Abstract
Mice lacking the vascular endothelial growth factor (VEGF) receptor flt-1 (VEGFR-1) die from vascular overgrowth, caused primarily by aberrant endothelial cell division (Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL: Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood 2002, 99:2397–2407). Because a second high-affinity VEGF receptor, flk-1, produces a positive endothelial proliferation signal, it was logical to ask whether flt-1 affects developmental blood vessel formation by modulating signaling through flk-1. Differentiated embryonic stem cell cultures lacking flt-1 (flt-1−/−) had increased flk-1 tyrosine phosphorylation, indicating that flk-1 signaling is up-regulated in the mutant background. The selective flk-1 inhibitor SU5416 partially rescued the flt-1−/− mutant phenotype, and this rescue was accompanied by a decrease in the relative amount of flk-1 tyrosine phosphorylation. Thus reduced flk-1 signal transduction can partially compensate for the lack of flt-1. The flt-1−/− mutant phenotype was also partially rescued by Flt-1/Fc, a truncated flt-1 that binds and sequesters the VEGF ligand. Taken together, these data show that down-regulation of flk-1 signaling by two different strategies partially rescues the developmental vascular overgrowth seen in the absence of flt-1, and they support a model whereby flt-1 modulates the flk-1 signal at an early point in the pathway.
The regulation of blood vessel formation is critical both developmentally and in the adult.1,2 In the adult, normal processes such as wound healing and pathological processes such as tumor expansion and metastasis require the production of new blood vessels. We have begun to understand how blood vessel formation is regulated at the molecular level, and vascular endothelial growth factor (VEGF) is clearly central to this process.3 Mouse embryos lacking even one copy of the VegfA gene die as embryos with vascular defects, and vascular development in differentiating embryonic stem (ES) cells is compromised in VegfA+/− and VegfA−/− ES cells in a dose-dependent manner.4–6 Moreover, modestly elevated levels of VEGF result in vascular abnormalities,7 and large doses of VEGF compromise both vascular development and neovascularization in adult organisms.8,9 These findings suggest that VEGF signaling must be precisely controlled during vascularization to result in proper vessels. The location and duration of VEGF expression provide one level of control, but other components of the pathway are also likely to be involved in fine tuning the signal.
Two high-affinity VEGF receptors, flk-1 and flt-1, are candidates to mediate fine tuning of the pathway.10,11 Both receptors are membrane-spanning receptor tyrosine kinases that bind VEGF-A with high affinity, but their effects on VEGF signaling are very different. Mice or ES cells lacking flk-1 have little or no blood vessel formation, suggesting that VEGF effects on endothelial cells are mediated through flk-1.12,13 VEGF signaling through flk-1 produces several cellular responses, including a strong mitogenic signal and a survival signal for endothelial cells and their precursors.14–17
In contrast, VEGF binding to flt-1 does not produce a strong mitogenic signal, and flt-1−/− mice die at mid-gestation with vascular overgrowth and disorganization.18,19 We recently showed that flt-1 impacts developmental blood vessel formation by negatively modulating endothelial cell division.20 Thus it was logical to ask whether agents that affect flk-1 signaling could rescue the phenotypic effects of the flt-1 mutation. Our results show that two agents, the potent flk-1 small molecule inhibitor SU5416 and a Flt-1/Fc chimeric protein that binds and sequesters VEGF, partially rescue the vascular overgrowth phenotype of flt-1−/− ES cell-derived blood vessels. Flk-1 tyrosine phosphorylation is elevated in the flt-1 mutant blood vessels and this increase is blocked by the flk-1 inhibitor. These results indicate that flt-1 negatively modulates developmental blood vessel formation by dampening signaling through flk-1.
Materials and Methods
Cell Culture, Differentiation, and Antibody Staining
ES cells were maintained and differentiated as described.20,21 SU5416 was resuspended in dimethyl sulfoxide at a concentration of 10 mmol/L, and Flt-1/Fc (recombinant mouse VEGFR-1 (flt-1)/Fc; R & D Systems, Minneapolis, MN) was resuspended in phosphate-buffered saline (PBS)/0.1% bovine serum albumin at a concentration of 10 μg/ml. Both solutions were added to growth medium immediately before feeding the cultures every second day from day 5 (SU5416) or day 3 (Flt-1/Fc). Day 8 cultures were fixed in fresh cold MeOH:acetone (1:1) for 5 minutes and then processed for antibody staining as described.20,21 All cultures were reacted with rat anti-mouse PECAM-1 (Mec 13.3; Pharmingen B-D, San Diego, CA) at 1:1000 dilution and then with donkey anti-rat tetramethyl-rhodamine isothiocyanate (Jackson Immunoresearch, West Grove, PA) at a 1:200 dilution. Cultures were stored in PBS at 4°C. Image analysis was as described.20,21
Protein Analysis
Cell lysates were collected from day 7 or 8 differentiated ES cells using RIPA buffer [150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5, 1% Nonidet P-40, 0.25% Na deoxycholate, 1 mmol/L Na orthovanadate, 1 mmol/L NaF, and complete mini ethylenediaminetetraacetic acid-free protease cocktail inhibitor tablets (Boehringer Mannheim, Indianapolis, IN)]. Flk-1 protein was immunoprecipitated from 6 mg of total protein with 1.5 μg anti-flk polyclonal antibody (sc-504; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Protein A agarose beads were added and the lysates incubated for 2 hours at 4°C. Immunoprecipitates were washed with RIPA buffer followed by PBS and loaded onto a 5% polyacrylamide gel. After transfer to polyvinylidene difluoride (Amersham, Arlington Heights, IL), phosphorylated flk-1 was detected by incubation with a monoclonal anti-pTyr antibody (clone PY20; BD Transduction, San Jose, CA) at 1:1000, followed by washing and detection with enhanced chemiluminescence (Amersham). The membrane was then stripped and total flk-1 was detected using anti-flk-1 antibody [sc-6251 at 1:100 (Santa Cruz Biotechnology) or no. 555307 at 1:500 (B-D Pharmingen)], and enhanced chemiluminescence detection. The signal from the upper band was quantitated by densitometry using NIH Image, and p-Tyr levels were normalized to flk-1 levels.
Results
Blockade of the Flk-1 Signaling Pathway Partially Rescues the Flt-1 Mutant Vascular Phenotype
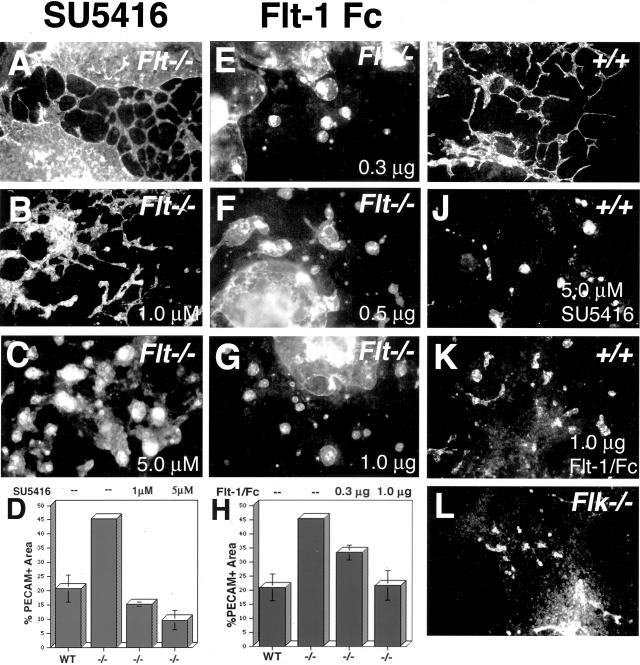
The flk-1 signal transduction pathway was experimentally perturbed in two distinct ways (Figure 1). First, the selective flk-1 inhibitor SU5416 was added to ES cell cultures as they differentiated to form primitive blood vessels. SU5416 is a small molecule inhibitor that freely traverses the membrane and binds the ATP-binding pocket of flk-1, preventing phosphorylation and downstream signaling.22–24 As expected, control wild-type cultures incubated with the inhibitor showed a severe disruption of normal blood vessel formation (compare Figure 1, I and J), similar to the phenotype seen when flk-1 is genetically ablated (compare Figure 1, J and L). The vascular overgrowth seen in the flt-1−/− cultures (Figure 1A) was partially rescued by incubation with SU5416 (Figure 1, B and C). Quantitation of the vascular area showed dose-dependent rescue of the overgrowth, with 1 μmol/L of inhibitor approximating wild-type levels of vasculature (Figure 1D). The flt-1−/− vessels also appeared the most normal, but not completely normal, when incubated with 1 μmol/L of SU5416 (Figure 1B). Thus, perturbation of flk-1 signaling partially rescued the flt-1 mutant vascular phenotype.
Figure 1.
Inhibition of flk-1 signaling partially rescues the flt-1 mutant phenotype of ES cell-derived blood vessels. ES cells were differentiated to day 8 as described in Materials and Methods and then fixed and stained with anti-PECAM-1 to visualize blood vessels by immunofluorescence. A–C, E–G: flt-1−/− ES differentiation cultures; I–K: +/+ wild-type ES differentiation cultures; L: flk-1−/− ES differentiation culture. Treatments: A, I, and L, untreated; B, 1 μmol/L of SU5416; C and J, 5 μmol/L of SU5416; E, 0.3 μg/ml of Flt-1/Fc; F, 0.5 μg/ml of Flt-1/Fc; G and K, 1 μg/ml of Flt-1/Fc. D and H: Quantitation of vessel area was as described in Materials and Methods.
The experiment was repeated with a second inhibitor with a completely different mode of action. Flt-1/Fc is a small protein that binds and sequesters the ligand VEGF-A to prevent it from binding flk-1.25 Because vascular development in ES cell cultures is VEGF-dependent,6 we reasoned that modulating effective VEGF-A levels with this inhibitor might have similar effects to incubation with SU5416. This was the case, and wild-type vascular development was perturbed while flt-1−/− mutant vascular development was partially rescued by addition of Flt-1/Fc (Figure 1; E to G, K). Quantitation of the vascular area also showed that this partial rescue was dose-dependent, with 1 μg/ml of inhibitor approximating wild-type levels of vasculature (Figure 1H). However, the phenotypic rescue of the flt-1 mutant vessels was less dramatic with Flt-1/Fc than with SU5416 (compare Figure 1, B and F). Thus, perturbation of the flk-1 signal transduction pathway by two mechanistically distinct inhibitors partially rescues the flt-1 mutant vasculature, suggesting that flt-1 impinges on the flk-1 signaling pathway.
Flk-1 Tyrosine Phosphorylation Levels Correlate with the Vascular Phenotype in Flt-1 Mutant and Rescued Vessels
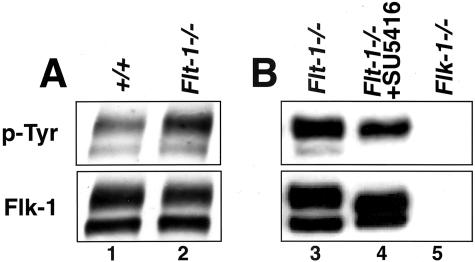
Because flk-1 phosphorylation is one of the first biochemical events associated with ligand engagement, we hypothesized that flk-1 phosphorylation was normally sensitive to levels of endogenous flt-1, and also that partial rescue of the flt-1 mutant vascular phenotype would be accompanied by a reduction in the level of flk-1 tyrosine phosphorylation. We immunoprecipitated flk-1 from lysates prepared from ES cell differentiation cultures, then blotted with an antibody to phosphotyrosine to determine the amount of phospho-flk. A flk-1 antibody was used to determine total levels of flk-1, and the ratio of phospho-flk to total flk-1 was calculated to control for variations in the amount of flk-1 in the different cultures (Figure 2). The doublet seen in the flk-1 lanes represents differential glycosylation of flk-1, and only the upper form is significantly phosphorylated.26 Thus, only the upper band was used for quantitation. The ratio of phospho-flk to total flk was elevated by threefold in the flt-1 mutant ES cell differentiation cultures (Figure 2A). This finding indicates that flt-1 normally negatively modulates flk tyrosine phosphorylation. Compared to control flt-1−/− cultures, flt-1−/− mutant cultures incubated with the inhibitor SU5416 showed a threefold decrease in the ratio of phospho-flk to total flk (Figure 2B), which is consistent with the mode of action of the inhibitor. The partial rescue of the flt-1 mutant phenotype in the presence of relatively reduced amounts of phospho-flk suggests that flt-1 normally affects flk-1 signaling biochemically by modulating flk phosphorylation during vascular development.
Figure 2.
Flk-1 tyrosine phosphorylation is elevated by the flt-1 mutation and sensitive to flk-1 inhibitor. Lysates of day 7 or 8 differentiated ES cell cultures were immunoprecipitated with anti-flk-1 antibody and then subjected to Western blot analysis with anti-p-Tyr antibody. Blots were then stripped and reprobed with anti-flk-1 antibody. Detection was with enhanced chemiluminescence. A: Lysates of wild-type (lane 1) and flt-1−/− (lane 2) ES cell cultures. B: Lysates of flt-1−/− (lane 3), flt-1−/− with 5 μmol/L of SU5416 (lane 4), and flk-1−/− (lane 5) ES cell cultures. The doublet represents different glycosylation levels of the flk-1 protein.26 The reproducible increase in the mobility of the upper band in the presence of SU5416 is unexplained but documented in several other studies.22,36
Discussion
Although it has been known for some time that the VEGF receptor flt-1 negatively modulates blood vessel formation,18,27 it has not been clear how this effect is achieved at the cellular and molecular levels. We recently showed that flt-1 negatively modulates endothelial cell proliferation during embryonic development and in differentiating ES cell cultures.20 Here we show that this negative modulation by flt-1 impinges on the flk-1 signaling pathway by affecting the level of flk-1 tyrosine phosphorylation. The data supporting this model are that two distinct modes of perturbation of flk-1 signaling partially rescue flt-1−/− mutant blood vessel formation, and phenotypic rescue is accompanied by reduced levels of flk-1 tyrosine phosphorylation. Moreover, elevated levels of flk-1 tyrosine phosphorylation in the flt-1 mutant blood vessels show that the absence of flt-1 leads to up-regulation of the first measurable parameter of flk-1 signal transduction. This finding has implications for the molecular mechanism of flt-1 action.
Studies in cultured cells have provided evidence for two potential molecular models of flt-1 action. Signal transduction through the flt-1 receptor is thought to negatively regulate flk-1-dependent functions such as proliferation and migration.28–30 However, a mouse that has no wild-type flt-1 protein, but instead is homozygous for a mutant flt-1 that is missing most of its cytoplasmic domain, is viable.31 This finding suggests that the signaling capacity of flt-1 may not be important developmentally. A soluble form of flt-1 (sflt-1) can be produced by alternative splicing,32,33 suggesting that one role for flt-1 involves sequestration of the VEGF ligand by sflt-1. Our data do not rigorously distinguish between these models, but the finding that flk-1 tyrosine phosphorylation is elevated in the flt-1 mutant background strongly indicates that an early step in flk-1 signal transduction is the target of flt-1 action developmentally. This is consistent with a model whereby flt-1 acts as a sink to modulate the amount of VEGF ligand available to bind to flk-1. Thus in the absence of flt-1 more ligand is available to bind flk-1, and the first step of signal transduction, receptor tyrosine phosphorylation, is up-regulated. This up-regulation is predicted to lead to increased signaling through all of the numerous pathways that are affected by flk-1 signal transduction, including proliferation, survival, migration, and permeability.
This is the first demonstration that the vascular overgrowth seen in the absence of flt-1 can be modulated by manipulations of the flk-1 pathway. The inability to completely rescue the flt-1 mutant phenotype with either inhibitor suggests that either flt-1 impinges on pathways other than flk-1 signaling, or that the inhibitors cannot act optimally. We favor the latter hypothesis because several studies show that rigorous control of the amplitude and timing of VEGF signaling in local environments is required for proper vascular development.4,6–8 In this context, the increased efficacy of the small molecule inhibitor in rescue relative to the Flt-1/Fc protein may result from deposition of some endogenous VEGF into local matrix, where it may be inaccessible for sequestration by Flt-1/Fc. Alternatively, Flt-1/Fc may incompletely rescue the flt-1 mutant phenotype because it is not spatially and/or temporally regulated properly. Recent studies suggest that a VEGF gradient may be important for proper vessel formation,34,35 and we have recently demonstrated that flt-1 affects vessel morphogenesis (JP Kearney et al, in press). Thus flt-1 may normally affect presentation of VEGF in a spatial context that is not reproduced by exogenous administration of Flt-1/Fc. In any case, the data presented here provides further understanding of how the organism normally modulates critical parameters of blood vessel formation. Two high-affinity VEGF receptors have opposing effects on this process. Moreover, the negative modulation by flt-1 impinges on flk-1 signaling at an early step in the pathway, suggesting that all downstream effects of this pathway are tightly modulated as new blood vessels form.
Acknowledgments
We thank Guo-Hua Fong and Janet Rossant for the flt-1−/− and flk-1−/− ES cells, respectively; and the members of the Bautch Lab and the Carolina Cardiovascular Biology Center for fruitful discussions.
Footnotes
Address reprint requests to Victoria L. Bautch, Ph.D., Professor of Biology, Dept. of Biology, CB#3280, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599. E-mail: bautch@med.unc.edu.
Support by the National Institutes of Health (R01 HL43174 to V.L.B.), Glaxo-SmithKline (to V.L.B.), a National Science Foundation predoctoral fellowship (to D.M.R.), and predoctoral fellowships DAMD 17-02-1-0524 (to D.M.R.) and DAMD 17-00-1-0379 (to J.B.K.).
Current address of M.P.R.: Piedmont Research Center, 3300 Gateway Center, Morrisville, NC 27560.
References
- Beck L, Jr, D’Amore PA. Vascular development: cellular and molecular regulation. EMBO J. 1997;11:365–373. [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Bautch VL, Redick SD, Scalia A, Harmaty M, Carmeliet P, Rapoport R. Characterization of the vasculogenic block in the absence of vascular endothelial growth factor-A. Blood. 2000;95:1979–1987. [PubMed] [Google Scholar]
- Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Little CD. Exogenous vascular endothelial growth factor induces malformed and hyperfused vessels during embryonic neovascularization. Proc Natl Acad Sci USA. 1995;92:7657–7661. doi: 10.1073/pnas.92.17.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. EMBO J. 1999;13:9–22. [PubMed] [Google Scholar]
- Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001:RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- Schuh A, Faloon P, Hu Q, Bhimani M, Choi K. In vitro hematopoietic and endothelial potential of flk-1−/− embryonic stem cells and embryos. Proc Natl Acad Sci USA. 1999;96:2159–2164. doi: 10.1073/pnas.96.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–2407. doi: 10.1182/blood.v99.7.2397. [DOI] [PubMed] [Google Scholar]
- Bautch VL. Embryonic stem cell differentiation and the vascular lineage. Methods Mol Biol. 2002;185:117–125. doi: 10.1385/1-59259-241-4:117. [DOI] [PubMed] [Google Scholar]
- Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK, Cherrington JM. Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des. 2000;15:29–41. [PubMed] [Google Scholar]
- Mendel DB, Schreck RE, West DC, Li G, Strawn LM, Tanciongco SS, Vasile S, Shawver LK, Cherrington JM. The angiogenesis inhibitor SU5416 has long-lasting effects on vascular endothelial growth factor receptor phosphorylation and function. Clin Cancer Res. 2000;6:4848–4858. [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shibuya M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J Biol Chem. 2000;275:16986–16992. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- Zeng H, Dvorak HF, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2001;276:26969–26979. doi: 10.1074/jbc.M103213200. [DOI] [PubMed] [Google Scholar]
- Zeng H, Zhao D, Mukhopadhyay D. KDR stimulates endothelial cell migration through heterotrimeric G protein Gq/11-mediated activation of a small GTPase RhoA. J Biol Chem. 2002;277:46791–46798. doi: 10.1074/jbc.M206133200. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itokawa T, Nokihara H, Nishioka Y, Sone S, Iwamoto Y, Yamada Y, Cherrington J, McMahon G, Shibuya M, Kuwano M, Ono M. Antiangiogenic effect by SU5416 is partly attributable to inhibition of flt-1 receptor signaling. Mol Cancer Ther. 2002;1:295–302. [PubMed] [Google Scholar]