figure 6.
Expression of HEC-GlcNAc6ST in HEV-like vessels at sites of inflammation. Cryostat sections from hyperplastic AKR thymus (A–C), NOD pancreas (D–F), NOD lacrimal glands (G–I), and NOD salivary glands (J–L) were dual stained for MECA-79 (A, D, G, J; Cy3, red) and HEC-GlcNAc6ST (B, E, H, K; Cy2, green). C, F, I, and L are the respective overlays. Scale bar, 25 μm.
NOD mice spontaneously develop autoimmune diabetes with a pathology similar to that seen in human type I insulin-dependent diabetes mellitus.21,37 In these mice, mononuclear islet infiltrates develop starting at 3 weeks of age and progressively acquire characteristics of secondary lymphoid organs. Similar infiltrates also develop in the salivary and lacrimal glands of these mice, with consequent progressive destruction of these glands.23 Consistent with the observations made in these previous reports, we confirmed the presence of MECA-79+ HEV-like vessels in the inflamed regions of pancreas, lacrimal glands, and salivary glands (Figure 6). The high-walled vessels with strong pericellular MECA-79 staining were also strongly positive for HEC-GlcNAc6ST in the characteristic Golgi pattern found for lymph node HEVs.
In the RIP-BLC transgenic mouse, BLC (a B-cell chemokine also known as CXCL13) is expressed ectopically in the pancreas under the control of the rat insulin promoter.34,38 The result is the formation of lymph node-like aggregates with segregated B-cell and T-cell areas and HEV-like structures. These are similar to aggregates that form in the inflammatory settings of the NOD pancreas and salivary glands and the RIPLTαβ mouse,19 with one notable difference that B cells are the predominant infiltrating cells. As shown in Figure 7, the presence of luminal MECA-79 epitopes again correlated with HEC-GlcNAc6ST expression. To establish that this relationship was causal, we crossed the RIP-BLC transgene onto a HEC-GlcNAc6ST-null background. In the RIP-BLC+/HEC-GlcNAc6ST-null mice, MECA-79 staining of the HEV-like vessels disappeared from the luminal aspect but was retained abluminally. With respect to the degree of infiltration, there was a trend toward reduced size and frequency of pancreatic infiltrates on the HEC-GlcNAc6ST-null background (28% and 22% reduction, respectively) but these differences did not reach statistical significance. As in the RIP-BLC mice, the lymphoid aggregates formed on the null background showed a segregation of B and T cells, with the predominance of the former (not shown).
Figure 7.
Contribution of HEC-GlcNAc6ST to luminal MECA-79 epitopes and lymphoid aggregates in the pancreas of RIP-BLC mice. Cryostat sections from infiltrated pancreas of RIP-BLC/HEC-GlcNAc6ST+/+ (A, C, and E) and RIP-BLC/HEC-GlcNAc6ST−/− (B, D, and F) mice were dual stained for MECA-79 (Cy3, red) and HEC-GlcNAc6ST (Cy2, green). Shown are the bright-field images (A, B), the MECA-79 staining (C, D), and the merged MECA-79 and anti-HEC-GlcNAc6ST staining (E, F). Scale bar, 25 μm.
Discussion
In this study, we used a polyclonal anti-peptide antibody to study the expression of HEC-GlcNAc6ST, a member of the GlcNAc6ST subfamily of sulfotransferases comprised of four members in mouse and five in human.31,35 Confirming previous in situ hybridization and Northern blot analyses,29,30 we demonstrated that this sulfotransferase was indeed highly restricted in its expression at the protein level. Specific staining was observed only for HEVs in lymph nodes and HEV-like vessels in several instances of lymphoid neogenesis. In further agreement with previous gene expression analyses, staining was absent in a panel of normal tissues. In contrast, the other members of the GlcNAc6ST family are predicted to have a wider tissue distribution based on Northern analysis31,35 and the National Center for Biotechnology Information-expressed sequence tag (EST) database. As predicted for a carbohydrate-modifying sulfotransferase, staining for HEC-GlcNAc6ST co-localized with the Golgi compartment. HEC-GlcNAc6ST was not detected in the HEVs of PPs.
To study the relationship of HEC-GlcNAc6ST expression to functional ligands for L-selectin, we used MECA-79 as a reporter for a major class of L-selectin ligands. Two patterns of MECA-79 staining were discerned: luminal staining, which was correlated with the expression of HEC-GlcNAc6ST; and abluminal staining, which did not require the expression of this sulfotransferase though it was reduced in its absence. The unambiguous assignment of luminal and abluminal aspects of HEVs was accomplished by a two-stage protocol in which intravascular administration of MECA-79 was followed by staining of the cryostat sections with a biotinylated form of MECA-79. In the absence of HEC-GlcNAc6ST, as occurs in the lymph nodes of the null mouse as well and naturally in PP HEVs, abluminal staining was clearly present whereas luminal MECA-79 epitopes were present at very low to undetectable levels. Despite this paucity of luminal MECA-79 staining, a proportion of functional L-selectin ligands persists on venules within the lymph nodes of HEC-GlcNAc6ST-null mice32,33 and in the HEVs of normal PPs.39,40 Whether these ligands carry essential sulfation modifications is unknown. With respect to the abluminal ligands that are found in the absence of HEC-GlcNAc6ST, another enzyme in the same subfamily, GlcNAc6ST-1, is the prime candidate as the relevant sulfotransferase. This enzyme is detected at the mRNA level in lymph node HEVs41 and can contribute to the elaboration of L-selectin ligand activity, the MECA-79 epitope, and the 6-sulfo sLex determinant in transfected cells.42,43 The function of the abluminal ligands is not understood at this time.
MECA-79 staining of HEV-like vessels in inflammatory sites has been confirmed in several instances to reflect L-selectin ligand activity: MECA-79 reactivity has been correlated with positive staining by an L-selectin/IgG chimera,44 L-selectin-dependent attachment of lymphocytes in an in vitro adhesion assay,21,22,45 and L-selectin-dependent homing of lymphocytes to the inflamed organ.22,23 In the homing studies, short-term lymphocyte accumulation was inhibited by intravenous administration of MECA-79.22,23 Previously, the presence of HEC-GlcNAc6ST protein in HEV-like vessels has been noted within the inflamed pancreas of RIP-LTαβ mice, in which both the LTα and LTβ are expressed under the control of the rat insulin promoter, generating expression of the LTαβ complex.19 The HEC-GlcNAc6ST-positive vessels exhibit both abluminal and luminal MECA-79 staining. Here, we have extended the correlation between the expression of HEC-GlcNAc6ST and strong luminal staining by MECA-79 to several other examples of lymphoid neogenesis, including two spontaneous models, ie, the AKR thymoma and the NOD mouse. Moreover, by crossing the RIP-BLC transgene in the context of the HEC-GlcNAc6ST-null background, we have established that this sulfotransferase is required for the presence of abundant luminal epitopes. In the absence of HEC-GlcNAc6ST, there was a trend to a reduction in the frequency (22% reduction) and size of the infiltrates (28% reduction). We previously noted a similar level of reduction (≈20%) in the cellularity of MNs in HEC-GlcNAc6ST-null mice.32 In common with the HEVs of mesenteric nodes, the HEV-like vessels in the infiltrates of RIP-BLC mice are mosaic, expressing both PNAd and MAdCAM-1, sometimes simultaneously.34 The adhesion mechanisms induced to compensate for the absence of HEC-GlcNAc6ST may be similar at the two sites. It should be noted that the HEV-like vessels in NOD pancreas and AKR thymus are also chimeric for PNAd and MAdCAM-1.21,22
As mentioned in the Introduction, a number of studies have reported MECA-79+ vessels in inflammatory lesions in the human, frequently but not always, in the context of organized lymphoid tissue.14,15 The first reports of MECA-79+ vessels in human inflammatory lesions were anecdotal, involving small numbers of samples. Subsequent studies by Renkonen and co-workers20,46–49 of several inflammatory conditions (rejecting heart and kidney allografts, peribronchial specimens from asthmatics, thyroiditis, and psoriasis) have demonstrated the consistent induction of MECA-79+ vessels in association with inflammatory infiltrates in large numbers of independent specimens. Similarly, Salmi and colleagues50 reported that this epitope is present on a subpopulation of synovial vessels in the majority of chronic arthritis patients. It remains to be determined whether the MECA-79+ vessels within these various lesions express HEC-GlcNAc6ST, in analogy with our findings in ectopic lymphoid aggregates in mouse.
Acknowledgments
We thank Minoru Fukuda for providing the cDNA for mouse HEC-GlcNAc6ST/L-selectin ligand sulfotransferase, Sara Michie for providing sections of AKR mice and NOD salivary and lacrimal glands, Jeff Bluestone for providing the NOD pancreas, and Kristy Red-Horse for assistance with Western blotting.
Footnotes
Address reprint requests to Steven Rosen, Department of Anatomy, University of California, San Francisco, CA 94143-0452. E-mail: sdr@itsa.ucsf.edu.
Supported by the American Heart Association (postdoctoral fellowship to A.B.) and the National Institutes of Health (grants R01 GM57411 and R37 GM23547 to S.D.R., R01AI45073 to J.G.C., and NCIRO1 CA16885 and R01 DK57731 to N.H.R.).
Present address of A.B.: Thios Pharmaceuticals, 5980 Horton St., Emeryville CA 94608.
Present address of N.B.: Roswell Park Cancer Institute, Buffalo, NY 14263.
References
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res. 1999;19:119–125. doi: 10.1007/BF02786481. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation and beyond. Ann Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- Hemmerich S, Leffler H, Rosen SD. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- Satomaa T, Renkonen O, Helin J, Kirveskari J, Makitie A, Renkonen R. O-glycans on human high endothelial CD34 putatively participating in L-selectin recognition. Blood. 2002;99:2609–2611. doi: 10.1182/blood.v99.7.2609. [DOI] [PubMed] [Google Scholar]
- Scudder PR, Shailubhai K, Duffin KL, Streeter PR, Jacob GS. Enzymatic synthesis of a 6-sulfated sialyl-Lewisx which is an inhibitor of L-selectin binding to peripheral addressin. Glycobiology. 1994;4:929–933. doi: 10.1093/glycob/4.6.929. [DOI] [PubMed] [Google Scholar]
- Mitsuoka C, Sawada-Kasugai M, Ando-Furui K, Izawa M, Nakanishi H, Nakamura S, Ishida H, Kiso M, Kannagi R. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis X. J Biol Chem. 1998;273:11225–11233. doi: 10.1074/jbc.273.18.11225. [DOI] [PubMed] [Google Scholar]
- Mitoma J, Petryniak B, Hiraoka N, Yeh JC, Lowe JB, Fukuda M. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis X-type L-selectin ligand activity. J Biol Chem. 2003;278:9953–9961. doi: 10.1074/jbc.M212756200. [DOI] [PubMed] [Google Scholar]
- Streeter PR, Rouse BTN, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD. Endothelial ligands for L-selectin: from lymphocyte recirculation to allograft rejection. Am J Pathol. 1999;155:1013–1020. doi: 10.1016/S0002-9440(10)65201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J-P, Springer TA. High endothelial venules: specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Samulowitz U, Kuhn A, Brachtendorf G, Nawroth R, Braun A, Bankfalvi A, Bocker W, Vestweber D. Human endomucin: distribution pattern, expression on high endothelial venules, and decoration with the MECA-79 epitope. Am J Pathol. 2002;160:1669–1681. doi: 10.1016/S0002-9440(10)61114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkonen J, Tynninen O, Hayry P, Paavonen T, Renkonen R. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am J Pathol. 2002;161:543–550. doi: 10.1016/S0002-9440(10)64210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA, Simell O, Michie SA. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid binding to islet endothelium. J Clin Invest. 1993;92:2509–2515. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie SA, Streeter PR, Butcher EC, Rouse RV. L-selectin and alpha 4 beta 7 integrin homing receptor pathways mediate peripheral lymphocyte traffic to AKR mouse hyperplastic thymus. Am J Pathol. 1995;147:412–421. [PMC free article] [PubMed] [Google Scholar]
- Mikulowska-Mennis A, Xu B, Berberian JM, Michie SA. Lymphocyte migration to inflamed lacrimal glands is mediated by vascular cell adhesion molecule-1/alpha(4)beta(1) integrin, peripheral node addressin/l-selectin, and lymphocyte function-associated antigen-1 adhesion pathways. Am J Pathol. 2001;159:671–681. doi: 10.1016/s0002-9440(10)61738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabien N, Bergerot I, Orgiazzi J, Thivolet C. Lymphocyte function associated antigen-1, integrin alpha 4, and L-selectin mediate T-cell homing to the pancreas in the model of adoptive transfer of diabetes in NOD mice. Diabetes. 1996;45:1181–1186. doi: 10.2337/diab.45.9.1181. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of HEV-ligands by L-selectin and MECA 79, an adhesion-blocking mAb. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly P, Thall AD, Petryniak B, Rogers CE, Mith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E- and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- Homeister JW, Thall AD, Petryniak B, Maly P, Rogers CE, Smith PL, Kelly RJ, Gersten KM, Askari SW, Cheng G, Smithson G, Marks RM, Misra AK, Hindsgaul O, von Andrian UH, Lowe JB. The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- Bistrup A, Bhakta S, Lee JK, Belov YC, Gunn MD, Zuo F-R, Huang C-C, Kannagi R, Rosen SD, Hemmerich S. Sulfotransferases of two specificities function in the reconstitution of high-endothelial-cell ligands for L-selectin. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Petryniak B, Nakayama J, Tsuboi S, Suzuki M, Yeh J-C, Izawa D, Tanaka T, Miyasaka M, Lowe JB, Fukuda M. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewis x, an L-selectin ligand displayed by CD34. Immunity. 1999;11:79–89. doi: 10.1016/s1074-7613(00)80083-7. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Rosen SD. Carbohydrate sulfotransferases in lymphocyte homing. Glycobiology. 2000;10:849–856. doi: 10.1093/glycob/10.9.849. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Bistrup A, Singer MS, Zante AV, Lee JK, Tsay D, Peters M, Carminati JL, Brennan TJ, Carver-Moore K, Leviten M, Fuentes ME, Ruddle NH, Rosen SD. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15:237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- van Zante A, Gauguet J-M, Bistrup A, Tsay D, von Andrian UH, Rosen SD. Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse. J Exp Med. 2003;198:1289–1300. doi: 10.1084/jem.20030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Hiraoka N, Akama TO, Fukuda MN. Carbohydrate-modifying sulfotransferases: structure, function, and pathophysiology. J Biol Chem. 2001;276:47747–47750. doi: 10.1074/jbc.R100049200. [DOI] [PubMed] [Google Scholar]
- De Graffenried CL, Bertozzi CR. Golgi localization of carbohydrate sulfotransferases is a determinant of L-selectin ligand biosynthesis. J Biol Chem. 2003;278:40282–40295. doi: 10.1074/jbc.M304928200. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Gagnerault MC, Lepault F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J Immunol. 1994;152:5969–5978. [PubMed] [Google Scholar]
- Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- Bargatze RF, Jutila MA, Butcher EB. Distinct roles of L-selectin and integrins alpha4beta7 and LFA-1 in lymphocyte interactions with Peyer’s patch HEV in situ: the multi-step model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Ramos CL, Steeber DA, Muller W, Wagner N, Tedder TF, Ley K. The roles of L-selectin, beta 7 integrins, and P-selectin in leukocyte rolling and adhesion in high endothelial venules of Peyer’s patches. J Immunol. 1998;161:2449–2456. [PubMed] [Google Scholar]
- Uchimura K, Muramatsu H, Kadomatsu K, Fan QW, Kurosawa N, Mitsuoka C, Kannagi R, Habuchi O, Muramatsu T. Molecular cloning and characterization of an N-acetylglucosamine-6-O-sulfotransferase. J Biol Chem. 1998;273:22577–22583. doi: 10.1074/jbc.273.35.22577. [DOI] [PubMed] [Google Scholar]
- Kimura N, Mitsuoka C, Kanamori A, Hiraiwa N, Uchimura K, Muramatsu T, Tamatani T, Kansas GS, Kannagi R. Reconstitution of functional L-selectin ligands on a cultured human endothelial cell line by cotransfection of α1→3 fucosyltransferase VII and newly cloned GlcNAcβ: 6-sulfotransferase cDNA. Proc Natl Acad Sci USA. 1999;96:4530–4535. doi: 10.1073/pnas.96.8.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori A, Kojima N, Uchimura K, Muramatsu T, Tamatani T, Berndt MC, Kansas GS, Kannagi R. Distinct sulfation requirements of selectins disclosed using cells that support rolling mediated by all three selectins under shear flow. L-selectin prefers carbohydrate 6-sulfation to tyrosine sulfation, whereas p-selectin does not. J Biol Chem. 2002;277:32578–32586. doi: 10.1074/jbc.M204400200. [DOI] [PubMed] [Google Scholar]
- Onrust SV, Hartl PM, Rosen SD, Hanahan D. Modulation of L-selectin ligand expression during an immune response accompanying tumorigenesis in transgenic mice. J Clin Invest. 1996;97:54–64. doi: 10.1172/JCI118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-D, Karin N, Tisch R, Steinman L, McDevitt HO. Inhibition of insulitis and prevention of diabetes in nonobese diabetic mice by blocking L-selectin and very late antigen 4 adhesion receptors. Proc Natl Acad Sci USA. 1993;90:10494–10498. doi: 10.1073/pnas.90.22.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppila S, Paavonen T, Nieminen MS, Häyry P, Renkonen R. Endothelial L-selectin ligands are likely to recruit lymphocytes into human rejecting heart transplants. Am J Pathol. 1999;155:1303–1310. doi: 10.1016/S0002-9440(10)65232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppila S, Paavonen T, Laitinen A, Laitinen LA, Renkonen R. Endothelial sulfated sialyl Lewis x glycans, putative L-selectin ligands, are preferentially expressed in bronchial asthma but not in other chronic inflammatory lung diseases. Am J Respir Cell Mol Biol. 2000;23:492–498. doi: 10.1165/ajrcmb.23.4.4113. [DOI] [PubMed] [Google Scholar]
- Turunen JP, Majuri ML, Seppo A, Tiisala S, Paavonen T, Miyasaka M, Lemstrom K, Penttila L, Renkonen O, Renkonen R. De novo expression of endothelial sialyl Lewis(a) and sialyl Lewis(x) during cardiac transplant rejection: superior capacity of a tetravalent sialyl Lewis(x) oligosaccharide in inhibiting L-selectin-dependent lymphocyte adhesion. J Exp Med. 1995;182:1133–1141. doi: 10.1084/jem.182.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirveskari J, Paavonen T, Hayry P, Renkonen R. De novo induction of endothelial L-selectin ligands during kidney allograft rejection. J Am Soc Nephrol. 2000;11:2358–2365. doi: 10.1681/ASN.V11122358. [DOI] [PubMed] [Google Scholar]
- Salmi M, Rajala P, Jalkanen S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J Clin Invest. 1997;99:2165–2172. doi: 10.1172/JCI119389. [DOI] [PMC free article] [PubMed] [Google Scholar]