Abstract
Endotoxin (lipopolysaccharide, LPS)-induced tumor necrosis factor-α (TNF-α) release from Kupffer cells is critically involved in the pathogenesis of alcohol-induced liver injury. We recently reported that inhibition of alcohol-induced plasma endotoxin elevation contributes to the protective action of zinc against alcoholic hepatotoxicity. The present study was undertaken to determine whether zinc interferes with the endotoxin-TNF-α signaling pathway, and possible mechanism(s) by which zinc modulates the endotoxin-TNF-α signaling. Administration of LPS to metallothionein (MT)-knockout (MT-KO) mice and 129/Sv wild-type (WT) controls at 4 mg/kg induced hepatic TNF-α elevation at 1.5 hours, followed by liver injury at 3 hours. Zinc pretreatment (two doses at 5 mg/kg) attenuated TNF-α production and liver injury in both MT-KO and WT mice, indicating a MT-independent action of zinc. Immunohistochemical detection of the phosphorylation of I-κB and nuclear factor (NF)-κB in the liver of MT-KO mice demonstrated that zinc pretreatment abrogated LPS-induced NF-κB activation in the Kupffer cells. Fluorescent microscopy of superoxide by dihydroethidine and of zinc ions by Zinquin in the liver of MT-KO mice showed that zinc pretreatment increased the intracellular labile zinc ions and inhibited LPS-induced superoxide generation. These results demonstrate that zinc inhibits LPS-induced hepatic TNF-α production through abrogation of oxidative stress-sensitive NF-κB pathway, and the action of zinc is independent of MT. Thus, zinc may be beneficial in the treatment of LPS-induced liver injuries, such as sepsis and alcoholism.
Tumor necrosis factor-α (TNF-α) is a cytokine involved in alcoholic liver disease.1–3 The production of TNF-α by exposure to alcohol has been repeatedly demonstrated both in animal models and in patients with alcoholic hepatitis.4–6 These studies often reported elevations in serum TNF-α protein and hepatic TNF-α mRNA levels. Neutralizing TNF-α with a polyclonal antibody resulted in suppression of hepatic necrosis and inflammation caused by chronic alcohol exposure.7 Blocking TNF-α signaling in a TNF-α receptor-1 knockout mouse model also led to attenuation of alcohol-induced liver injury.8,9 These reports strongly suggest that TNF-α plays a critical role in alcohol-induced liver injury.
Kupffer cells are the main source of TNF-α after alcohol exposure. Endotoxin has been shown to trigger TNF-α production in alcohol-induced liver injury. Previous investigations demonstrated that endotoxin activates Kupffer cells by binding to the CD14/Toll-like receptor 4 on Kupffer cells, whose activation leads to increased activity of NADPH oxidase, nuclear factor (NF)-κB activation, and, eventually, TNF-α production.10–12 Thus, preventing endotoxin-induced Kupffer cell activation and TNF-α production may be an important strategy in the prevention of alcohol-induced liver injury.
Zinc is an essential trace element involved in many physiological functions, including catalytic and structural roles in metalloenzymes, as well as regulatory roles in diverse cellular processes such as synaptic signaling and gene expression. Many reports indicate that zinc acts as an effective hepatoprotective agent under a variety of toxic conditions.13–15 These reports showed that the action of zinc is associated with metallothionein (MT) induction. MT has been known as the major protein responsible for zinc homeostasis. MT has one-third cysteine residues and functions as an antioxidant. It has been long debated whether protection by zinc treatment results from zinc per se or from MT induction. Our recent studies with a MT-transgenic and MT-knockout (MT-KO) mouse models demonstrated that zinc, independent of MT, provides effective protection against acute alcohol-induced liver injury.16,17 Furthermore, it was found that zinc prevented alcohol-induced alterations in gut permeability, which was involved in zinc inhibition of alcohol-induced endotoxin release from gut to blood stream.18 However, it is unclear whether zinc can modulate the endotoxin-TNF-α signaling in the liver. Therefore, the present study was undertaken to determine whether zinc interferes with the endotoxin-TNF-α signaling pathway and the possible mechanism(s) by which zinc interacts with the endotoxin-TNF-α signaling.
Materials and Methods
Animals
Homozygous MT-KO mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were produced on the 129/Sv genetic background. The MT-KO mice lacking MT-I and MT-II, the major mouse hepatic MT isoforms, were produced by a gene-targeting technique.19 Both MT-KO and 129/Sv wild-type (WT) controls were housed in the animal quarters at the University of Louisville Research Resources Center. They were maintained at 22°C with a 12-hour light/dark cycle and had free access to rodent chow and tap water. The experimental procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association for the Accreditation of Laboratory Animal Care.
Treatments
Nine-week-old male mice (23 to 25 g body weight) were used in the following experiments. In experiment 1 the effects of zinc pretreatment on lipopolysaccharide (LPS)-induced TNF-α production and liver injury were studied. MT-KO and WT mice were divided into eight groups by a 2 × 2 × 2 factorial design (+/−MT, +/−zinc, +/−LPS). For zinc treatment, two doses of zinc sulfate in saline at 5 mg of zinc ion/kg were administrated intraperitoneally at 36 hours and 12 hours before LPS (from E. Coli Serotype 0111: B4, Sigma, St. Louis, MO) treatment (4 mg/kg). Saline was used for controls for both zinc and LPS treatments. To assess intrahepatic TNF-α level, liver samples were harvested at 1.5 hours after LPS administration. To evaluate liver injury, plasma and liver samples were harvested at 3 hours after LPS administration. In experiment 2 the effects of zinc pretreatment on LPS signal transduction in the liver were studied. MT-KO mice were divided into four groups by a 2 × 2 factorial design (+/−zinc, +/−LPS). Two doses of zinc sulfate in saline at 5 mg of zinc ion/kg were administrated intraperitoneally at 36 hours and 12 hours before LPS treatment (4 mg/kg). Saline was used for controls of both zinc and LPS treatments. Dihydroethidine (Molecular Probes, Eugene, OR) at 5 mg/kg and Zinquin ethyl ester (Sigma) at 2.5 mg/kg were injected via tail vein immediately after LPS challenge for in situ detections of superoxide and zinc ions in the liver. Liver samples were harvested at 1 hour after LPS administration. At the end of each experiment, the mice were anesthetized with sodium pentobarbital (0.05 mg/g body weight). Blood was drawn using a heparinized syringe from the dorsal vena cava, and plasma was obtained by centrifugation using a plasma separator tube. The liver was perfused with saline and tissue samples were processed for both pathological and biochemical analysis.
TNF-α Quantification
Liver samples for TNF-α assay were prepared according to a previous report20 with some minor modifications. Briefly, liver samples were disintegrated in 4 vol of ice-cold Ripa buffer.20 After incubation on ice for 30 minutes, samples were centrifuged twice at 25,000 × g for 15 minutes at 4°C. The resulting supernatants were used for the assay. The TNF-α levels in the liver were detected by immunoassay using a murine kit (BioSource Int., Camarillo, CA) and were expressed as pg/g liver tissue.
Alanine Aminotransferase (ALT) Assay
Plasma ALT (EC 2.6.1.2.) activity was colorimetrically measured by using a Sigma Diagnostic kit (procedure no. 505, Sigma) following the instructions provided by the manufacture.
Histopathological Examination
Liver tissues were fixed with 10% neutral formalin and embedded in paraplast. Tissue sections of 5 μm were stained by hematoxylin and eosin.
MT Assay
Tissue MT concentrations were determined by a cadmium-hemoglobin affinity assay. Briefly, liver tissues were homogenized in 4 vol of 10 mmol/L Tris-HCl buffer, pH 7.4, at 4°C. After centrifugation of the homogenate at 10,000 × g for 15 minutes, 200 μl of supernatant were transferred to microtubes for MT analysis, as described previously.21
Measurement of Zinc
Hepatic zinc concentrations were determined by inductively coupled argon-plasma emission spectroscopy (model 1140; Jarrel-Ash, Waltham, MA) after lyophilization and digestion of the tissues with nitric acid and hydrogen peroxide.22 Zinc concentrations in the liver were expressed as μg/g dry tissue.
Immunohistochemical Detection of p-I-κB, p-NF-κB, Kupffer Cell, and TNF-α in the Liver
Cryostat liver sections were cut at 5 μm, air-dried, and fixed in acetone for 20 minutes at −20°C. For p-I-κB and p-NF-κB immunoperoxidase staining, endogenous peroxidase activity was quenched by incubating sections in 3% H2O2. Nonspecific binding sites were blocked by 5% normal goat serum for 30 minutes. Sections were incubated with polyclonal rabbit anti-TNF-α (BioSource), rabbit anti-phospho-I-κB (p-I-κB; Cell Signaling Technology, Beverly, MA), or rabbit anti-phospho-NF-κB (p-NF-κB, Cell Signaling) overnight at 4°C, followed by incubation with DAKO EnVision+ horseradish peroxidase-conjugated goat anti-rabbit IgG (DAKO, Carpinteria, CA) for 30 minutes. The antibody binding sites were visualized by incubation with a diaminobenzidine-H2O2 solution. For double-immunofluorescence staining of Kupffer cells and TNF-α, TNF-α staining was first performed by incubation with rabbit anti-TNF-α and Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), and Kupffer cells were then stained by incubation with monoclonal rat anti-mouse pan tissue-fixed macrophages (clone EM8; Research Diagnostics, Flanders, NJ) and fluorescein isothiocyanate-conjugated donkey anti-rat IgG (Jackson ImmunoResearch Laboratories). For double-immunofluorescence staining of TNF-α and p-NF-κB, p-NF-κB was first stained by incubation with rabbit anti-p-NF-κB and Cy3-conjugated donkey anti-rabbit IgG, and TNF-α staining was then followed by incubation with monoclonal rat anti-mouse TNF-α (BD PharMingen, San Diego, CA) and fluorescein isothiocyanate-conjugated donkey anti-rat IgG. All of the reactions of primary antibodies were conducted at 4°C overnight, and the fluorescence conjugates were conducted at room temperature for 1 hour.
Double-Fluorescence Labeling of Superoxide and Zinc Ion
Dihydroethidine was used for in situ detection of superoxide production in the liver. Dihydroethidine is oxidized to ethidine (red fluorescence) selectively by superoxide, but not by other reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl radicals, or peroxynitrite.23,24 Intracellular zinc ions were detected by Zinquin. Zinquin is a membrane-permeable, blue fluorescent probe that fluoresces on binding to zinc ions.25,26 For simultaneous in situ detection of superoxide and zinc ions in the liver, dihydroethidine (Molecular Probes) at 5 mg/kg and Zinquin ethyl ester (Sigma) at 2.5 mg/kg were injected via tail vein 1 hour before tissue harvest, as described above. Cryostat sections of liver were cut at 5 μm and mounted on glass slides. The fluorescence was detected with a Nikon 2000S fluorescent microscope.
Statistics
All data are expressed as mean ± SD (n = 4 to 6). The data were analyzed by analysis of variance and Newman-Keuls multiple-comparison test. Experiments involved in factorial designs were analyzed accordingly. Differences between groups were considered significant at P < 0.05.
Results
Zinc Treatment Attenuates LPS-Induced TNF-α Production and Liver Injury Independent of MT
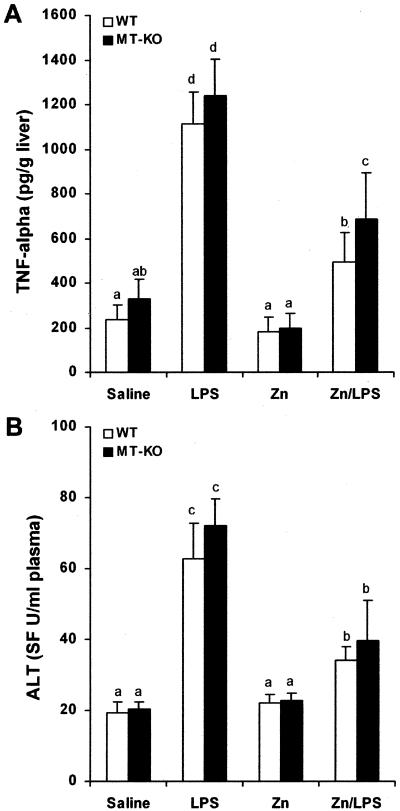
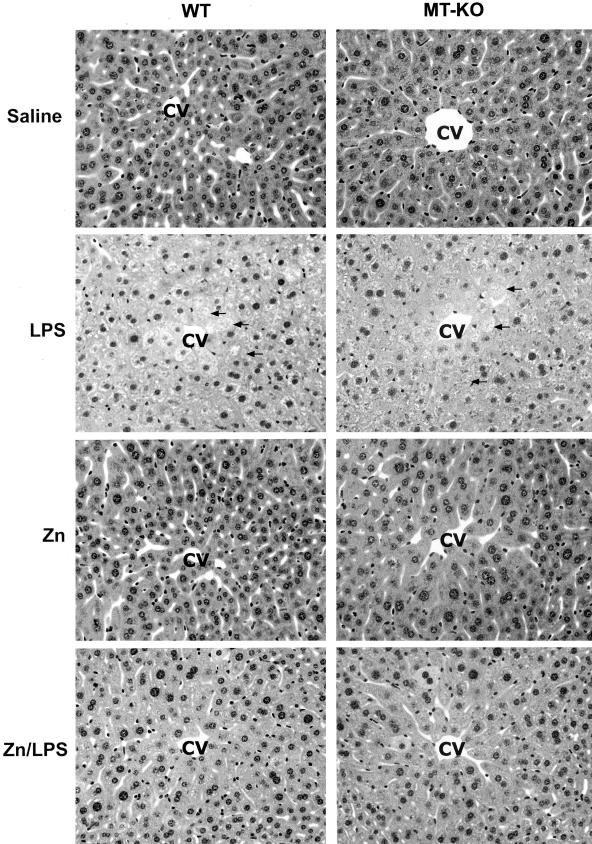
To determine the role of zinc in inhibition of LPS-induced TNF-α production and liver injury, WT mice were treated with zinc before LPS administration. TNF-α was measured at 1.5 hours and liver injury was assessed at 3 hours after LPS administration based on our preliminary time-course study at 1.5, 3, 6, and 12 hours, in which TNF-α production was peaked at 1.5 hours and liver injury at 3 hours after LPS administration (data not shown). As shown in Figure 1A, LPS administration induced a 5-fold increase in TNF-α concentrations in the liver. Zinc pretreatment significantly inhibited LPS-induced TNF-α production in the liver. Plasma ALT activity also was significantly elevated by LPS administration, whereas zinc pretreatment attenuated LPS-induced increase in plasma ALT activity (Figure 1B). Corresponding to plasma ALT elevation, histopathological observation demonstrated that zinc pretreatment suppressed LPS-induced necrotic cell death in the liver (Figure 2). To determine whether zinc inhibition of LPS-induced liver injury is mediated by MT production, MT-KO mice were treated in the same manner as the WT mice. As shown in Figure 1A, zinc significantly inhibited LPS-induced TNF-α production in the MT-KO mice, although the value was higher than in WT mice. Furthermore, the inhibitory effect of zinc on LPS-induced liver injury in the MT-KO mice was comparable to that observed in the WT mice (Figure 1B, Figure 2).
Figure 1.
Effects of zinc pretreatment on LPS-induced hepatic TNF-α production and plasma ALT activity elevation. MT-KO and WT mice were administrated with two doses of zinc sulfate at 5 mg of zinc ion/kg, followed by one dose of LPS at 4 mg/kg. Hepatic TNF-α level at 1.5 hours after LPS challenge was measured by immunoassay. Plasma ALT activity at 3 hours after LPS challenge was measured using a Sigma Diagnostic kit. A: Effects of zinc on LPS-induced TNF-α production in the liver. B: Effects of zinc on LPS-induced elevation in plasma ALT activity. Results are means ± SD (n = 4 to 6). Significant difference (P < 0.05) is identified by different letters.
Figure 2.
Effect of zinc on LPS-induced histopathological changes in the liver. MT-KO and WT mice were administrated with two doses of zinc sulfate at 5 mg of zinc ion/kg, followed by one dose of LPS at 4 mg/kg. Histopathological changes were observed at 3 hours after LPS challenge. LPS-induced necrotic damages (arrows) in the liver were inhibited by zinc pretreatment in both WT and MT-KO mice. CV, Central vein. H&E. Original magnifications, ×260.
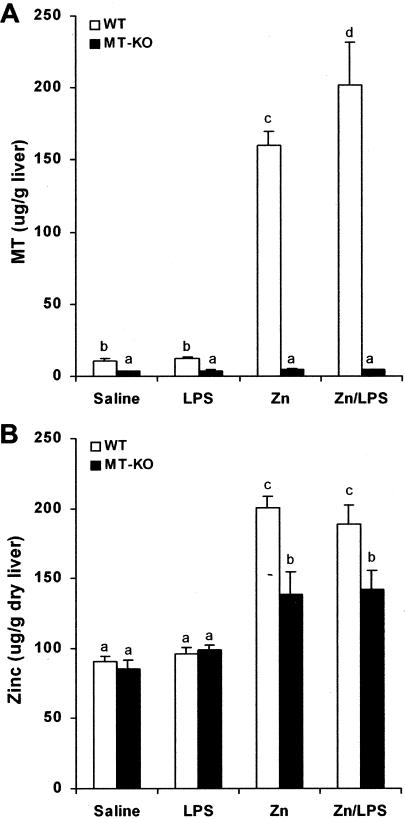
To confirm the MT-independent action of zinc in hepatic protection from LPS damage, MT and zinc concentrations in the liver of WT and MT-KO mice were compared. As shown in Figure 3A, zinc treatment induced more than a 15-fold increase in hepatic MT concentrations in WT mice with or without LPS treatment. However, LPS per se did not affect MT concentrations in the liver. When the same treatment was applied to MT-KO mice, only trace amounts of hepatic MT were detected in all groups of MT-KO mice. On the other hand, zinc treatment elevated hepatic zinc concentrations by 2-fold in the WT mice and 1.5-fold in the MT-KO mice (Figure 3B). LPS per se did not cause significant change in hepatic zinc concentrations in either WT or MT-KO mice.
Figure 3.
Effects of zinc pretreatment on hepatic MT and zinc concentrations. MT-KO and WT mice were administrated with two doses of zinc sulfate at 5 mg of zinc ion/kg, followed by one dose of LPS at 4 mg/kg. MT and zinc concentrations in the liver were measured by a cadmium-hemoglobin affinity assay and by inductively coupled argon plasma emission spectroscopy, respectively, at 1.5 hours after LPS challenge. A: MT concentrations in the liver. B: Zinc concentrations in the liver. Results are means ± SD (n = 4 to 6). Significant difference (P < 0.05) is identified by different letters.
Zinc Treatment Abrogates LPS-Induced NF-κB Activation
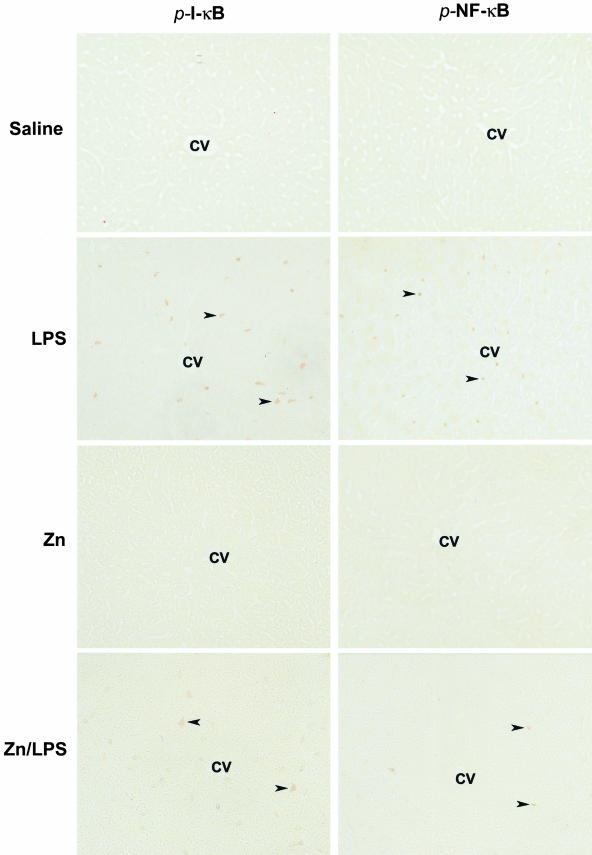
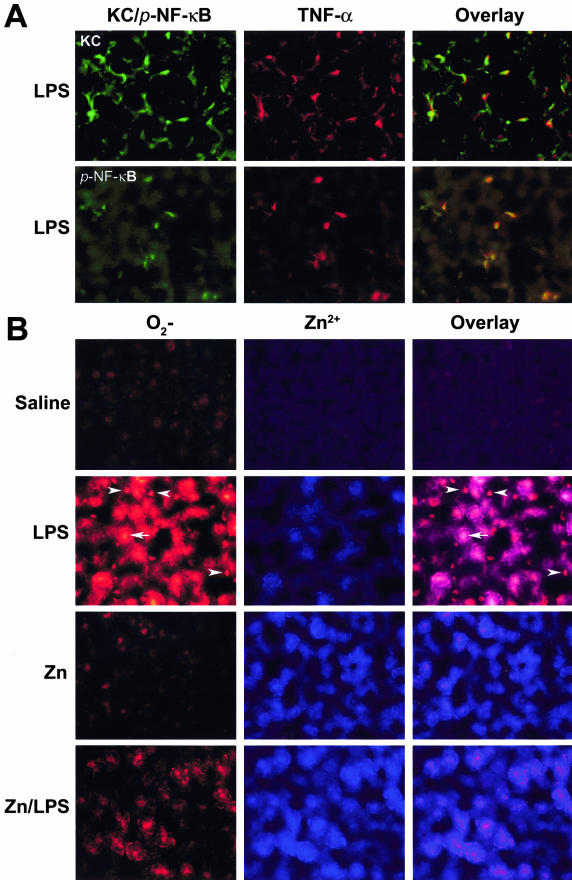
To determine the molecular mechanism by which zinc inhibits LPS-induced TNF-α production, the effects of zinc on NF-κB activation were monitored by immunohistochemical staining of p-I-κB and p-NF-κB at 1 hour after LPS challenge to WT mice. As shown in Figure 4, there were no p-I-κB immunoreactive cells that were detected in the livers of control or zinc-treated mice. In the LPS-treated mice, there were numerous positive cells found in the liver, and the positive staining was apparently in the Kupffer cells that localize on the sinusoid wall. In the zinc/LPS-treated mice, there were only a few weakly stained cells found in the liver. Correspondingly, a large number of Kupffer cells in the LPS-treated mice showed immunoreactivity to p-NF-κB, and the positive staining was localized mainly in the nuclei. Zinc pretreatment abrogated LPS-induced NF-κB activation; there were only a few Kupffer cells showing weak staining.
Figure 4.
In situ detection of LPS-induced NF-κB activation in the liver by immunohistochemistry. MT-KO mice were administrated with one dose of LPS at 4 mg/kg. Livers were removed at 1 hour after LPS challenge, and cryostat sections were cut at 5 μm. NF-κB activation was monitored by immunohistochemical staining of phospho-I-κB (p-I-κB) and phospho-NF-κB (p-NF-κB). Arrowheads show positive cells immunoreactive to p-I-κB and p-NF-κB. CV, Central vein. Original magnifications, ×260.
To elucidate the link between TNF-α production and NF-κB activation in the Kupffer cells, double-immunofluorescence staining of Kupffer cell/TNF-α and p-NF-κB/TNF-α were performed in the liver of MT-KO mice. As shown in Figure 5A, TNF-α and p-NF-κB were co-localized in the Kupffer cells.
Figure 5.
Double-fluorescence labeling of TNF-α/Kupffer cell, p-NF-κB/TNF-α, and superoxide/zinc ions in the liver. MT-KO mice were administrated with two doses of zinc sulfate at 5 mg of zinc ion/kg, followed by one dose of LPS at 4 mg/kg. Livers were removed at 1 hour after LPS challenge, and cryostat sections were cut at 5 μm. A: Co-localization of TNF-α/Kupffer cell (KC) and p-NF-κB/TNF-α by immunofluorescence staining. B: Simultaneous detection of superoxide (O2−) by dihydroethidine and zinc ions (Zn2+) by Zinquin. Arrowheads show the Kupffer cells and arrows show hepatocytes. Original magnifications, ×520.
Zinc Inhibits LPS-Induced Oxidative Stress
To understand the mechanism by which zinc abrogates LPS-induced NF-κB activation, in situ detections of superoxide by dihydroethidine and zinc ions by Zinquin in the liver of MT-KO mice were performed at 1 hour after LPS challenge. As shown in Figure 5B, only weak ethidine fluorescence was found in the control and zinc-treated livers. However, strong ethidine fluorescence was detected in the livers of LPS-treated mice. This LPS-induced ethidine fluorescence was attenuated by zinc pretreatment. The Zinquin fluorescence was slightly increased by LPS treatment, whereas strong Zinquin fluorescence was found in the livers of zinc- and zinc/LPS-treated mice. Co-localization analysis of ethidine and Zinquin fluorescence demonstrated that the increased availability of intracellular zinc ions correlates with decreased superoxide accumulation after LPS administration.
Discussion
The results presented in the present study demonstrated that zinc pretreatment provided effective protection against LPS-induced liver injury through inhibition of TNF-α production, and the protective action of zinc was independent of MT. The mechanistic link through in situ detection of p-I-κB, p-NF-κB, superoxide, and zinc ions was found to be likely that zinc pretreatment increased the availability of intracellular zinc ions and abrogated LPS-induced oxidative stress and NF-κB activation in the Kupffer cells. Our results are the first to demonstrate that zinc protection, independent of MT, against LPS-induced hepatotoxicity is mediated via abrogating NF-κB activation and TNF-α production in the Kupffer cells.
TNF-α has been repeatedly reported to be specifically responsible for LPS-induced liver injury.12 A convinced evidence is that TNF-α neutralization with an anti-TNF-α antibody prevented liver injury in LPS-treated rat.27 This mechanism has been demonstrated in alcoholic liver disease.1–6,10,11 Alcohol administration increases intestinal permeability, thereby causing elevation of blood LPS.28–30 Because the LPS/TNF-α signaling pathway is critically involved in the pathogenesis of alcoholic liver injury, abrogating this pathway should attenuate the progression of alcoholic liver injury. In vitro studies have generated strong evidence that LPS activates the inflammatory response in Kupffer cells through oxidative stress.31
A number of antioxidants and herbal extracts have been reported to suppress LPS-induced TNF-α production from Kupffer cells and macrophages, including N-acetyl-l-cysteine,32–34 α-tocopherol,32,33 dilinoleoylphosphatidylcholine,35 dimethyl sulfoxide,36 pyrrolidine dithiocarbamate,37 and extracts of ginkgo biloba38 and green tea.39 In vitro studies using Kupffer cells or macrophages demonstrated that treatments with N-acetyl-l-cysteine (NAC), α-tocopherol, or dilinoleoylphosphatidylcholine inhibit LPS-induced TNF-α release.32,34,35 Similarly, in vivo treatments with pyrrolidine dithiocarbamate, ginkgo biloba, or green tea attenuated LPS-induced elevation in serum TNF-α level.37–39 However, less attention was paid to the causes and effects of LPS-induced intrahepatic TNF-α production and liver injury. The present study demonstrated that zinc attenuated LPS-induced TNF-α production in the liver, leading to inhibition of liver injury.
Zinc is a well-known MT inducer. Many investigations have demonstrated that zinc treatment provides protection against a variety of toxic chemicals.13–15 Because zinc treatment is always associated with MT induction, it has been postulated that zinc acts as a hepatoprotective agent through induction of MT synthesis. In this study, we indeed noted that zinc increased hepatic MT concentrations by 15-fold. The question, then, was whether MT mediates the zinc protective action. By using an MT-KO mouse model, we recently demonstrated that zinc, independent of MT, provides protection against alcohol-induced oxidative liver injury.17 In the present study, zinc treatment also prevented LPS-induced liver injury in the MT-KO mice in association with attenuation of intrahepatic TNF-α production. Because zinc treatment increased hepatic zinc level, but not MT, in MT-KO mice, the protective action by zinc treatment against LPS hepatotoxicity is apparently independent of MT induction. However, MT is important in maintaining high levels of zinc in the liver.
The signaling transduction cascade that regulates the LPS-induced TNF-α gene expression in Kupffer cells has been widely studied. Although several transcription factors have been implicated in TNF-α gene expression, the oxidative stress-sensitive transcription factor NF-κB likely plays a central role.10–12,40 Under unstimulated conditions, NF-κB is retained in the cytoplasm by binding to inhibitors such as I-κB. Induction of phosphorylation of I-κB leads to NF-κB release and translocation to the nucleus, thereby activating TNF-α gene transcription.41 Many in vitro studies have repeatedly demonstrated that the I-κB/NF-κB pathway is involved in LPS-induced TNF-α biosynthesis in Kupffer cells. Down-regulation of TNF-α release from LPS-activated Kupffer cells by antioxidants such as N-acetyl-l-cysteine, α-tocopherol, dimethyl sulfoxide, and pyrrolidine dithiocarbamate or herbal extracts such as ginkgo biloba and green tea has been shown to be accompanied by the inhibition of NF-κB activation.32–39 However, this I-κB/NF-κB pathway has yet to be elucidated in vivo. In the present study, phosphorylations of I-κB and NF-κB in Kupffer cells after LPS challenge were demonstrated by immunohistochemistry. Zinc treatment blocked the LPS-induced I-κB/NF-κB signaling cascade. This result indicates that zinc down-regulates LPS-induced TNF-α gene expression by the modulating I-κB/NF-κB signaling pathway.
Although the role of NF-κB activation is well characterized, the precise mechanism by which LPS induces NF-κB activation has yet to be elucidated. One well-studied pathway is that endotoxin activates Kupffer cells through the endotoxin receptor CD14/toll-like receptor 4 complex to produce ROS via NADPH oxidase.3,31 It has been postulated that ROS serve as a common mechanism for all stimuli of NF-κB activation and are central to LPS-induced cellular signaling events.42,43 An alternative explanation is that ROS generation causes an increase in the cellular ratio of oxidized to reduced thiol, which regulates NF-κB activation.44 N-acetyl-l-cysteine, a ROS scavenger and a thiol-modulating agent, often has been used to test these hypothesis. N-acetyl-l-cysteine treatment has been shown to reduce LPS-induced ROS generation including superoxide, hydrogen peroxide, and hydroxyl radical.34 Several reports have demonstrated that the inhibitory effect of N-acetyl-l-cysteine on LPS-induced Kupffer cells/macrophages activation results from a direct ROS scavenging action.45,46 These studies indicate that ROS may be the key mediator in LPS-induced NF-κB activation. In the present study, superoxide generation in the liver after LPS challenge was detected before TNF-α production. We have recently reported that oxidative stress plays a critical role in alcoholic hepatotoxicity and zinc has an inhibitory effect on alcohol-induced oxidative stress in the liver.17,47 The present study further demonstrated the antioxidant action of zinc in protection against LPS-induced oxidative stress in the liver.
Zinquin was used to determine whether zinc pretreatment increases the availability of intracellular zinc ions under LPS-induced oxidative stress. Zinquin fluorescence is highly specific for zinc ions, and is sensitive to nanomolar zinc ions.48 LPS treatment slightly increased Zinquin fluorescence in the liver in association with superoxide accumulation. Because exogenous nitric oxide or a cysteine-reactive agent has been shown to cause zinc release from thiol groups,49,50 the LPS-induced Zinquin fluorescence likely indicates an increase in free zinc ions rather than the loosely-bound zinc ions. On the other hand, the strong Zinquin fluorescence in the liver of zinc-treated mice indicates the increase in intracellular labile zinc ions. Co-localization of labile zinc ions and superoxide demonstrated that the elevation of intracellular labile zinc ions correlates with the inhibition of superoxide accumulation after LPS challenge. This result suggests that inhibition of oxidative stress is involved in the abrogation of LPS-induced I-κB/NF-κB signaling cascade.
In conclusion, the results of the present study demonstrated that zinc provides effective production against LPS hepatotoxicity by inhibition of TNF-α production. The inhibitory effect of zinc on LPS-induced TNF-α production results from modulation of oxidative stress-sensitive I-κB/NF-κB signaling cascade. Our results, thus, suggest that zinc may have a therapeutic potential in the prevention and/or treatment of LPS-mediated liver injury under conditions such as sepsis and alcoholism.
Acknowledgments
We thank Xinguo Sun, Gwen Dahlen, Kay Keehr, and Laura Idso for technical assistance.
Footnotes
Address reprint requests to Zhanxiang Zhou, Ph.D., University of Louisville School of Medicine, Department of Medicine, 511 South Floyd St., MDR 525, Louisville, KY 40292. E-mail: z0zhou01@louisville.edu.
Supported in part by the National Institutes of Health (grants AA13601 to Z.Z., AA10496 to C.J.M., AA10762 to C.J.M., and HL59225 to Y.J.K.), the University of Louisville School of Medicine, and the Veterans Administration, Louisville, Kentucky.
Y.J.K. is a Distinguished University Scholar of the University of Louisville, Louisville, KY.
References
- McClain CJ, Hill D, Schmidt J, Diehl AM. Cytokines in alcoholic liver disease. Semin Liver Dis. 1993;13:170–182. doi: 10.1055/s-2007-1007347. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Barve S, Barve S, Deaciuc I, Hill D. Tumor necrosis factor and alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:248S–252S. doi: 10.1097/00000374-199805001-00006. [DOI] [PubMed] [Google Scholar]
- Thurman RG. Mechanisms of hepatic toxicity. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- McClain C, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Rahemtulla A, Miao L, Fogt F, Matsumoto H, Tahan SR, Su GL. Activation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology. 1999;30:934–943. doi: 10.1002/hep.510300402. [DOI] [PubMed] [Google Scholar]
- Hill DB, Barve S, Joshi-Barve S, McClain C. Increased monocyte nuclear factor-kappaB activation and tumor necrosis factor production in alcoholic hepatitis. J Lab Clin Med. 2000;135:387–395. doi: 10.1067/mlc.2000.106451. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alpha attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- Yin M, Gabele E, Wheeler MD, Connor H, Bradford BU, Dikalova A, Rusyn I, Mason R, Thurman RG. Alcohol-induced free radicals in mice: direct toxicants or signaling molecules? Hepatology. 2001;34:935–942. doi: 10.1053/jhep.2001.28888. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gabele E, Rusyn I, Yamashina S, Froh M, Adachi Y, Iimuro Y, Bradford BU, Smutney OM, Connor HD, Mason RP, Goyert SM, Peters JM, Gonzalez FJ, Samulski RJ, Thurman RG. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53–61. doi: 10.1016/s0741-8329(02)00212-4. [DOI] [PubMed] [Google Scholar]
- Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol. 2001;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- Dhawan D, Goel A. Further evidence for zinc as a hepatoprotective agent in rat liver toxicity. Exp Mol Pathol. 1995;63:110–117. doi: 10.1006/exmp.1995.1035. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Hartley D, Klaassen CD, Shehin-Johnson SE, Lucas A, Cohen SD. Metallothionein-I/II knockout mice are sensitive to acetaminophen-induced hepatotoxicity. J Pharmacol Exp Ther. 1999;289:580–586. [PubMed] [Google Scholar]
- Kimura T, Fujita I, Itoh N, Muto N, Nakanishi T, Takahashi K, Azuma J, Tanaka K. Metallothionein acts as a cytoprotection against doxorubicin toxicity. J Pharmacol Exp Ther. 2000:299–302. [PubMed] [Google Scholar]
- Zhou Z, Sun X, Kang YJ. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med. 2002;227:214–222. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, Lambert JC, Saari JT, Kang YJ. Metallothionein-independent zinc protection from alcoholic liver injury. Am J Pathol. 2002;160:2267–2274. doi: 10.1016/S0002-9440(10)61174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Exp Phamarcol Ther. 2003;305:880–886. doi: 10.1124/jpet.102.047852. [DOI] [PubMed] [Google Scholar]
- Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Schumann J, Koerber K, Kiemer AK, Vollmar AM, Sass G, Papadopoulos T, Bang R, Klein SD, Brune B, Tiegs G. Low-molecular-weight hyaluronic acid induces nuclear factor-kappaB-dependent resistance against tumor necrosis factor alpha-mediated liver injury in mice. Hepatology. 2001;34:535–547. doi: 10.1053/jhep.2001.27218. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest. 1997;100:1501–1506. doi: 10.1172/JCI119672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FH, Zimmerman TJ, Shuler TR. Interactions among nickel, copper, and iron in rats: liver and plasma contents of lipid and trace elements. Biol Trace Elem Res. 1982;4:1225–1243. doi: 10.1007/BF02783253. [DOI] [PubMed] [Google Scholar]
- Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski PD, Forbes IJ, Betts WH. Correlation of apoptosis with a change in intracellular labile Zn, using Zinquin, a new specific fluorescent probe for Zn. Biochem J. 1993;296:403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P, Zalewski PD, Philcox JC, Forbes IJ, Ward AD, Lincoln SF, Mahadevan I, Rofe AM. Measurement of zinc in hepatocytes by using a fluorescent probe, Zinquin: relationship to metallothionein and intracellular zinc. Biochem J. 1994;303:781–786. doi: 10.1042/bj3030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastin CE, McClain CJ, Lee EY, Bagby GJ, Chawla RK. Choline deficiency augments and antibody to tumor necrosis factor-alpha attenuates endotoxin-induced hepatic injury. Alcohol Clin Exp Res. 1997;21:1037–1041. [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- Landmann R, Scherer F, Schumann R, Link S, Sansano S, Zimmerli W. LPS directly induces oxygen radical production in human monocytes via LPS binding protein and CD14. J Leukoc Biol. 1995;57:440–449. doi: 10.1002/jlb.57.3.440. [DOI] [PubMed] [Google Scholar]
- Fox ES, Brower JS, Bellezzo JM, Leingang KA. N-acetylcysteine and alpha-tocopherol reverse the inflammatory response in activated rat Kupffer cells. J Immunol. 1997;158:5418–5423. [PubMed] [Google Scholar]
- Bellezzo JM, Leingang KA, Bulla GA, Britton RS, Bacon BR, Fox ES. Modulation of lipopolysaccharide-mediated activation in rat Kupffer cells by antioxidants. J Lab Clin Med. 1998;131:36–44. doi: 10.1016/s0022-2143(98)90075-0. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Land SC. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-alpha biosynthesis. Br J Pharmacol. 2002;135:520–536. doi: 10.1038/sj.bjp.0704467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine decreases LPS-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats: respective roles of MAPKs and NF-kappaB. Biochem Biophys Res Commun. 2002;294:849–853. doi: 10.1016/S0006-291X(02)00586-7. [DOI] [PubMed] [Google Scholar]
- Chang CK, Albarillo MV, Schumer W. Therapeutic effect of dimethyl sulfoxide on ICAM-1 gene expression and activation of NF-kappaB and AP-1 in septic rats. J Surg Res. 2001;95:181–187. doi: 10.1006/jsre.2000.6033. [DOI] [PubMed] [Google Scholar]
- Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- Wadsworth TL, McDonald TL, Koop DR. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced signaling pathways involved in the release of tumor necrosis factor-alpha. Biochem Pharmacol. 2001;62:963–974. doi: 10.1016/s0006-2952(01)00734-1. [DOI] [PubMed] [Google Scholar]
- Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128:2334–2340. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-κB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- Victor VM, Rocha M, De la Fuente M. Regulation of macrophage function by the antioxidant N-acetylcysteine in mouse-oxidative stress by endotoxin. Int Immunopharmacol. 2003;3:97–106. doi: 10.1016/s1567-5769(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Sprong RC, Winkelhuyzen-Janssen AM, Aarsman CJ, van Oirschot JF, van der Bruggen T, van Asbeck BS. Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am J Respir Crit Care Med. 1998;157:1283–1293. doi: 10.1164/ajrccm.157.4.9508063. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003;163:1137–1346. doi: 10.1016/s0002-9440(10)63473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrni CJ, O’Halloran TV. Aqueous coordination chemistry of quinoline-based fluorescence probe for the biological chemistry of zinc. J Am Chem Soc. 1999;121:11448–11458. [Google Scholar]
- Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am J Physiol. 2002;282:L185–L192. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- Haase H, Beyersmann D. Intracellular zinc distribution and transport in C6 rat glioma cells. Biochem Biophys Res Commun. 2002;296:923–928. doi: 10.1016/s0006-291x(02)02017-x. [DOI] [PubMed] [Google Scholar]